Abstract
Context:
Ranibizumab and bevacizumab are used widely for treating patients with choroidal neovascular membrane (CNVM) secondary to age-related macular degeneration (AMD).
Aims:
To determine and compare the efficacy and safety of intravitreal ranibizumab and bevacizumab in treatment of CNVM due to AMD.
Settings and Design:
Prospective comparative case series carried out in an eye institute and eye department of a hospital in Kolkata, India.
Materials and Methods:
One hundred and four eyes with CNVM due to AMD were randomized into two groups. Group A (n=54; 24 occult) received monthly intravitreal ranibizumab injections (0.5 mg in 0.05 ml) and Group B (n=50; 22 occult) received monthly bevacizumab injections (1.25 mg in 0.05 ml) for 3 consecutive months and then as per study criteria. Data analysis done using SPSS software. P-value of <0.05 was considered statistically significant.
Results:
The mean best corrected visual acuity (BCVA) in the ranibizumab group increased from 58.19 Early Treatment Diabetic Retinopathy Study (ETDRS) letters at baseline to 64 ETDRS letters at month 3 (P<0.001). In bevacizumab group mean BCVA increased from 56.80 to 61.72 ETDRS letters at month 3 (P<0.001). At the end of 18 months, there was no statistically significant difference between groups A and B with respect to change in BCVA (P=0.563) or central macular thickness (CMT; P=0.281), as measured by optical coherence tomography (Stratus OCT 3000). No significant sight-threatening complications developed.
Conclusions:
Ranibizumab and bevacizumab are equally safe and efficacious in treating CNVM due to AMD.
Keywords: Ranibizumab, bevacizumab, choroidal neovascular membrane, age-related macular degeneration, intravitreal injection, central macular thickness, best corrected visual acuity
Vascular endothelial growth factor-A (VEGF – A) has been proved to play a major role in the pathogenesis of neovascular age-related macular degeneration (AMD).[1,2] The major anti-VEGFs in use today for the treatment of choroidal neovascular membrane (CNVM) secondary to AMD are pegaptanib sodium, ranibizumab and bevacizumab. The other drugs being evaluated for this purpose include VEGF Trap, combrestatin, sirolimus, squalamine (inhibits plasma membrane ion channels), vatalanib and pazopanib (receptor tyrosine kinase inhibitors) and bevasiranib (small interfering RNA).
Ranibizumab is a recombinantly produced, humanized, antibody (Fab) fragment that binds all biologically active isoforms of VEGF-A.[3] It has only one antigen-binding domain. Ranibizumab received United States Food and Drug Administration (USFDA) approval for the treatment of neovascular AMD on June 30, 2006. Bevacizumab is a humanized full-length antibody that is derived from the same monoclonal antibody as ranibizumab.[4] It has two antigen-binding domains and a longer half life of about 17-21 days. On February 26, 2004, the USFDA approved bevacizumab as a first-line treatment for intravenous use in patients with metastatic colorectal cancer.[5]
Although ranibizumab is USFDA-approved for the treatment of CNVM secondary to AMD, it is indeed a costly treatment option. On the other hand, bevacizumab has been widely used off label for the treatment of CNV (at a dose of 1.25 mg in 0.05 ml)[6] and is a relatively inexpensive treatment option when a single vial of bevacizumab is shared among multiple patients on the same day. Infact, ranibizumab should be atleast 2.5 times more efficacious than bevacizumab for it to be cost-effective.[7]
The cost difference between ranibizumab and bevacizumab could be highly significant for people who have limited or no health insurance coverage and this aspect assumes a greater significance in the Indian scenario. Thus, there is a very urgent need of carrying out large multicentric randomized controlled prospective studies comparing these two drugs with respect to their efficacies and safety profile. Two such studies are already underway (IVAN[8] and CATT[9] ),but the complete results are not yet available. Our study aimed to determine and compare the efficacy and safety of intravitreal ranibizumab and bevacizumab in treatment of CNVM due to AMD in the Indian scenario.
