Abstract
PKCζ (protein kinase C-ζ), a member of protein kinase C family, plays an important role in cell proliferation, differentiation, and apoptosis. It acts as a downstream molecule for TNF-α (tumor necrosis factor) signal transduction and also regulates the expression of CD1d, an HLA-class I-like molecule. The interaction of CD1d with natural killer T (NKT) cells has been shown to be important in their Th1 cytokine production in psoriasis. In this study, we examined PKCζ in psoriasis in order to define its role in the pathogenesis of the disease. We found that T-cell receptor (TCR) Vα24 + Vβ11 + NKT cells and CD1d molecules within psoriatic skin were increased. Moreover, there was an associated increase in PKCζ mRNA and protein expression with membrane translocation in psoriasis lesions compared to uninvolved skin. Furthermore, cultured keratinocytes exhibited increased PKCζ activity and membrane translocation upon stimulation by TNF-α, a cytokine known to play an important role in the pathogenesis of psoriasis. These results implied that PKCζ is an important transduction molecule downstream of TNF-α signaling and is associated with increased expression of CD1d that may enhance CD1d–NKT cell interactions in psoriasis lesions. This makes PKCζ a tempting target for possible pharmacological intervention in modifying the downstream effects of TNF-α in psoriasis.
INTRODUCTION
Psoriasis is a chronic inflammatory skin disorder characterized by erythematous plaques with silvery scales. Histologically, the lesions exhibit proliferation of epidermal keratinocytes (KCs), inflammatory cell infiltration, and increased angiogenesis of the superficial dermal vessels (Gaspari, 2006). There is evidence suggesting that infiltration of inflammatory cells, especially T lymphocytes, plays a major role in the development of the lesions in predisposed individuals, as the pathology develops following infiltration of lymphocytes and the Th1 cytokines they release, for example, IFN-γ and tumor necrosis factor-α (TNF-α) (Krueger and Bowcock, 2005; Gaspari, 2006; Lowes et al., 2007). Other immune cells, such as natural killer T (NKT) cells, dendritic cells, and a recently defined population of CD4 + T cells, Th17 cells, are increasingly recognized to contribute to or may even play a major part in the pathogenesis of the disease. The role of T cells and the cytokines in the pathogenesis of psoriasis is illustrated clinically by improvement of lesions after administration of cyclosporin to inhibit T-cell cytokine production, or the use of new biologicals to interfere with T-cell activation or to block the effects of cytokines such as TNF-α (Nickoloff and Stevens, 2006). A mouse xenograft model also demonstrated that T cells and other immunocytes, including cells with NKT cell features, from the blood or residing in the engrafted skin from the patients can reproduce typical lesions in the psoriatic engraftment (Nestle and Nickoloff, 2005).
NKT cells are cells of the innate immune system and have important functions in immunological tolerance, infection, anticancer immune response, and autoimmunity. NKT cells have limited T-cell receptor (TCR) gene usage, TCR Vα24, and Vβ11 in humans, and have the unique ability to release promptly large quantities of cytokines of both Th1 and Th2 types upon recognition of glycolipid antigens presented in the context of MHC class I-like molecule, CD1d (Godfrey et al., 2004). Increased number of NKT cells in psoriasis lesions has been reported in several recent studies, implicating them as cells of possible pathogenic significance (Bonish et al., 2000; Cameron et al., 2002; Vissers et al., 2004; Liao et al., 2006; Ottaviani et al., 2006). Nickoloff and co-workers (Bonish et al., 2000) demonstrated that psoriatic KCs overexpressed CD1d and NKT cells can be activated to elaborate IFN-γ when cultured with CD1d overexpressing KCs. These results provided a pathogenetic link between psoriatic KCs, which overexpress CD1d and NKT cells infiltrating the lesions. NKT cells as cells of innate immunity are of particular interest in view of the recent contentions that, rather than acquired immunity, it is innate immunity that is central to the pathogenesis of psoriasis (Bos, 2007). Therefore, studies on NKT cells and their antigen-presenting molecule CD1d are important in unraveling the role played by innate immunity in general and NKT cells in particular in the pathogenesis of psoriasis.
Protein kinase C-ζ (PKCζ) is a member of the PKC family and is involved in the signal transduction of TNF-α (Moscat et al., 2006). There is little information on the activities of PKCζ in psoriasis despite numerous studies on TNF-α in psoriasis. Our previous study has shown that PKCζ regulated CD1d mRNA expression and protein synthesis (Fishelevich et al., 2006). The question of interest therefore is whether TNF-α can initiate increased PKCζ activity as the downstream signal molecule, which, apart from relaying TNF-α signal to trigger a cascade of cellular events leading to psoriatic pathology, may also upregulate CD1d expression observed in psoriasis and thus be important in modulating interactions between CD1d-overexpressing KCs and the infiltrating NKT cells.
Therefore, in this report, changes in PKCζ in psoriasis and the mechanisms relating to its activation were studied. We show that NKT cells within the hyperplastic psoriatic epithelia are increased. Moreover, PKCζ mRNA and protein are increased in psoriasis lesions with membrane translocation of the phosphorylated form of PKCζ compared with uninvolved skin. Finally, primary KCs and HaCaT cells show PKCζ increase and membrane and nuclear translocation upon stimulation by TNF-α. These results imply that PKCζ is an important signal transduction molecule downstream of TNF-α stimulation, and also plays a role in the increased CD1d expression and potential involvement in KC–NKT cell interactions in psoriatic lesions.
