Abstract
Cardiovascular disease is increased in individuals with type 1 or type 2 diabetes mellitus (DM). Left ventricular hypertrophy (LVH), which is an ominous prognostic sign and an independent risk factor for cardiac events, is often present in type 2 DM patients. The aim of our cross-sectional study was to evaluate the prevalence of LVH, and risk factors for its development, in normotensive type 2 diabetic patients without antihypertensive medication. The objectives of the study were to find out the prevalence of high left ventricular mass (LVM) in normotensive type 2 diabetic patients and compare it with nondiabetics and to uncover the risk factors for the development of high LVM in normotensive type 2 diabetic patients. A total of 130 age- and sex-matched subjects were selected (65 cases, diabetic normotensive, and 65 controls, nondiabetic normotensive) and baseline data were collected. LVM and left ventricular mass index (LVMI) were calculated using echocardigraphic parameters and body surface area. LVMI was significantly higher in patients with type 2 DM compared with age-, sex-matched healthy population (104.9 ± 21 vs. 78.5 ± 22.7 g/m2, respectively; P < 0.05). BMI, HbA1c, and duration of diabetes were significantly associated with LVH whereas sexes, age, PPBS, were not.
Keywords: Diabetes mellitus, left ventricular mass index, left ventricular hypertrophy, normotensive
INTRODUCTION
Cardiovascular disease is increased in individuals with type 1 or type 2 diabetes mellitus (DM). The Framingham Heart Study revealed a marked increase in peripheral arterial disease (PAD), congestive heart failure (CHF), coronary artery disease (CAD), myocardial infarction (MI), and sudden death (risk increase from one- to fivefold) in DM. Risk factors for macrovascular disease in diabetic individuals include dyslipidemia, hypertension, obesity, reduced physical activity, and cigarette smoking. Additional risk factors more prevalent in the diabetic population include microalbuminuria, macroalbuminuria, an elevation of serum creatinine, and abnormal platelet function.
Cardiovascular complications account for the highest mortality in diabetic patients, mainly due to CAD and CHF. Diabetes is associated with a high prevalence of hypertension, dyslipidemia, and microalbuminuria, all known independent cardiovascular risk factors. Even in populations with low cardiovascular risk, diabetes is associated with an increased incidence of cardiovascular death.[1]
Left ventricular hypertrophy (LVH), which is an ominous prognostic sign and an independent risk factor for cardiac events, is often present in type 2 DM patients. The possible contributions of hyperinsulinemia and hyperglycemia to left ventricular mass (LVM) have been suggested in the normotensive and hypertensive subjects without diabetes but there have been few studies that examined the risk factors related to LVM in type 2 DM patients without hypertension.
Echocardiography provides a reliable noninvasive estimation of LVM and has been proven to be a more sensitive tool for the detection of LVH than other techniques.
The aim of our cross-sectional study was to evaluate the prevalence of LVH, and risk factors for its development, in normotensive type 2 diabetic patients without antihypertensive medication.
The study had following aims:
Comparison of LVM between normotensive diabetic and age- and sex-matched normotensive, nondiabetic populations.
To evaluate the prevalence of LVH, and risk factors for its development, in normotensive type 2 DM patients without antihypertensive medication.
Determination of LVM in normotensive type 2 DM patients and age- and sex-matched, similar nondiabetic patients.
To find out the prevalence of high LVM in normotensive type 2 diabetic patients.
To uncover the risk factors for the development of high LVM in normotensive type 2 diabetic patients.
MATERIALS AND METHODS
This was a 1-year (from May 2008 to April 2009) hospital-based, matched, cross-sectional, observational study based on clinical workup and investigations, and was conducted in Medical College and Hospital, Kolkata. The study population included patients from diabetes OPD, medicine OPD, and medicine wards. A total of 130 patients included 65 cases who were type 2 diabetes patients with normal blood pressure and a similar number of healthy, age- and sex-matched nondiabetic controls. The inclusion criteria were as follows: (1) patients on oral or injectable antidiabetic therapy among already diagnosed diabetic patients and (2) patients not on antidiabetic therapy but fulfilling the American Diabetic Association definition for DM. Exclusion criteria were as follows: (1) patients of known hypertension with and without drugs; (2) patients of known ischemic heart disease, CHF, cardiomyopathy, thyroid disorder, and renal involvement; (3) patients with COPD; and (4) known case type 1 DM and patients with dyslipedemia.