Materials and Methods
Approval from the institutional ethical committee was obtained for performing this prospective randomized study. Patients attending outpatient department of a tertiary hospital in Kolkata with complaints of dimness or blurring or distortion of vision, were subjected to complete ophthalmological examination. Patients were subjected to visual acuity testing by early treatment diabetic retinopathy study (ETDRS) chart, intraocular pressure measurement by applanation tonometry, gonioscopy, Amsler Grid assessment, slit-lamp examination and biomicroscopy after pupillary dilatation using +90 diopter (D) lens and direct and indirect ophthalmoscopy. Patients suspected to have wet AMD were referred to a tertiary eye institute in Kolkata for digital fundus fluorescein angiography (after adequate medical clearance) and optical coherence tomography (OCT), macular scan, to reach a definite diagnosis of CNVM formation.
Patients fulfilling all the following criteria were included:
Patients aged more than 50 years.
Patients with baseline best corrected visual acuity (BCVA) between 35 and 70 ETDRS letters.
All cases of CNVM with classic and occult lesions.
All cases of subfoveal and juxtafoveal CNVM.
Cases with active leakage pattern.
Baseline central macular thickness (CMT) greater than or equal to 250 μm measured by Stratus OCT 3000 (Carl Zeiss Meditec, USA) using six radial lines of 6 mm each at the macular region. The CMT assessment was done using calipers.
No previous treatment for CNVM in either eye.
Exclusion criteria included any one of the following:
Macular scarification.
Coexisting other ocular pathology (like advanced cataract, high myopia, chorio-retinal atrophic patches, diabetic retinopathy, glaucoma)
One-eyed patients.
History of ocular surgery within last 6 months.
History of cerebrovascular accident and myocardial infarction.
Patients fulfilling the criteria of our study were informed of the nature of treatment that they would be offered, the potential risks, benefits, adverse effects, alternative treatment options and possible treatment outcomes. This was explained in their native language. Patients who wished to enroll signed a consent form. In the hospital where this study was carried out, the patients did not pay for their treatment and the hospital took care of the financial aspect of all treatments (including the cost of ranibizumab and bevacizumab injections) given to the patients. The consultant ophthalmologist had the discretion to prescribe suitable treatment for his/her patient after discussing the same. Informed consent was also obtained on subsequent treatment visits requiring intravitreal injections.
The study aimed to enroll a total of 120 patients over a period of about 6 months. This number was arrived at by the investigators after considering the sample size of the available literature of relevant studies at the time of initiating this study. Using random number tables, 60 numbers were randomly picked up from 1 to 120 and assigned to group A while the remaining sixty numbers were assigned to group B. This randomization of the 120 numbers into two groups was done before initiation of enrolment itself. Upon initiation of enrollment, the patients were numbered sequentially based on the serial order of enrolment in the study. Depending on the enrolment number, the patients were automatically assigned to either group A or B based on the prior randomization of numbers 1-120 into two equal groups using random number tables.
The patients in group A were given intravitreal injections of 0.5 mg ranibizumab (Lucentis) in 0.05 ml in the operation theatre, taking full aseptic measures on three consecutive months (Months 0, 1 and 2). Similarly, the patients in group B were given intravitreal injections of 1.25 mg bevacizumab (Avastin) in 0.05 ml in the operation theatre taking full aseptic measures on three consecutive months (Months 0, 1 and 2). The injections were given through the pars plana route 3.5-4.00 mm behind the limbus by the investigators, who were blinded to the type of injection. The patients were followed up for 6 hours after injection to check for visual acuity, intraocular pressure and status of the optic disc.
The patients were followed up monthly for the next 18 months. All assessors were masked to the group of patient they were following up. Visual acuity and assessment of CMT using Stratus OCT 3000 using six radial lines of 6 mm each at the macular region was performed along with measurement of blood pressure and enquiry about any unusual new extremity pain for each patient at each follow-up visit. Repeat injections (of the same drug as the initial one, at the same dose) were given to any patient who showed an increase in CMT of more than 100 μm after the initial three injections in either group or a fall in BCVA by more than 5 ETDRS letters.
The primary outcome measures were the changes in BCVA and CMT from baseline (month 0) to month 18. The results obtained were statistically analyzed using SPSS (Statistical Package for the Social Sciences, version 10) and the null hypothesis was rejected for P-value less than 0.05.
