Abstract
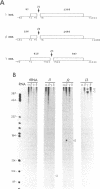
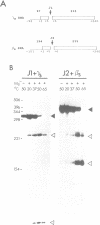
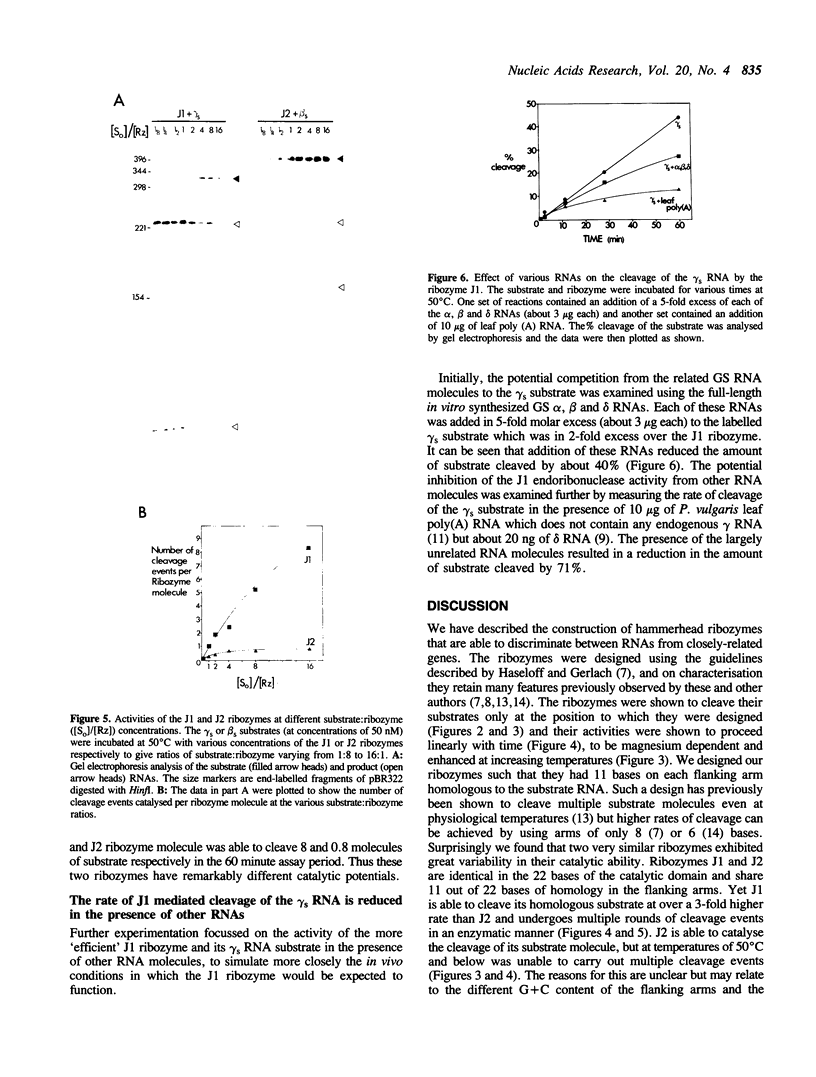
In Phaseolus vulgaris L. (French bean) glutamine synthetase (GS) is encoded by four closely-related genes termed gln-alpha, gln-beta, gln-gamma and gln-delta. We have constructed and characterised in vitro a number of hammerhead ribozymes designed to cleave individual RNAs encoded by these genes. The three ribozymes, termed J1, J2 and J3, were targeted to cleave RNA at the start of the gamma and beta, and the middle of the gamma, GS open reading frames respectively. All three ribozymes successfully discriminated between the four (alpha, beta, gamma and delta) highly homologous sequences, even though the targeted sites of cleavage shared up to 18 out of 22 identical bases with other gene family members. The ribozyme-mediated cleavage reactions were Mg2+ dependent and enhanced at higher temperatures, although the J1 ribozyme retained considerable activity at physiological temperatures. Both J1 and J2 demonstrated a time-dependent cleavage of their targeted GS RNAs, although these two ribozymes differed markedly in their ability to cleave multiple substrate molecules. The rate of cleavage by J1 was found to be reduced in the presence of related GS RNAs and by total leaf poly(A) RNAs. The implications of these results for ribozyme activity in vivo are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belinsky M. G., Dinter-Gottlieb G. Non-ribozyme sequences enhance self-cleavage of ribozymes derived from Hepatitis delta virus. Nucleic Acids Res. 1991 Feb 11;19(3):559–564. doi: 10.1093/nar/19.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron F. H., Jennings P. A. Specific gene suppression by engineered ribozymes in monkey cells. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9139–9143. doi: 10.1073/pnas.86.23.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M., Platz J., O'Leary M., Sookdeo C., Cannon F. Organ-specific modulation of gene expression in transgenic plants using antisense RNA. Plant Mol Biol. 1990 Jul;15(1):39–47. doi: 10.1007/BF00017722. [DOI] [PubMed] [Google Scholar]
- Cech T. R. The chemistry of self-splicing RNA and RNA enzymes. Science. 1987 Jun 19;236(4808):1532–1539. doi: 10.1126/science.2438771. [DOI] [PubMed] [Google Scholar]
- Colman A. Antisense strategies in cell and developmental biology. J Cell Sci. 1990 Nov;97(Pt 3):399–409. doi: 10.1242/jcs.97.3.399. [DOI] [PubMed] [Google Scholar]