Parameters studied
Patient particulars such as age, sex, height, weight, body surface area (BSA), body mass index (BMI), and blood pressure were measured, and routine and relevant investigations like fasting blood glucose, postprandial blood glucose, HbA1c, serum urea and creatinine, urine RE/ME, urinary albumin creatinine ratio (UACR), lipid profile, thyroid profile, chest x-ray, ECG, and echocardiography were also assessed.
Body mass index
Estimation of BMI was done by taking weight in kilograms on a balance scale; the height was recorded in centimeters:
BMI = WEIGHT (KG)/HEIGHT (CM)2
Body surface area (m2)
BSA was measured using the following formula:
(0.0001) × (71.84) × (Weight in kg) 0.425 × (Height in cm) 0.725
Blood pressure measurement
Recommended criteria for the diagnosis of hypertension are an average awake blood pressure of 135/85 mmHg and a sleeping blood pressure of 120/75 mmHg. These levels approximate a clinic blood pressure of 140/90 mmHg.
We have taken BP more than 140/90 mmHg as hypertensive and less than 140/90 mmHg as normotensive.
Laboratory investigations
Fasting plasma glucose
Venus blood samples were drawn in the morning following an overnight (minimum 8 h) fast and the oxalated blood sample was sent to the laboratory and was estimated using the GOD/POD method (Caltek Diagnostics Pvt. Ltd, India).
Postprandial plasma glucose
An oral glucose tolerance test was performed 2 h after the ingestion of a standard 75 g of anhydrous glucose.
Serum urea was estimated by the urease-Berthelot method. For serum creatinine, the alkaline picrate (Jaffe's reaction) method was used. Serum lipid levels were measured using Hitachi 912 analyzer. Routine and microscopic urine examinations were done in all cases.
Hemoglobin A1c
This was measured using boronate affinity chromatography by Micromat II (Biorad).
Urinary albumin concentration
Microalbuminuria estimation was done by the evaluation of the UACR. Normoalbuminuria was defined as a ratio less than 30.
Echocardiography
M-mode and pulsed Doppler echocardiography were performed according to the recommendations of the American Society of Echocardiography using Vingmed CFM725 equipped with a 3.25-MHz transducer.
Left ventricular dimensions
LV dimensions were measured from 2D-guided M-mode echocardiograms of the LV at the level of mitral leaflet tips or the papillary muscle using the parasternal view. The thicknesses of the left ventricular posterior wall and the ventricular septum (from the leading edge to the trailing edge) were measured. These values were used to calculate the LV mass. The LV end-diastolic and end-systolic dimensions were measured at the level of tips of the mitral leaflets as the largest and the smallest LV dimensions, respectively.
Left ventricular mass
The following equation provides a reasonable determination of LVM in grams:
LV mass (ASE method) = 0.8 (1.04([LVID+PWT+IVST]3 – [LVID]3)) + 0.6 g
where LVID is the left ventricle internal dimension, PWT is the posterior wall thickness, IVST is the interventricular septal thickness, 1.04 is the specific gravity of the myocardium, and 0.8 is the correction factor. All measurements were made at end-diastole (at the onset of the R wave) in centimeters.[2]
For comparison, the LVM index (LVMI) was calculated by dividing the LVM with the surface area. Left ventricular wall motion was inspected in each of the 16 segments defined by the American Society of Echocardiography. All measurements were averaged over five cycles.
The upper limit of LVM was 162 g in females and 224 g in males.[3,4] The upper limit of the LVMI was 95 g/m2 in females and 115 g/m2 in males.[3,4]
Data collection in controls
In the age- and sex-matched healthy control group, anthropometric measurements were taken and fasting and postprandial blood sugar, urea, creatinine, urine albumin, ECG, chest x-ray, lipid profile, thyroid profile, and LVM by echocardiography were estimated.
Statistical methods
Data are expressed as mean ± SD for continuously distributed variables, and are in absolute numbers and percentages for the discrete variables.
Tests of significance
Unpaired Student's t-test
This is a statistical significance test for comparing one set of data with another, by comparing two means to see if they are significantly different, the data belonging to two different samples.
Software developed by Open Source Epidemiologic Statistics for Public Health (version 2, OpenEpi) was used to perform a t-test (http://www.openepi.com). A P-value of <0.05 was considered significant.