Results
One hundred and twenty eyes of 120 patients were enrolled for the study which extended from 01-04-07 to 01-04-09. Sixteen patients were lost to follow-up. Ultimately, group A had 54 cases and group B had 50 cases. So, 90% of the patients completed 18-months follow-up in group A and 83.33% of the patients completed 18-months follow-up in group B.
Results from 54 eyes with CNVM due to AMD were analyzed in the ranibizumab group (group A) out of which 24 eyes had occult CNVM. Results from 50 eyes were analyzed in the bevacizumab group (group B) out of which 22 eyes had occult CNVM. The results from the two groups were compared. The mean age of patients in the ranibizumab group was 63.48 years and that in the bevacizumab group was 64.36 years and there was no statistically significant difference between the two groups with respect to age of patients (P=0.76). There were 22 males (40.74%) and 32 females in the ranibizumab group and 28 males (56%) and 22 females in the bevacizumab group.
Minor complications developed in 7.29% patients in the ranibizumab group whereas 11.10% patients developed minor complications in the bevacizumab group. The mean number of intravitreal injections required in the ranibizumab group was 5.6 and that in the bevacizumab group was 4.3.
The mean BCVA in the ranibizumab group increased from 58.19 ETDRS letters at baseline to 64 ETDRS letters after the first three intravitreal ranibizumab injections [Table 1]. The change in BCVA in ranibizumab group was statistically significant from baseline to month 3 (P<0.001) [Table 1]. The BCVA at the end of 18 months in this group was 61.74 ETDRS letters (P=0.048) [Table 1].
Table 1.
Assessment of change in best corrected visual acuity in Early Treatment Diabetic Retinopathy Study letters at baseline, months 3, 6, 12 and 18 in ranibizumab group (group A, 54 cases) and bevacizumab group (group B, 50 cases)
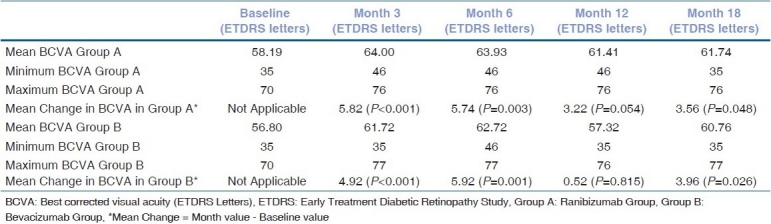
The mean BCVA in the bevacizumab group increased from 56.80 ETDRS letters at baseline to 61.72 ETDRS letters after the first three intravitreal bevacizumab injections [Table 1]. This change was also statistically significant from baseline to month 3 (P<0.001) [Table 1]. The BCVA at the end of 18 months in this group was 60.76 ETDRS letters (P=0.026) [Table 1].
In the ranibizumab group, 56% patients maintained BCVA within +/- 5 ETDRS letters from baseline to the end of the 18th month of follow-up (referred to as maintenance of vision), whereas 11% patients had a loss of >5 ETDRS letters at the end of 18 months (referred to as deterioration of vision). The remaining 33% patients had an increase of >5 ETDRS letter at the end of follow-up period (referred to as improvement of vision). Similarly in the bevacizumab group, 60% patients maintained vision within +/- 5 ETDRS letters, 32% improved more than 5 letters and 8% reported a deterioration of >5 ETDRS letters equivalent at the end of 18 months.
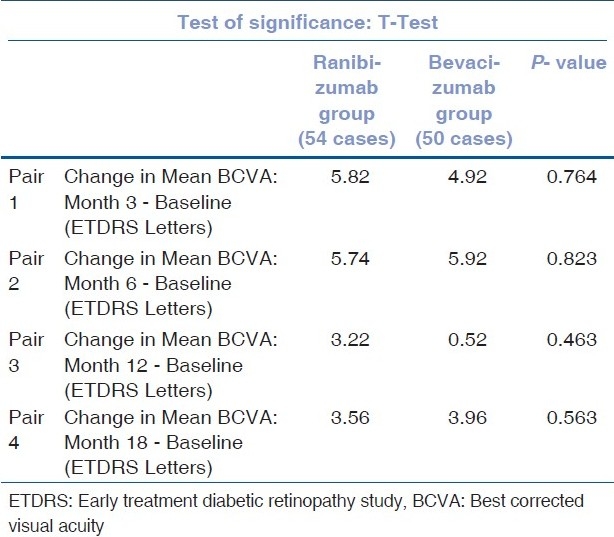
The change in mean BCVA (ETDRS letters) between ranibizumab and bevacizumab groups was compared [Fig. 1] and the test of significance carried out using the ‘t’ test. It is seen that the P-values at months 3, 6, 12 and 18 were 0.764, 0.823, 0.463 and 0.563, respectively. Thus, there was no statistically significant difference between the BCVA changes in the two groups [Table 2].