RESULTS
Increased TCR Vα24 + Vβ11 + NKT cells in psoriasis
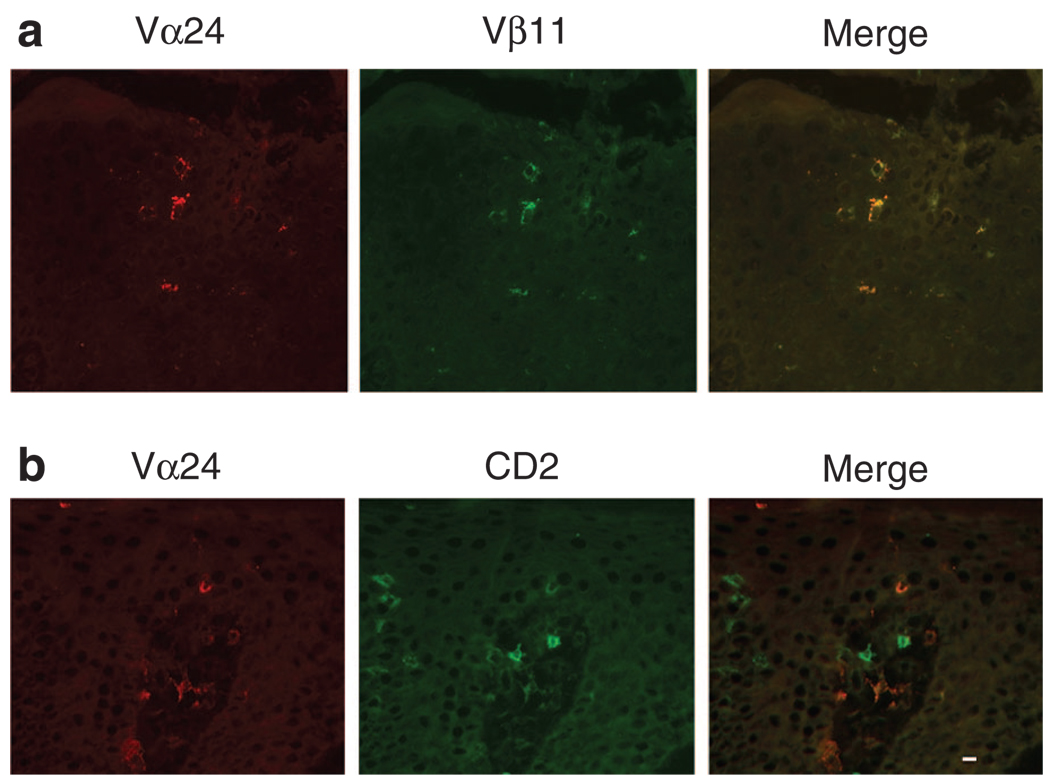
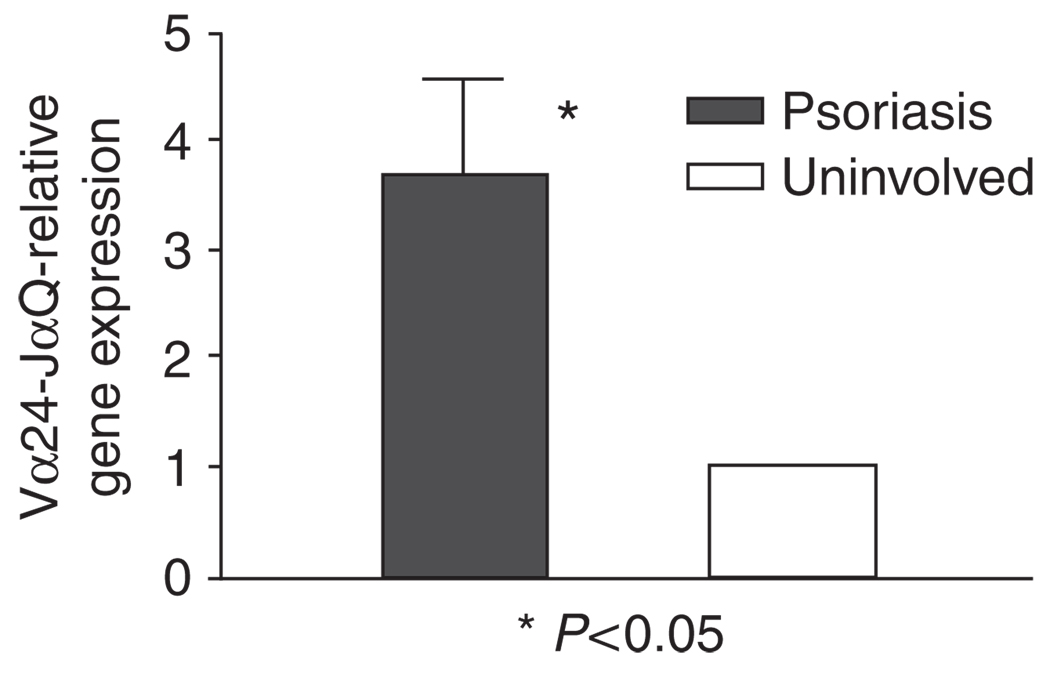
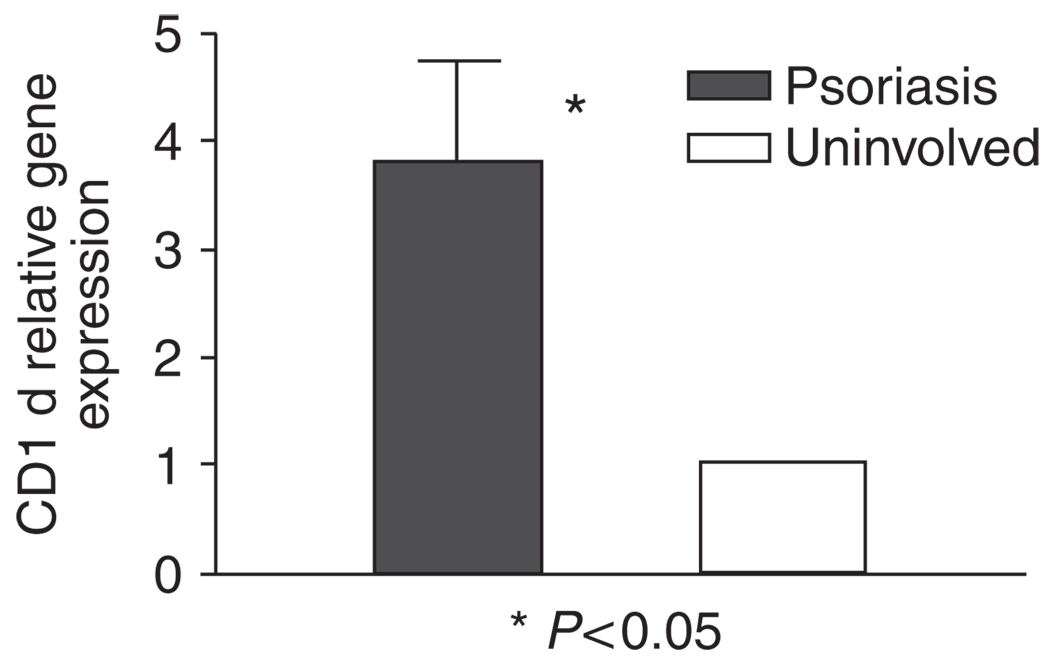
A number of studies have shown that NKT cells are associated with psoriatic lesions, including early, active, and relapsing lesions (Bonish et al., 2000; Cameron et al., 2002; Vissers et al., 2004; Liao et al., 2006; Ottaviani et al., 2006), although the exact role played by these cells is yet to be defined precisely. We hypothesize that NKT–KC interactions could be involved in the pathogenesis in psoriasis. Snap-frozen skin biopsy specimens were double-stained with anti-CD2 and anti-Vα24, or with anti-Vα24 and anti-Vβ11 mAbs. Vα24 + CD2 + or Vα24 + Vβ11 + double positive NKT cells were increased in psoriasis (4.7 NKT cells per millimeter of linear epidermis, n = 7, P < 0.0001) compared with healthy adult skin (0.3 NKT cell per millimeter, n = 6) (Figure 1). All Vα24- or Vβ11-positive cells were also positive for both markers. Although Vα24 + CD2 + double positive NKT cells were present in both epidermal and dermal compartments in psoriasis, the epidermis is the dominant compartment showing an enrichment of NKT cells compared with the dermis. We next sought to confirm whether the NKT cells in psoriasis observed by immunohistochemistry expressed the Vα24-JαQ chain, a unique combination characteristic of the “classical, invariant” NKT cells (Norris et al., 1999). Indeed, in all six psoriasis plaques, but not in the corresponding uninvolved skin examined, Vα24-JαQ was increased, both by nested PCR (not shown) and real-time PCR (Figure 2) (P < 0.05). These data confirmed at the transcript level that NKT cells were increased in psoriasis plaques than in uninvolved skin of these patients. The expression of CD1d was more extensive in psoriasis, spanning much of the full thickness, by immunohistochemistry, whereas in normal skin CD1d was expressed in the upper epidermis (data not shown). When skin from the six patients were assessed by quantitative real-time PCR, CD1d transcripts were increased in psoriatic plaques for more than 2- to 7-fold (P < 0.05) compared with uninvolved skin from each of the six patients (Figure 3).