Frequency table
The frequency table procedure can be used for the followingTo test the hypothesis that for one classification table, all classification levels have the same frequency
To test the relationship between two classification factors.
In the frequency table dialog box, one or two discrete variables with the classification data must be identified. Classification data may be either numeric or alphanumeric (string) values.
Chi-square test
When we want to test the hypothesis that for one single classification table (e.g., gender), all classification levels have the same frequency, then we have to identify only one discrete variable in the dialog form. In this case, the null hypothesis is that all classification levels have the same frequency. If the calculated P-value is low (P<0.05), then the null hypothesis is rejected and the alternative hypothesis that there is a significant difference between the frequencies of the different classification levels must be accepted.
When we want to study the relationship between two classification factors (e.g., gender and profession), then we have to identify the two discrete variables in the dialog form. In this case, the null hypothesis is that the two factors are independent. If the calculated P-value is low (P<0.05), then the null hypothesis is rejected and the alternative hypothesis that there is a relation between the two factors is accepted. o0 penEpi, version 2, was used to perform a chi-square test.
RESULTS AND ANALYSIS
Demographic profile
To exclude the effect of age over LVM, people were selected from the same age group in both case and control groups. The range of age was 44–60 years with a mean age of 53 years.
Among 65 DM patients, there were 37 male patients and they constituted 56% of the total diabetic group; female patients were 28 in number and they constituted 44% of the total diabetic group. Male-female distribution was same in the control population.
All structural measurements of the left ventricle - left ventricular internal dimension in diastole [(LVID(D)], left ventricular posterior wall thickness (LVPWT), and interventricular septal thickness (IVST) - were higher in type 2 DM patients than control subjects and the difference was also statistically significant (P < 0.0001). The increased LVM and LVMI in cases can be explained by these [Table 2].
Table 2.
Echocardiographic profile of diabetes mellitus patients and control subjects
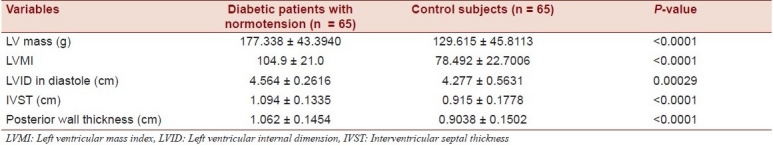
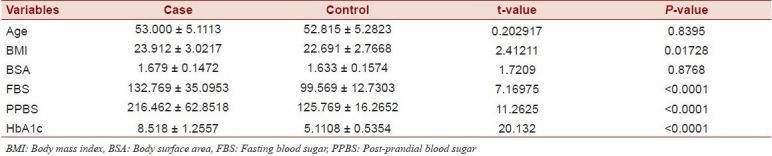
Table 1.
Clinical and laboratory data of cases and controls
High LVMI means LVMI more than 115 g/m2 in the case of males and LVMI more than 95 g/m2 in the case of females means high LVMI [Tables 4–6].
Table 4.
No. of subjects with high LVMI in type 2 DM and control patients

Table 6.
Clinical and laboratory characteristics of 65 normotensive type 2 diabetic patients not on antihypertensive medication, according to the left ventricular mass index
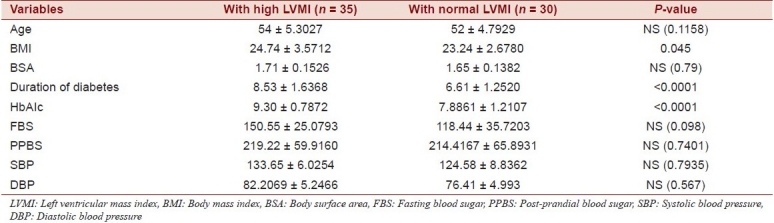
Table 3.
Prevalence of LVMI in type 2 DM and control patients

Table 5.
Relation between duration of diabetes and LVMI

DISCUSSION
Heart disease occurs eventually in a majority of patients with DM and continues to be the outstanding factor in overall diabetic morbidity and mortality. Increased LVM may contribute to the increased cardiovascular risk because LVH is an ominous prognostic sign and an independent risk factor for sudden death, ventricular dysarrhythmia, myocardial ischemia, coronary heart disease and heart failure. Angiotensin-converting enzymes inhibitors are effective both in controlling blood pressure and reversing LVH.[5] Hypertension occurs about twice as often in individuals with diabetes as it does in the nondiabetic population, and up to 50% diabetic individuals become hypertensive.