Figure 1.
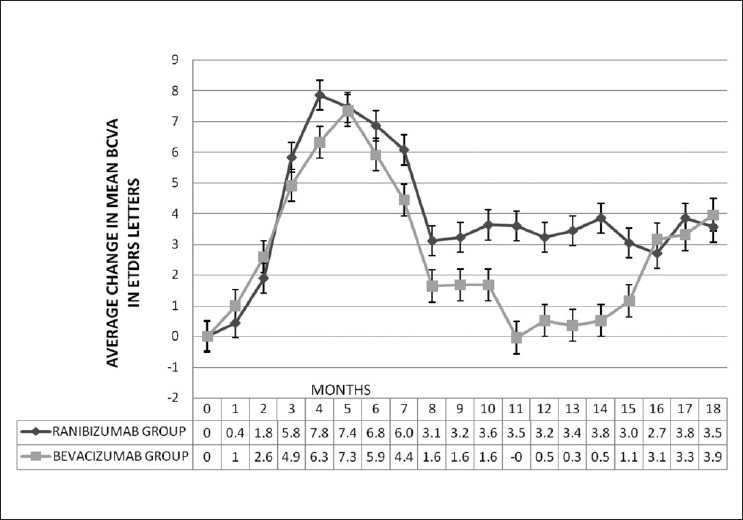
Average change in mean best corrected visual acuity (ETDRS letters) over 18 months in ranibizumab group (Group A) and bevacizumab group (Group B) (Respective intravitreal injections administered at months 0, 1 and 2 and then as per study criteria)
Table 2.
Comparison of change in mean best corrected visual acuity in ETDRS letters- RANIBIZUMAB vs. BEVACIZUMAB group
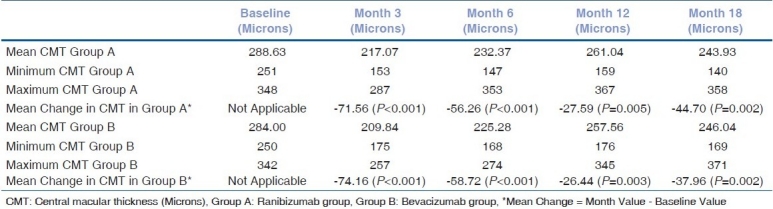
The change in average CMT (measured in μm using calipers in the macular radial line scan protocol of Stratus OCT 3000) in the ranibizumab and bevacizumab groups was also analyzed [Table 3]. The average CMT in ranibizumab group decreased from 288.63 μm at baseline to a low of 217.07 μm at month 3 (P<0.001). The average CMT at the end of the 18th month of follow-up in this group was 243.93 μm (P=0.002) [Table 3]. Similarly, the average CMT in bevacizumab group decreased from 284 μm at baseline to a low of 209.84 μm at the end of month 3 (P<0.001). The average CMT at the end of the 18th month of follow-up in this group was 246.04 μm (P=0.002) [Table 3]. Thus there is a statistically significant decrease in average CMT in both the groups, postinjection and the statistically significant decrease is maintained over 18 months.
Table 3.
Assessment of change in central macular thickness in μm letters at baseline, months 3, 6, 12 and 18 in ranibizumab group (group A, 54 cases) and bevacizumab group (group B, 50 cases)
The changes in mean CMT between the ranibizumab and bevacizumab groups [Fig. 2] were plotted graphically and it is seen that the two graphs almost superimpose one another.
Figure 2.