Figure 1. NKT cells increased in psoriasis.
(a) Increase of Vα24 + Vβ11 + double positive NKT cells in the epidermis of a psoriatic plaque. All Vα24-positive cells in psoriasis were also Vβ11-positive. The isotype controls were negative and Vα24 and Vβ11 single labeling showed no isotype cross-reactivity (not shown). (b) CD2 + Vα24 + double positive NKT cells (merge), as well as Va24 (red) or CD2 (green) single positive cells, in the psoriatic epidermis and dermal papilla. Bar = 10 µm.
Figure 2. Infiltrating lymphocytes in psoriatic plaques express increased invariant Vα24-JαQ transcripts.
Relative Vα24-JαQ gene expression was measured using real-time PCR to analyze gene expression from six psoriasis (lesional and paired uninvolved skin). There is significant increase in relative Vα24-JαQ gene expression in psoriasis lesions (P < 0.05, Wilcoxon signed-rank test). Values are mean ± SD, error bar = 1 SD.
Figure 3. CD1d gene expression is increased in psoriatic plaques.
Increased CD1d mRNA in psoriatic plaques was shown compared with uninvolved skin in the six patients by real-time PCR (P < 0.05, Wilcoxon rank test). Values are mean ± SD, error bar=1 SD.
Increased PKCζ in psoriasis
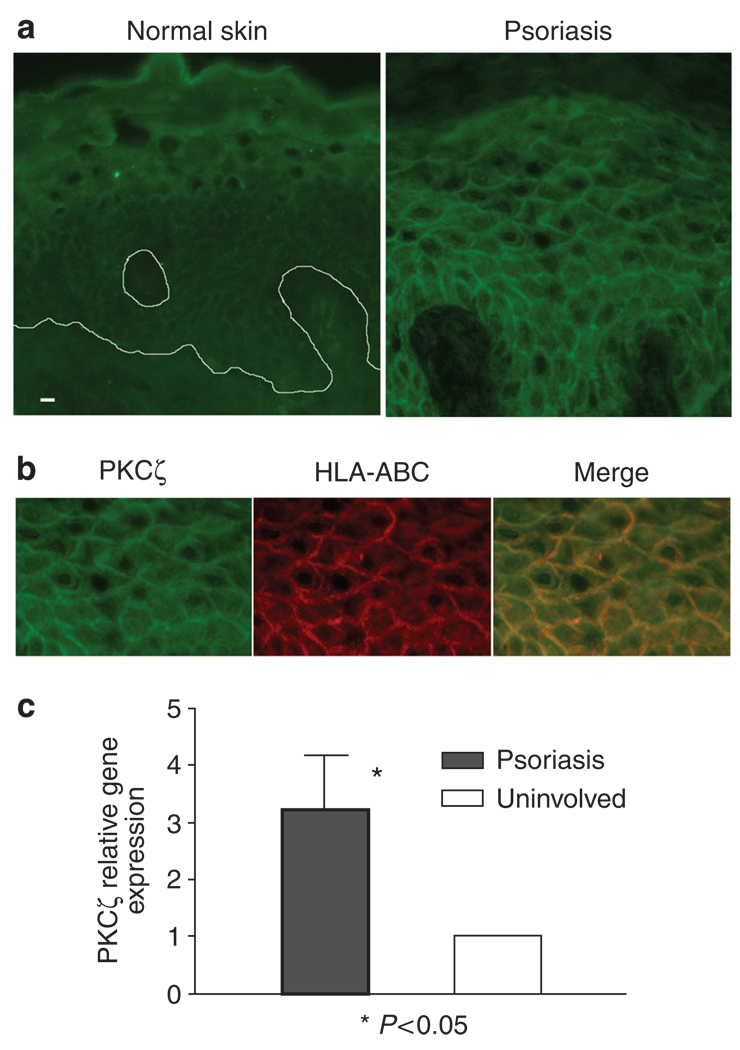
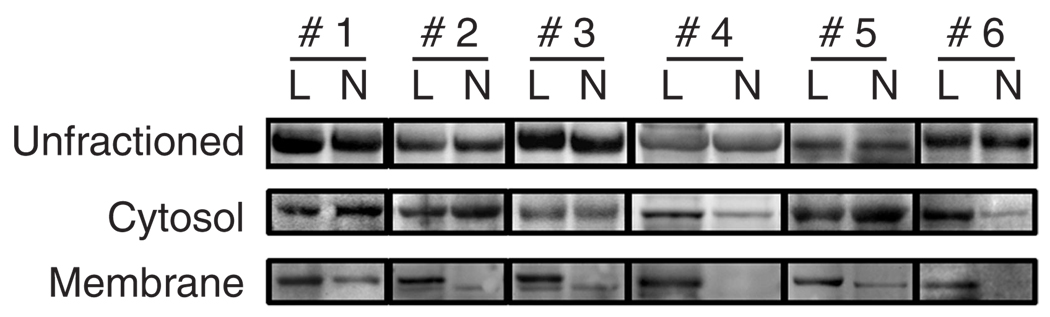
PKCζ is required for TNF-α signaling and nuclear factor-κB (NF-κB) activation (Moscat et al., 2006), both important in the pathogenesis of psoriasis (Schottelius et al., 2004; Lizzul et al., 2005). We have shown that in KCs (Fishelevich et al., 2006) and monocytes (our unpublished data), CD1d expression after cytokine stimulation was associated with increase in PKCζ activity, and CD1d expression at both mRNA and protein levels could be reduced by inhibiting PKCζ activity. We speculated that, as a result of TNF-α stimulation, there could be increased PKCζ activity, which might be an underpinning factor for the increase in CD1d expression on KCs in psoriasis. We therefore examined fresh and archival tissues of psoriasis for evidence of increased PKCζ expression. Immunofluorescence of snap-frozen samples for PKCζ in psoriasis specimens (n = 6) not only showed more extensive and stronger cytoplasmic staining spanning almost the full thickness of the hyperproliferative epithelia, but also a distinct membrane staining pattern, which colocalized with HLA-ABC antigen, compared with normal controls (n = 5) (Figure 4a and b). The epidermis of normal skin is moderately positive for PKCζ with a cytoplasmic staining pattern, mainly in the upper epidermis (n = 5). PKCζ gene expression was assayed using a quantitative real-time PCR in six pairs of psoriasis plaques and uninvolved skin, and was found to be increased significantly in all the psoriasis samples compared with the corresponding uninvolved skin (P < 0.05) (Figure 4c). To further compare the differences of PKCζ in psoriasis, western blot analysis of paired lysates from diseased and uninvolved skin (n = 6) was performed for PKCζ and its activated form, phospho-PKCζ, but no statistically significant differences in PKCζ or phospho-PKCζ were detected when whole lysates were studied (Figure 5). Since activation of PKCζ is associated with translocation of the enzyme from cytosol to the membrane (Nakanishi et al., 1993; Le Good et al., 1998), we therefore examined the translocation of this kinase to the membranes by ultracentrifugation fractionation of whole-tissue lysates from these cases. Phospho-PKCζ reactivity was significantly increased in the membrane (P < 0.01) but not the cytosolic fractions of the diseased skin, compared with their uninvolved counterparts (Figure 5). These data suggest that PKCζ is indeed activated in psoriatic plaques, resulting in its translocation from the cytoplasm to the plasma membrane.