- Cotten M., Birnstiel M. L. Ribozyme mediated destruction of RNA in vivo. EMBO J. 1989 Dec 1;8(12):3861–3866. doi: 10.1002/j.1460-2075.1989.tb08564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Schaffner G., Birnstiel M. L. Ribozyme, antisense RNA, and antisense DNA inhibition of U7 small nuclear ribonucleoprotein-mediated histone pre-mRNA processing in vitro. Mol Cell Biol. 1989 Oct;9(10):4479–4487. doi: 10.1128/mcb.9.10.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M. The in vivo application of ribozymes. Trends Biotechnol. 1990 Jul;8(7):174–178. doi: 10.1016/0167-7799(90)90168-w. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of virusoid RNA is performed by the proposed 55-nucleotide active site. Cell. 1987 Jul 3;50(1):9–16. doi: 10.1016/0092-8674(87)90657-x. [DOI] [PubMed] [Google Scholar]
- Goodchild J., Kohli V. Ribozymes that cleave an RNA sequence from human immunodeficiency virus: the effect of flanking sequence on rate. Arch Biochem Biophys. 1991 Feb 1;284(2):386–391. doi: 10.1016/0003-9861(91)90313-8. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Herschlag D. Implications of ribozyme kinetics for targeting the cleavage of specific RNA molecules in vivo: more isn't always better. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6921–6925. doi: 10.1073/pnas.88.16.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Hayase Y., Imura A., Iwai S., Miura K., Ohtsuka E. Synthesis and hybridization studies on two complementary nona(2'-O-methyl)ribonucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6131–6148. doi: 10.1093/nar/15.15.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi M., Iwai S., Ohtsuka E. Construction of a series of several self-cleaving RNA duplexes using synthetic 21-mers. FEBS Lett. 1988 Feb 15;228(2):228–230. doi: 10.1016/0014-5793(88)80004-8. [DOI] [PubMed] [Google Scholar]
- Lamb J. W., Hay R. T. Ribozymes that cleave potato leafroll virus RNA within the coat protein and polymerase genes. J Gen Virol. 1990 Oct;71(Pt 10):2257–2264. doi: 10.1099/0022-1317-71-10-2257. [DOI] [PubMed] [Google Scholar]
- May M. J., Hartley M. R., Roberts L. M., Krieg P. A., Osborn R. W., Lord J. M. Ribosome inactivation by ricin A chain: a sensitive method to assess the activity of wild-type and mutant polypeptides. EMBO J. 1989 Jan;8(1):301–308. doi: 10.1002/j.1460-2075.1989.tb03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol J. N., van der Krol A. R., van Tunen A. J., van Blokland R., de Lange P., Stuitje A. R. Regulation of plant gene expression by antisense RNA. FEBS Lett. 1990 Aug 1;268(2):427–430. doi: 10.1016/0014-5793(90)81298-3. [DOI] [PubMed] [Google Scholar]
- Rossi J. J., Sarver N. RNA enzymes (ribozymes) as antiviral therapeutic agents. Trends Biotechnol. 1990 Jul;8(7):179–183. doi: 10.1016/0167-7799(90)90169-x. [DOI] [PubMed] [Google Scholar]
- Sarver N., Cantin E. M., Chang P. S., Zaia J. A., Ladne P. A., Stephens D. A., Rossi J. J. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990 Mar 9;247(4947):1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]