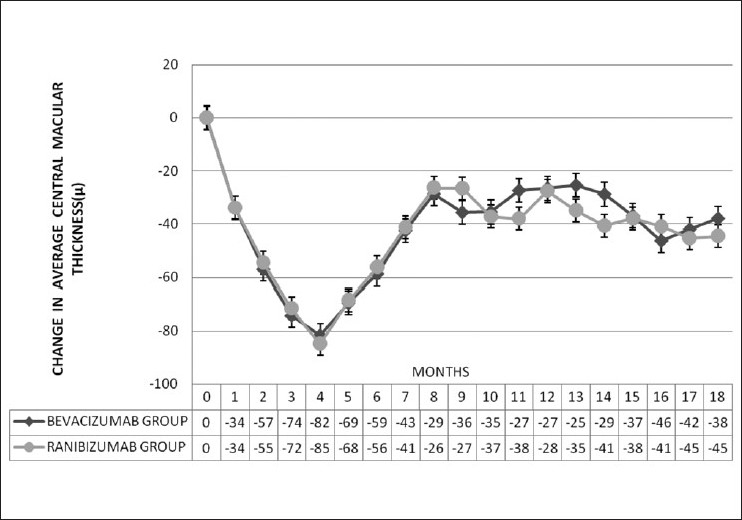
Change in average central macular thickness (μm) over 18 months in ranibizumab group (group A) and bevacizumab group (group B) (Respective intravitreal injections administered at months 0, 1 and 2 and then as per study criteria)
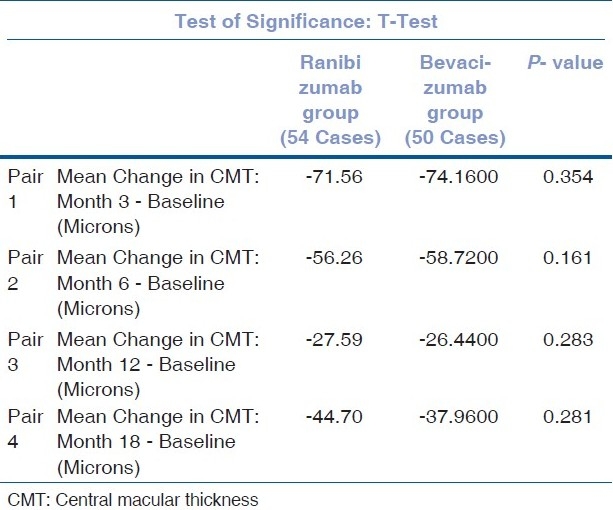
When the mean change in CMT between ranibizumab and bevacizumab groups is compared, no statistically significant difference is detected between the two groups at month 3 (P=0.354), 6 (P=0.161), 12 (P=0.283) and 18 (P=0.281) [Table 4].
Table 4.
Comparison of change in mean CMT-RANIBIZUMAB vs. BEVACIZUMAB group
Discussion
Safety and efficacy of intravitreal ranibizumab in neovascular AMD has been well elaborated in the ANCHOR[10] and MARINA[11] Trials. ANCHOR study showed that 94.3% and 96.4% of patients receiving 0.3 mg and 0.5 mg of ranibizumab lost fewer than 15 letters (<3 lines) at 12 months compared with baseline.[10] The MARINA study showed that 94.5% and 94.6% of patients receiving 0.3 mg and 0.5 mg of ranibizumab lost fewer than 15 letters (<3 lines) at 12 months compared with baseline.[11]
Efficacy of bevacizumab has been demonstrated by Rosenfeld et al,[4] and Avery et al.[12] The study conducted by Avery showed that at 1, 4, 8 and 12 weeks the mean retinal thickness of central 1 mm was decreased by 61,92,89 and 67 μm, respectively (P<0.0001 for 1, 4 and 8 weeks and P<0.01 for 12 weeks).
In our study, loss of more than 15 ETDRS letters was observed in two patients (3.7%) in ranibizumab group and in no patients in bevacizumab group at the end of 18 months. However, 14 patients (25.93%) in ranibizumab group and six patients (12%) in bevacizumab group reported a gain of 15 or more ETDRS letters at the end of 18 month. Our present study showed that in the patients receiving ranibizumab, 62.96% showed an improvement (mean of 96.52 μm) and 37.04% showed deterioration (mean of 56.60 μm) of the CMT. In patients receiving bevacizumab, 60.0% showed an improvement (mean of 78.73 μm) and 40% showed deterioration (mean of 41.40 μm) of CMT.