Figure 4. Increased membrane expression of PKCζ by psoriatic plaques.
(a) Immunofluorescence of PKCζ in psoriasis and normal control skin showed increased cytoplasmic and membrane staining for PKCζ in psoriasis. Bar = 10 µm. (b) Double labeling with anti-HLA-ABC antibody showed membrane localization of PKCζ in psoriasis lesion. (c) When mRNA from six pairs of both lesional and uninvolved skin specimens was examined by real-time PCR, PKCζ gene expression was increased in all psoriasis samples compared with uninvolved skin (P < 0.05, Wilcoxon rank test). Values are mean ± SD, error bar = 1 SD.
Figure 5. Increased phosphorylated PKCζ in membrane fractions in psoriasis plaques.
Whereas no consistent difference was demonstrated in phosphorylated PKCζ in the whole lysates or in the cytosolic fractions of psoriasis and their uninvolved skin (n = 6), the membrane fractions of psoriasis lesions (L) showed significant increase in phospho-PKCζ compared with their uninvolved counterparts (N). P < 0.01, Student’s t-test. Values are mean ± SD, error bar = 1 SD.
TNF-α induces PKCζ activation and translocation in KCs
TNF-α is a cytokine critical for the development of psoriasis (Schottelius et al., 2004) and blockade of its activities with biological agents can lead to remarkable improvement of psoriatic skin lesions (Nickoloff and Stevens, 2006). TNF-α requires PKCζ as a downstream element in its signaling and it has been shown to stimulate PKCζ activity and translocation in a variety of cell types (Esteve et al., 2002; Kim and Rikihisa, 2002; Lisby and Hauser, 2002). To better understand the role played by TNF-α in PKCζ increase in psoriasis lesions, we studied cultured KCs for the effects of TNF-α stimulation in the activation and translocation of this protein kinase. Primary KCs showed increased expression of PKCζ and membrane staining by immunofluorescence by anti-PKCζ antibody after stimulation with TNF-α (not shown). Confocal imaging showed increased membrane staining and increased cytoplasmic and nuclear staining intensity of PKCζ in KC after TNF-α stimulation (Figure 6a–c). This membrane translocation and increased PKCζ staining after TNF-α stimulation are also corroborated by electron microscopy of immunoperoxidase staining of KCs (not shown) and HaCaT (Figure 6d). Immunoblotting of TNF-α-stimulated KC whole-cell lysates showed no significant increase of either PKCζ or phospho-PKCζ (Figure 7a). However, mitogen-activated protein kinase (MAPK) pathway, known to be activated as a result of PKCζ signaling, was activated by such treatment in KCs as well as in HaCaT (Figure 7a). This prompted us to investigate PKCζ in various cell fractions. As shown in Figure 7b, phospho-PKCζ was increased in both membrane and nuclear fractions of KCs, whereas changes of phospho-PKCζ in the cytosolic fractions remained insignificant. These findings supported the hypothesis that TNF-α is involved in the increased PKCζ activity and translocation in KCs.
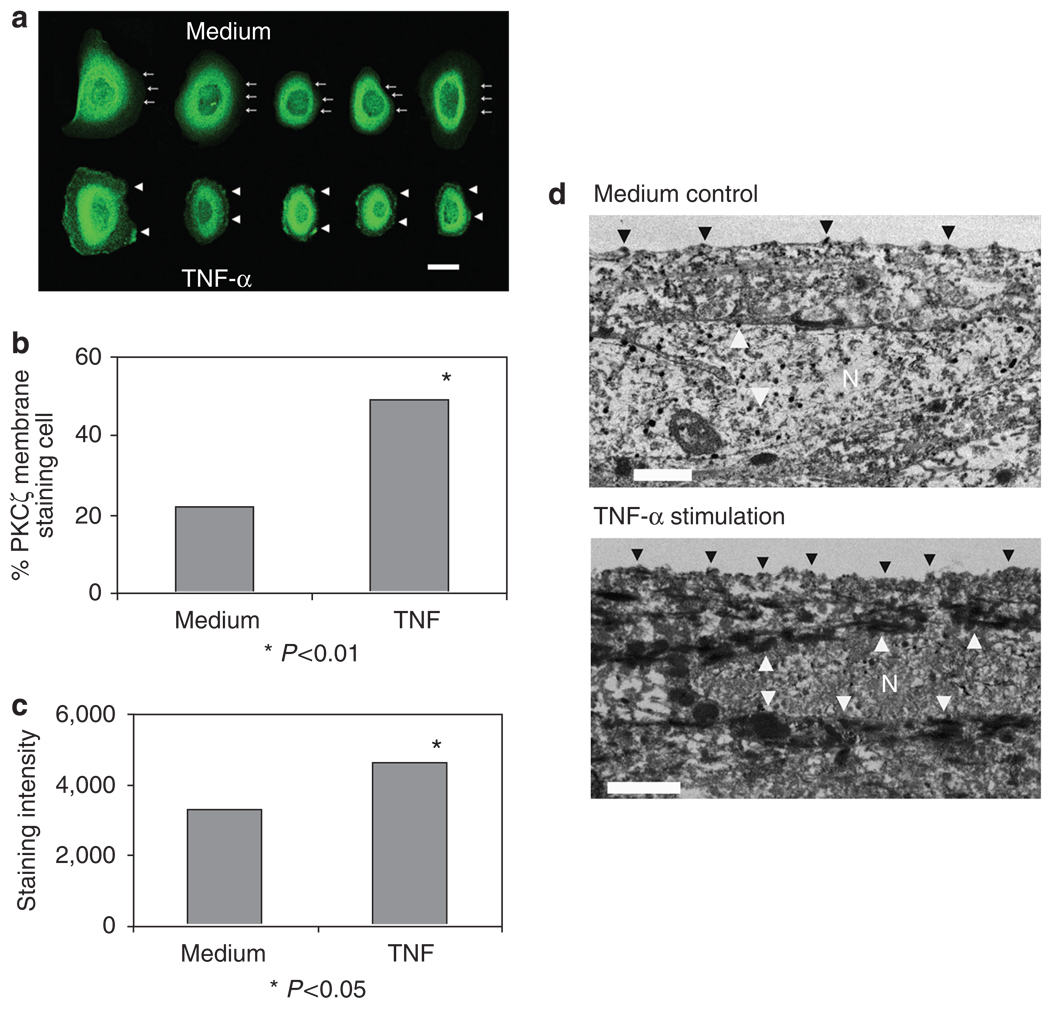
Figure 6. Increased PKCζ and membrane translocation in KCs stimulated with TNF.