In our study, no statistically significant difference was found in terms of change in BCVA or CMT between the ranibizumab or bevacizumab groups at 3, 6, 12 and 18 months [Tables 2 and 4]. Both were found to be equally efficacious with regard to bringing about improvement in BCVA (functional improvement) or improvement in CMT (structural improvement).
When we analyzed cases with predominantly classic CNVM only in the two groups (ranibizumab and bevacizumab groups), we found that the average increase in BCVA or average decrease in CMT in the two subgroups were better than the outcomes achieved in the whole group or the minimally classic and occult subgroups. At the end of 18 months, the ranibizumab group showed overall increase of 3.55 ETDRS letters over the baseline value, whereas predominantly classic ranibizumab subgroup showed an increase of 5.24 ETDRS letters equivalent over baseline. Similarly, in the bevacizumab group at the end of 18 months, overall increase of 3.96 ETDRS letters occurred over the baseline value, whereas the predominantly classic bevacizumab subgroup showed an increase of 5.4 ETDRS letters equivalent over baseline. This finding is in concurrence with the findings of the ANCHOR[10] and MARINA[11] trials where it was observed that predominantly classic CNVM reported a better improvement in visual acuity as compared to minimally classic and occult lesions. The probable reason for this is that predominantly classic lesions are diagnosed earlier because of the more rapid visual decline typical of this group, thereby allowing for earlier initiation of treatment so that more photoreceptors can be salvaged.[10] Also, the sub retinal pigment epithelium RPE location of occult lesions may hinder the anti-VEGF molecule's penetration to the VEGF receptor, thereby resulting in relatively lesser visual acuity gain.
In both the groups, no significant adverse effects were reported, with subconjunctival hemorrhage being the most common adverse effect in both groups, followed by increased intraocular pressure and mild ocular inflammation. These observations are in line with the observations of Mojica et al. and Fung et al.[13,14] There were no reported cases of endophthalmitis in the ranibizumab group in the PIER study.[15] According to the Fung et al.,[16] the incidence of endophthalmitis after using intravitreal injections of bevacizumab was 0.01%. Endophthalmitis, lens injury or retinal detachment was not observed in any patient in our study.
The mean number of injections required in our study in the bevacizumab group (4.3) was less than the number required in the ranibizumab group (5.6). This finding is similar to that of Fung et al.[16] and this can probably be explained by the fact that bevacizumab is a full-length molecule with a longer half life as compared to the fragmented molecule of ranibizumab which has a shorter half life. Repeat injections were not required in 25.93% in ranibizumab group and 21% in bevacizumab group.
There are few reported studies comparing head on head the efficacies of ranibizumab and bevacizumab till the time this article was written. Landa et al.[17] and Rosenfeld et al.[18] in their retrospective reviews concluded that there is no significant difference in the efficacies of ranibizumab and bevacizumab. Fong et al.[19]in a comparative retrospective case series concluded that both ranibizumab and bevacizumab groups showed similar improvement and stability of vision over time. Subramanian et al.[20]in their prospective randomized double masked single center study over 6 months concluded that visual outcomes of bevacizumab in wet AMD appear to be no different from ranibizumab. Their study had a total of 20 patients.
However, Chang et al.[21] concluded otherwise. In their retrospective comparative study, they concluded that short-term effectiveness of ranibizumab treatment, as measured by incremental improvement in OCT parameters, was significantly greater than bevacizumab treatment.
Our study, a prospective randomized trial conducted across two centers in Kolkata, India, with 104 subjects and a total of about 302 injections in the ranibizumab group and about 216 injections in the bevacizumab group, studied results over 18 months. Parameters studied included both change in BCVA and CMT and the adverse effects of the two drugs over 18 months. We found no statistically significant difference in the efficacy and safety of ranibizumab and bevacizumab when used as intravitreal injections for treatment of CNVM due to wet AMD.