(a) Confocal images: KCs cultured on coverslips stimulated with TNF-α showed prominent membrane staining for PKCζ (arrowheads) than the unstimulated cells (arrows at the dim cell boundaries). Bar = 10 µm. The picture is representative of three separate experiments. (b) The cells with membrane staining were increased (48.9%) significantly in TNF-α-stimulated cells compared with the medium alone (22.1%; P < 0.01 by Fisher’s exact test). One hundred cells were counted for each condition. (c) The fluorescence staining intensity of TNF-α stimulated cells increased significantly than those in medium alone. The intensities were measured by image analysis system of the fluorescence micrographs. P < 0.05, Student’s t-test. (d) Electron microscopic images of HaCaT on culture inserts stimulated with or without TNF-α, 100 ng ml−1, for 10 minutes. Cell stimulated with TNF-α showed stronger staining (arrowheads) for PKCζ in the plasma membrane as well as in the nuclear and perinuclear areas apart from cytoplasmic staining, compared with unstimulated control cells. Note: N denotes nucleus. Bar = 1 µm.
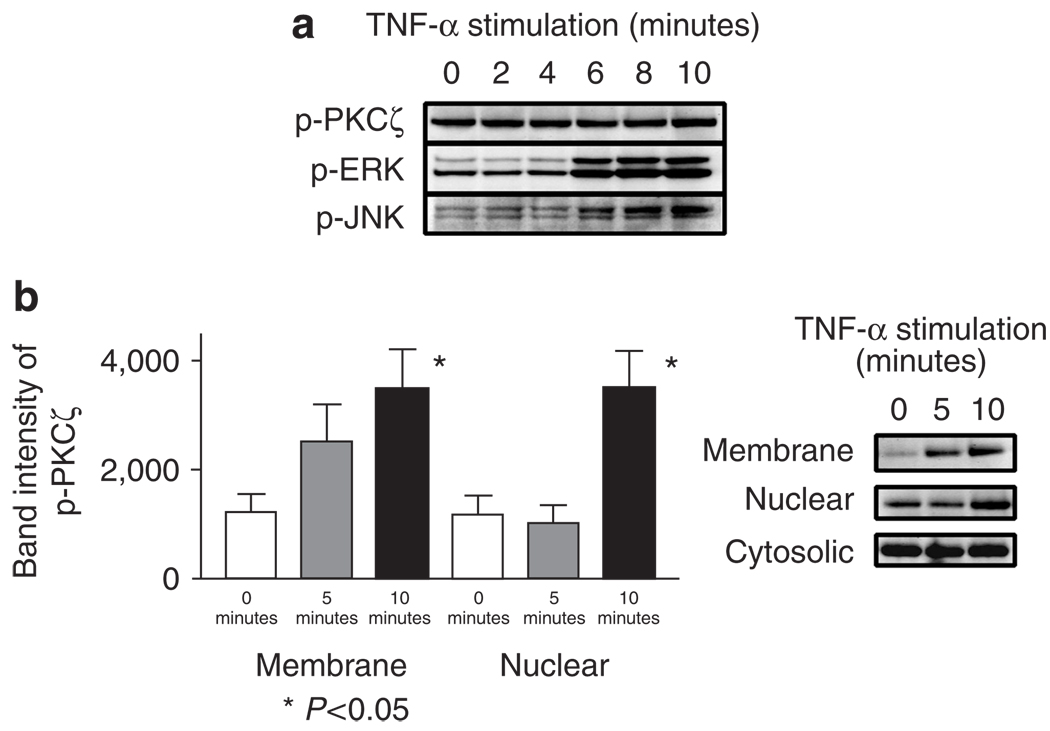
Figure 7. Increased phosphorylated PKCζ in membrane fractions of KCs stimulated with TNF.
(a) KCs were stimulated with 100 ng ml−1 of TNF-α for 0, 2, 4, 6, 8, and 10 minutes. While no significant change in the quantity of phospho-PKCζ was observed, pERK and pJNK were increased 6 minutes after TNF-α stimulation. (b) Increased phospho-PKCζ in the membrane and nuclear, but not in the cytosolic fractions, of primary KCs stimulated with TNF-α 100 ng ml−1 for 5 and 10 minutes (Student’s t-test). These blots are representations of three separate experiments. Values are mean ± SD, error bar = 1 SD.
DISCUSSION
There is considerable interest in NKT cells and their role in the pathophysiology of psoriasis. Increased NKT cells were consistently observed in psoriasis lesions by different groups (Bonish et al., 2000; Cameron et al., 2002; Vissers et al., 2004; Liao et al., 2006; Ottaviani et al., 2006). Experiments in severe combined immunodeficient mice have also shown that injection of human cells of NKT characteristics into transplanted psoriatic skin could drive lesion development (Nickoloff et al., 2000). Our present study showed increased densities of NKT cells using a set of precise markers for classical NKT cells, anti-Vα24 and anti-Vβ11 mAbs, in psoriatic lesions, especially in the epidermis, compared with healthy adult skin. CD1d molecules are HLA class I-like molecules whose antigen-binding clefts hold and present glycolipid antigens to NKT cells, which consequently release large quantities of cytokines and are of important immune regulatory significance (Godfrey et al., 2004). Among the cytokines released by activated NKT cells are IFN-γ and TNF-α, which are crucial in initiation and perpetuation of psoriatic lesions. It has been shown that NKT cells can indeed secrete IFN-γ rather than IL-4 when co-cultured with KC overexpressing CD1d (Bonish et al., 2000). These KCs may form a basis for pathological interactions with NKT cells, albeit in relative low numbers, infiltrating the psoriatic epidermis. Such an interaction might act as a trigger for NKT cytokine release, generating a cascade of events leading to the pathology of psoriasis.
The study of regulation of CD1d expression is of significance in the understanding of immunopathological role of NKT cells in psoriasis. In our previous report (Fishelevich et al., 2006), various factors regulating CD1d expression were studied and we established that PKCζ was an important enzyme for CD1d expression in KCs. In this study, we demonstrated that increased CD1d expression in psoriasis lesions was associated with increased PKCζ activation and gene expression. This indicates that PKCζ might be a crucial kinase controlling of CD1d expression in psoriasis. There have been similar reports showing the essential role PKCζ played in the expression of ICAM-1, which is also increased in psoriatic plaques (Rahman et al., 2000). PKC is a family of serine–threonine kinases with 11 members that can be classified into three groups: conventional PKCs (including α-, βI-, βII-, γ-isoforms), novel PKCs (including δ-, ε-, η-, θ-isoforms), and atypical PKCs (including ζ-, ι-isoforms) (Martelli et al., 2006; Moscat et al., 2006). PKCζ is an atypical isoform and regulates multiple cellular functions, including proliferation and differentiation, cell-cycle regulation, apoptosis, as well as tumorigenesis and autoimmunity (Martelli et al., 2006; Moscat et al., 2006). PKCζ is activated by phosphorylation of threonine at 410 and is then translocated to the plasma membrane or the nucleus (Martelli et al., 2006). Phospho-PKCζ can activate the MAPK pathway and nuclear factor NF-κB (Duran et al., 2003; Moscat et al., 2006). It has been shown that PKCζ was increased in squamous cell carcinoma and downregulation of PKCζ could inhibit cancer cell DNA synthesis and proliferation (Cohen et al., 2006). In osteoarthritis, chondrocytes overexpressing PKCζ produced increased amount of cartilage-destructive enzymes, whereas inhibition of PKCζ activity could reduce their production (LaVallie et al., 2006). PKCζ is also crucial for macrophage activation and expression of adhesion molecule ICAM-1, and metalloprotease-9 (Rahman et al., 2000; Esteve et al., 2002; Cuschieri et al., 2004). PKCζ is critical for CD1d expression in normal KCs and possibly its overexpression in psoriatic lesions. In psoriasis, however, PKCζ has a broader role to play than CD1d expression regulation, as it is important for signaling of TNF-α (Schottelius et al., 2004). PKCζ is one of the key downstream mediators of TNF-α signaling through to NF-κB activation and MAPK pathway in a variety of cell types (Berra et al., 1995; Moscat et al., 2006). For example, PKCζ is required for DNA synthesis and proliferation of epithelial KCs of the oral cavity via NF-κB and MAPK pathways (Cohen et al., 2006). Inhibition of NF-κB and MAPK or their upstream signaling mediator, PKCζ, can reduce KC proliferation. In this report, we further established that PKCζ in KC was activated by TNF-α and increased expression of this kinase in psoriasis lesions may be the result of TNF stimulation on the KCs in psoriatic epidermis. The effects of PKCζ activation on inflammation and KC proliferation in psoriasis are a topic that warrants further study.