Thus, from our present study we can conclude that both ranibizumab and bevacizumab are safe and efficacious treatment options as intravitreal injections in the treatment of CNVM due to AMD and that the two do not have statistically significant difference between them in terms of bringing about BCVA and CMT improvement.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–34. [PubMed] [Google Scholar]
- 2.Otani A, Takagi H, Oh H, Koyama S, Ogura Y, Matumura M, et al. Vascular endothelial growth factor family and receptor expression in human choroidal neovascular membranes. Microvasc Res. 2002;64:162–9. doi: 10.1006/mvre.2002.2407. [DOI] [PubMed] [Google Scholar]
- 3.Zarbin M, Szirth B. Current Treatment of Age-Related Macular Degeneration. Optom Vis Sci. 2007;84:559–72. doi: 10.1097/OPX.0b013e3180de4dd7. [DOI] [PubMed] [Google Scholar]
- 4.Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–47. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.First-line treatment of metastatic colorectal cancer [Internet]. FDA Approval for Bevacizumab – National Cancer Institute. [Last cited on 2010 Sep 18]. Available from: http://www.cancer.gov/cancertopics/druginfo/fdabevacizumab#Anchor-Approva-23287 .
- 6.Steinbrook R. The price of sight – ranibizumab, bevacizumab, and the treatment of macular degeneration. N Engl J Med. 2006;355:1409–12. doi: 10.1056/NEJMp068185. [DOI] [PubMed] [Google Scholar]
- 7.Raftery J, Clegg A, Jones J, Tan SC, Lotery A. Ranibizumab (Lucentis) versus bevacizumab (Avastin): Modelling cost effectiveness. Br J Ophthalmol. 2007;91:1244–6. doi: 10.1136/bjo.2007.116616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inhibit VEGF in Age-related choroidal Neovascularisation (IVAN) Trial Summary Page [Internet]. University of Bristol; c2002-2009. [Last cited on 2010 Mar 7]. Available from: http://cteu.bristol.ac.uk/trials/ivan/
- 9.Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) [Internet]. Center for Preventive Ophthalmology and Biostatistics. [Last cited on 2010 Mar 7]. Available from: http://www.med.upenn.edu/cpob/studies/CATT.shtml .
- 10.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. ANCHOR Study Group. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Bressler NM, Chang TS, Suñer IJ, Fine JT, Dolan CM, Ward J, et al. Vision-related function after ranibizumab treatment by better- or worse-seeing eye: Clinical trial results from MARINA and ANCHOR. Ophthalmology. 2010;117:747–56. doi: 10.1016/j.ophtha.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal Bevacizumab (Avastin) for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Mojica G, Hariprasad SM, Jager RD, Mieler WF. Short-term intraocular pressure trends following intravitreal injections of ranibizumab (Lucentis) for the treatment of wet age-related macular degeneration. Br J Ophthalmol. 2008;92:584. doi: 10.1136/bjo.2007.126193. [DOI] [PubMed] [Google Scholar]
- 14.Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: Using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90:1344–9. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–48. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–83. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Landa G, Amde W, Doshi V, Ali A, McGevna L, Gentile RC, et al. Comparative study of intravitreal bevacizumab (Avastin) versus ranibizumab (Lucentis) in the treatment of neovascular age-related macular degeneration. Ophthalmologica. 2009;223:370–5. doi: 10.1159/000227783. [DOI] [PubMed] [Google Scholar]
- 18.Stepien KE, Rosenfeld PJ, Puliafito CA, Feuer W, Shi W, Al-Attar L, et al. Comparison of intravitreal bevacizumab followed by ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2009;29:1067–73. doi: 10.1097/IAE.0b013e3181b1bb06. [DOI] [PubMed] [Google Scholar]
- 19.Fong DS, Custis P, Howes J, Hsu JW. Intravitreal Bevacizumab and Ranibizumab for Age-Related Macular Degeneration: A Multicenter, Retrospective Study. Opthalmol. 2010;117:298–302. doi: 10.1016/j.ophtha.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian ML, Ness S, Abedi G, Ahmed E, Daly M, Feinberg E, et al. Bevacizumab Vs Ranibizumab for age related macular degeneration: Early results of a prospective, double masked, randomized clinical trial. Am J Ophthamol. 2009;148:875–82. doi: 10.1016/j.ajo.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Chang TS, Kokame G, Casey R, Prenner J, Feiner L, Anderson N. Short-term effectiveness of intravitreal bevacizumab versus ranibizumab injections for patients with neovascular age-related macular degeneration. Retina. 2009;29:1235–41. doi: 10.1097/IAE.0b013e3181b20eed. [DOI] [PubMed] [Google Scholar]