It has been proposed that psoriasis is a disease caused by dysregulation of innate immunity, since TNF-α can be produced abundantly by cells of this innate arm of the immune system, such as macrophages, dendritic cells, and NKT cells, and neither the psoriasis antigen(s) nor the specific T cells for psoriasis have been identified unequivocally (Bos, 2007). Clinically, anti-TNF biological agents are highly effective for psoriasis in a large proportion of patients (Gaspari, 2006). The signal transduction of TNF-α to activate NF-κB is essential for execution of the proinflammatory effects by TNF, such as production of other inflammatory cytokines and chemokines (IL-1, IL-6, IL-8, and so on), and adhesion molecules, for example, ICAM-1 and VCAM, in the epidermis and the endothelial cells (Wajant et al., 2003; Schottelius et al., 2004). Increase in this activated phosphorylated form of NF-κB in psoriatic lesional KCs has been demonstrated in a recent study (Lizzul et al., 2005), and such elevated expression of NF-κB resumed to normal upon clinical improvement by anti-psoriatic treatment with a TNF blocker, etanercept. PKCζ can activate NF-κB by phosphorylating RelA p65 protein (Duran et al., 2003), crucial for its DNA binding after translocation into the nucleus. The DNA-bound NF-κB subsequently switches on the transcription of a host of important proinflammatory molecules (Schottelius et al., 2004). In our previous study, we found that PKCζ exerts its effects on CD1d synthesis also via NF-κB signaling (Fishelevich et al., 2006). The concerted effects of these molecules are activation of innate and acquired immunity, proliferation of KCs, as well as angiogenesis, and thus initiation and maintenance of psoriasis lesions.
In summary, in this study we found that there were increased NKT cells and their lipid antigen-presenting molecule CD1d within the psoriatic epidermis. Furthermore, PKCζ, which regulates CD1d expression, was increased in psoriasis and such increase could be stimulated by TNF-α, a cytokine critical in the pathogenesis of psoriasis. Taken together, PKCζ is critically located in the transduction pathway from TNF-α to activate NF-κB, the event crucial for the inflammatory process within the psoriasis lesions. This makes PKCζ an attractive target for pharmacological intervention for psoriasis, with possibly more selective inhibition of TNF-α effects.
MATERIALS AND METHODS
Reagents and antibodies
Anti-PKCζ rabbit polyclonal antibody, anti-pJNK, and anti-pERK mAbs were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA); anti-phospho-PKCζ rabbit polyclonal antibody was from Cell Signaling Technology (Beverly, MA); anti-CD1d mAb was from Biosource (Camarillo, CA); anti-TCR Vα24 and anti-Vβ11 mAbs were from Immunotech (Fullerton, CA); and anti-CD2-phycoerythrin or FITC mAbs, as well as goat anti-mouse IgG1 and IgG2a biotinylated antibodies, were from BD Pharmingen (San Diego, CA). Recombinant human TNF-α was purchased from Upstate Biotechnology (Lake Placid, NY). Protease inhibitor cocktail and phosphatase inhibitor cocktail were purchased from the Sigma Company (St Louis, MO).
Patient specimens
Patients without systemic treatment and not using topical treatments for at least 3 weeks were selected for the study after informed consent. Skin samples were obtained by punch biopsy under local lidocaine anesthesia. Psoriatic skin and uninvolved skin from 12 patients were homogenized and extracted for protein or RNA studies (six cases each). An additional seven cases of psoriasis lesional skin were collected for immunohistochemistry. Normal adult human skin specimens were obtained from healthy adult undergoing plastic surgery (n = 5). This study was reviewed and approved by the local institutional review board at University of Maryland, Baltimore. It followed the Declaration of Helsinki Principles for research involving human subjects.
Primary KC and HaCaT cell line
Single-cell suspension of KCs for primary culture was prepared by trypsinization of the epidermal sheets after separation from the dermis by dispase digestion of newborn foreskins, with approval of the local institutional review board. KCs were cultured in the presence of low concentration of CaCl2 (0.05mm) in Epi-Life growth medium supplemented with epidermal growth factor and pituitary extracts (Cascade Biologics, Portland, OR) at 37 °C with 5% CO2 in air. The human KC cell line HaCaT was cultured in DMEM with 10% fetal calf serum and antibiotics. For TNF-α stimulation study, KCs were serum- or growth factor-starved overnight, and incubated with TNF-α, 100 ng ml−1, for the indicated times before protein or RNA extraction.
Immunohistochemical procedures and confocal imaging
Frozen psoriasis samples were cut into 5-µm sections and fixed with cold acetone for 10 minutes. Cultured primary KCs or HaCaT cells were grown on coverslips and fixed with cold methanol for 10 minutes. PKCζ was stained with rabbit polyclonal antibody by indirect immunofluorescence for frozen sections, and cultured cells on coverslips. For double immunofluorescence of NKT cells, frozen sections were stained with primary antibody to TCR Vα24 by indirect immunofluorescence, followed by treatment with phycoerythrin- or FITC-conjugated anti-CD2 mAb. NKT cells were also detected for expression of both Vα24 (IgG1) and Vβ11 (IgG2a) using rhodamine-conjugated goat anti-mouse IgG and goat-anti-mouse IgG subclass antibodies and streptavidin–FITC. The staining was observed with Nikon Eclipse E600 epifluorescence microscope equipped with a digital camera. The images were documented using SpotTM imaging system (Diagnostic Instruments, Sterling Heights, MI). For laser-scanning confocal microscopy, stained cells on coverslips were examined with an LSM 510, Axiovert 100 laser confocal microscope (Zeiss, Carl Zeiss, Thornwood, NY) using an argon 458-nm laser for green fluorescence.
Immunoelectron microscopy
Cultured primary human KCs or HaCaT cells were grown in polyethylene terephthalate transparent inserts, pore size 0.4 µ. The inserts were then fixed in cold methanol and incubated with rabbit anti-human PKCζ antibody (Santa Cruz Biotechnology Inc.), and stained with Vectastain ABC immunoperoxidase procedure with EliteTM ABC substrate (Vector Laboratories, Burlingame, CA). Cells were then further fixed with 1/2 strength Karnovsky’s Fixative (1.6% paraformaldehyde, 2.5% glutaraldehyde in 0.1 m sodium cacodylate buffer). Following fixation, inserts were removed, post fixed in buffered 1% osmium tetroxide, impregnated, and embedded in epoxy resin (Polybed 812). Ultrathin epoxy sections (900 nm) prepared with a LEICA Ultracut ICU were nonspecifically stained with uranyl acetate and lead citrate, and analyzed with a JEOL JEM 1230 transmission electron microscope at 60,000 accelerating voltage.
Reverse transcription-PCR
Total RNAs from cultured KCs were prepared with the RNeasy kit (Qiagen, Valencia, CA) as per manufacturer’s instructions. Psoriasis samples were homogenized by Ultra-Turrax T8 homogenizer (IKA-Werke, Staufen, Germany) and RNA was isolated. Synthesis of cDNA was completed using standard methods. The expression of Vα24-JαQ TCR gene was examined using nested PCR amplification technique as described previously (Norris et al., 1999). Briefly, cDNA (1 µl) isolated from purified NKT cells from healthy adult peripheral blood (a generous gift from Dr D Unutmaz; Eger et al., 2006), biopsies from psoriasis, and normal skin were first amplified using Vα24 (5′-CACAAAGCAAAGCTCTCTGCACA) and Cα (5′-GCC ACAGCACTGTTGCTCTTG) primers to generate a 326-bp fragment. The amplicons were amplified in the second round using Vα24 and JαQ (5′-GAGTTCCTCTTCCAAAGTATAGCCT) primers, resulting in amplification of the 119-bp Vα24-JαQ fragment. The 18S gene was also amplified in the same biopsy samples using an equivalent volume of cDNA and the following primers: 5′-GTAACCCGTT GAACCCCATT (sense) and 5′-CCATCCAATCGGTAGTAGCG (antisense). For all amplifications, one cycle at 94 °C for 60 seconds followed by 30 cycles involving denaturation at 94 °C for 30 seconds, annealing at 56 °C for 40 seconds, and extension at 72 °C for 40 seconds were performed. The PCR products were visualized by 1.5% agarose gel electrophoresis with ethidium bromide staining.
Real-time PCR
Real-time PCR (LightCycler; Roche Applied Science, Indianapolis, IN) was used to examine different levels of expression of selected mRNA. The hybridization probes were labeled with fluorescein at the 5′ terminus (3FL) and with LightCycler Red at the 5′ terminus of the other probe. Amplification of a single PCR product was confirmed by gel electrophoresis and melting-curve analyses. The sequences of the sense, antisense, and internal hybridization probes are as follows: 18S (sense), 5′-AACCCGTTGAACCCCATT-3′; 18S (antisense), 5′-CCATCCAATCGGTAGTAGCG-3′; CD1d (sense), 5′-AGACATGGTATCTCCGAGCAAC-3′; CD1d (antisense), 5′-CTGAGCAGACCAGGACTGAA-3′; FL-probe, 5′-TAGTAGCTCCCACCCCAGTAGAGGAC-FL-3′; and LC-probe, 5′-LC Red640-ATGTCCTGGCCCTCTAGACTGCTGTGPH-3′. After denaturation, cDNA were subjected to 40–50 cycles of amplification, followed by melting-curve analysis. For quantification of Vα24-JαQ expression, real-time PCR was performed using the LightCycler FastStart DNA Master SYBR Green kit (Roche Applied Science) according to manufacturer’s instructions, and using real-time PCR conditions for 18S quantification. PKCζ real-time PCR was performed with Qiagen QuantiTect Gene Expression Assay using Hs-PRKCZ Assay Mix. The conditions recommended by the manufacturer were used, with an initial activation step of 15 minutes at 95 °C, followed by denaturation at 94 °C for 0 seconds, annealing/detection at 56 °C for 30 seconds, and extension at 76 °C for 30 seconds for 40–45 cycles.
Western blot
Cultured KCs were lysed by radioimmunoprecipitation assay buffer with protease and phosphatase inhibitor cocktails (Sigma Company). Psoriasis lesional and uninvolved skin or normal skin tissues were homogenized in radioimmunoprecipitation assay buffer supplemented with protease and phosphatase inhibitor cocktails. The protein concentration was measured by Lowry method. Equal quantities of total proteins (usually 20–30 µg of proteins) were loaded on the 10% Bis-Tris NuPage denaturing gel (Invitrogen Life Technologies, Carlsbad, CA) for electrophoresis. Gels were transferred to nitrocellulose membranes and were probed with appropriate antibodies and detected by WesternBreeze Chemiluminescent detection kit (Invitrogen Life Technologies). The enhanced chemiluminescence bands were detected using Chemi-Doc densitometer and Quantity-One software (Bio-Rad, Hercules, CA). The intensity of the bands was expressed in arbitrary units.
Cell fractionation
Cultured KCs were lysed in hypotonic buffer (10mm Tris-HCl pH 7.5, 1.0mm MgCl2, 10mm KCl, 10mm EDTA, 1.0mm dithiothreitol, 1.0mm Na3VO4, 100 × protease and phosphatase inhibitor cocktails) and centrifuged at 750 g for 10 minutes. The pellets were collected as the nuclear fraction and the supernatants were centrifuged at 100,000 g for 1 hour at 4 °C (Beckman Coulter, Fullerton, CA). The supernatants were regarded as the cytosolic fractions and the pellets were resuspended in the above hypotonic buffer with 1% Triton X-100 (Sigma Company) before pulse sonication (Sonic Dismembrator, Model 100; Fisher Scientific, Waltham, MA). The resuspensions were incubated at 4 °C for 30 minutes before centrifugation at 20,000 g for 20 minutes. The supernatants were collected as the plasma-membrane fractions. Fresh psoriasis biopsies were cut into small pieces and homogenized in radioimmunoprecipitation assay buffer, and then incubated with rotation at 4 °C for 30 minutes before centrifugation at 20,000 g at 4 °C for 10 minutes. The supernatants were subjected to centrifugation at 100,000 g for 1 hour. The membrane and cytosolic fractions were collected and the cell lysates or cell fractions were further analyzed by western blotting with equal loading of the total proteins in each lane in the same gel.
Statistical analysis
Quantitative and qualitative data were analyzed for statistically significant differences between control and treatment groups by t-test, Mann–Whitney rank-sum test, or χ2-test using GraphPad InStat software (P < 0.05 was considered significant).
ACKNOWLEDGMENTS
We acknowledge the generous gift of purified NKT cells from Dr D Unutmaz. This work was funded by NIH Grant R01-AR46108-05 and a VA Merit Award from the Department of Veteran Affairs (to AAG).
Abbreviations
- KC
keratinocyte
- NF-κB
nuclear factor-κB
- NKT
natural killer T
- PKCζ
protein kinase C ζ
- TNF
tumor necrosis factor
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Berra E, Díaz-Meco MT, Lozano J, Frutos S, Municio MM, Sánchez P, et al. Evidence for a role of MEK and MAPK during signal transduction by protein kinase C zeta. EMBO J. 1995;14:6157–6163. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonish B, Jullien D, Dutronc Y, Huang BB, Modlin R, Spada FM, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells. J Immunol. 2000;165:4076–4085. doi: 10.4049/jimmunol.165.7.4076. [DOI] [PubMed] [Google Scholar]
- Bos JD. Psoriasis, innate immunity, and gene pools. J Am Acad Dermatol. 2007;56:468–471. doi: 10.1016/j.jaad.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Cameron AL, Kirby B, Fei W, Griffiths CE. Natural killer and natural killer-T cells in psoriasis. Arch Dermatol Res. 2002;294:363–369. doi: 10.1007/s00403-002-0349-4. [DOI] [PubMed] [Google Scholar]
- Cohen EE, Lingen MW, Zhu B, Zhu H, Straza MW, Pierce C, et al. Protein kinase C zeta mediates epidermal growth factor-induced growth of head and neck tumor cells by regulating mitogen-activated protein kinase. Cancer Res. 2006;66:6296–6303. doi: 10.1158/0008-5472.CAN-05-3139. [DOI] [PubMed] [Google Scholar]
- Cuschieri J, Umanskiy K, Solomkin J. PKC-zeta is essential for endotoxin-induced macrophage activation. J Surg Res. 2004;121:76–83. doi: 10.1016/j.jss.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Duran A, Diaz-Meco MT, Moscat J. Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation. EMBO J. 2003;22:3910–3918. doi: 10.1093/emboj/cdg370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger KA, Sundrud MS, Motsinger AA, Tseng M, Van Kaer L, Unutmaz D. Human natural killer T cells are heterogeneous in their capacity to reprogram their effector functions. PLoS ONE. 2006;1:e50. doi: 10.1371/journal.pone.0000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve PO, Chicoine E, Robledo O, Aoudjit F, Descoteaux A, Potworowski EF, et al. Protein kinase C-zeta regulates transcription of the matrix metalloproteinase-9 gene induced by IL-1 and TNF-alpha in glioma cells via NF-kappa B. J Biol Chem. 2002;277:35150–35155. doi: 10.1074/jbc.M108600200. [DOI] [PubMed] [Google Scholar]
- Fishelevich R, Malanina A, Luzina I, Atamas S, Smyth MJ, Porcelli SA, et al. Ceramide-dependent regulation of human epidermal keratinocyte CD1d expression during terminal differentiation. J Immunol. 2006;176:2590–2599. doi: 10.4049/jimmunol.176.4.2590. [DOI] [PubMed] [Google Scholar]
- Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54(3 Suppl 2):S67–S80. doi: 10.1016/j.jaad.2005.10.057. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- Kim HY, Rikihisa Y. Roles of p38 mitogen-activated protein kinase, NF-kappaB, and protein kinase C in proinflammatory cytokine mRNA expression by human peripheral blood leukocytes, monocytes, and neutrophils in response to Anaplasma phagocytophila. Infect Immun. 2002;70:4132–4141. doi: 10.1128/IAI.70.8.4132-4141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 Suppl II:ii30–ii36. doi: 10.1136/ard.2004.031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVallie ER, Chockalingam PS, Collins-Racie LA, Freeman BA, Keohan CC, Leitges M, et al. Protein kinase Czeta is upregulated in osteoarthritic cartilage and is required for activation of NF-kappaB by tumor necrosis factor and interleukin-1 in articular chondrocytes. J Biol Chem. 2006;281:24124–24137. doi: 10.1074/jbc.M601905200. [DOI] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- Liao YH, Jee SH, Sheu BC, Huang YL, Tseng MP, Hsu SM, et al. Increased expression of the natural killer cell inhibitory receptor CD94/ NKG2A and CD158b on circulating and lesional T cells in patients with chronic plaque psoriasis. Br J Dermatol. 2006;155:318–324. doi: 10.1111/j.1365-2133.2006.07301.x. [DOI] [PubMed] [Google Scholar]
- Lisby S, Hauser C. Transcriptional regulation of tumor necrosis factor-alpha in keratinocytes mediated by interleukin-1beta and tumor necrosis factor-alpha. Exp Dermatol. 2002;11:592–598. doi: 10.1034/j.1600-0625.2002.110612.x. [DOI] [PubMed] [Google Scholar]
- Lizzul PF, Aphale A, Malaviya R, Sun Y, Masud S, Dombrovskiy V, et al. Differential expression of phosphorylated NF-kappaB/RelA in normal and psoriatic epidermis and downregulation of NF-kappaB in response to treatment with etanercept. J Invest Dermatol. 2005;124:1275–1283. doi: 10.1111/j.0022-202X.2005.23735.x. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Evangelisti C, Nyakern M, Manzoli FA. Nuclear protein kinase C. Biochim Biophys Acta. 2006;1761:542–551. doi: 10.1016/j.bbalip.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Moscat J, Rennert P, Diaz-Meco MT. PKCzeta at the crossroad of NF-kappaB and Jak1/Stat6 signaling pathways. Cell Death Differ. 2006;13:702–711. doi: 10.1038/sj.cdd.4401823. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Brewer KA, Exton JH. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- Nestle FO, Nickoloff BJ. From classical mouse models of psoriasis to a spontaneous xenograft model featuring use of AGR mice. Ernst Schering Res Found Workshop. 2005;50:203–212. doi: 10.1007/3-540-26811-1_11. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Bonish B, Huang BB, Porcelli S. Characterization of a T cell line bearing natual killer receptors and capable of creating psoriasis in a SCID mouse model system. J Dermatol Sci. 2000;24:212–225. doi: 10.1016/s0923-1811(00)00120-1. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Stevens SR. What have we learned in dermatology from the biologic therapies? J Am Acad Dermatol. 2006;54(3 Suppl 2):S143–S151. doi: 10.1016/j.jaad.2005.10.059. [DOI] [PubMed] [Google Scholar]
- Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, et al. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha 24-JalphaQ and gamma/delta T cell receptor bearing cells. Hum Immunol. 1999;60:20–31. doi: 10.1016/s0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Nasorri F, Bedini C, de Pita O, Girolomoni G, Cavani A. CD56brightCD16(−) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36:118–128. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- Rahman A, Anwar KN, Malik AB. Protein kinase C-zeta mediates TNF-alpha-induced ICAM-1 gene transcription in endothelial cells. Am J Physiol Cell Physiol. 2000;279:C906–C914. doi: 10.1152/ajpcell.2000.279.4.C906. [DOI] [PubMed] [Google Scholar]
- Schottelius AJG, Moldawer LL, Dinarello CA, Asadullah K, Sterry W, Edwards CK., III Biology of tumor necrosis factor-a—implications for psoriasis. Exp Dermatol. 2004;13:193–222. doi: 10.1111/j.0906-6705.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- Vissers WH, Arndtz CH, Muys L, Van Erp PE, de Jong EM, van de Kerkhof PC. Memory effector (CD45RO+) and cytotoxic (CD8+) T cells appear early in the margin zone of spreading psoriatic lesions in contrast to cells expressing natural killer receptors, which appear late. Br J Dermatol. 2004;150:852–859. doi: 10.1111/j.1365-2133.2004.05863.x. [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]