Our cross-sectional study demonstrated LVH to be a common association in normotensive type 2 diabetic patients predominantly without micro- or macrovascular complications and hypertension compared to the age- and sex-matched, normotensive, nondiabetic control population. None of the patients were receiving antihypertensive medication. LVM was indexed to the BSA to avoid the influence of obesity. In this study, it was observed that the mean of LVM and LVMI was statistically significantly high in diabetic patients in comparison to healthy control subjects. This indicates the association of high LVM in patients of DM. Hirayama et al. from Japan demonstrated in their study that LVM and LVMI were significantly greater in the normotensive type 2 DM patients than the normotensive control population.[6] So our results are at par with that study.
The prevalence of high LVM and high LVMI in all type 2 DM patients of our study was 44% and 53%, respectively. The prevalence of high LVM and high LVMI in male subjects with type 2 DM was 40% and 54%, respectively [Figure 1,2]. The prevalence of high LVM and high LVMI in female subjects with type 2 DM was 50% and 53%, respectively [Figure 3,4].
Figure 1.
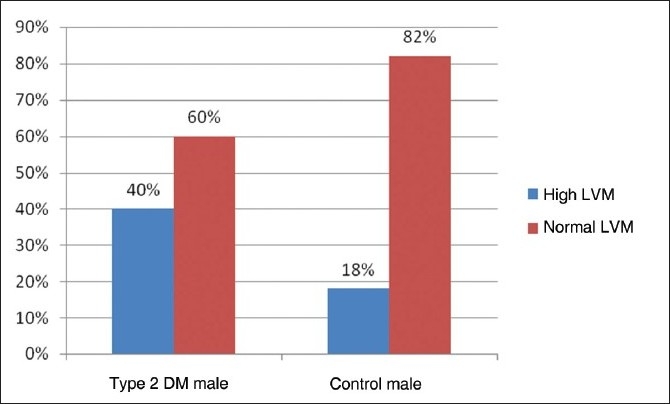
Bar diagram showing prevalence of left ventricular mass in male subjects of Type 2 DM and control patients
Figure 2.
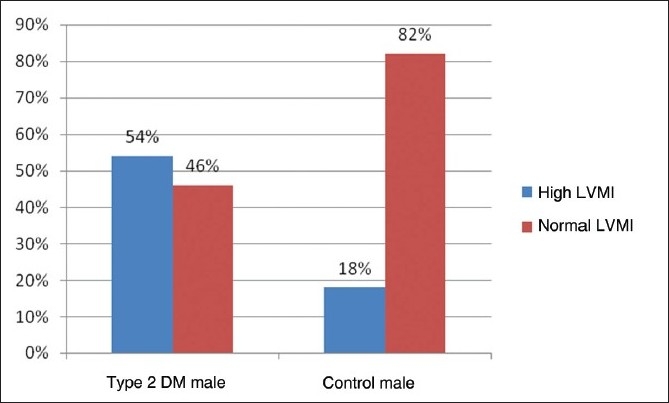
Bar diagram showing prevalence of left ventricular mass index in male subjects of Type 2 DM and control patients
Figure 3.
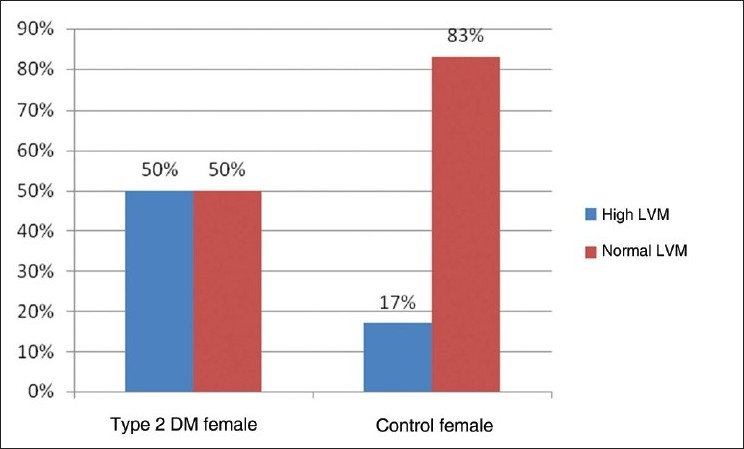
Bar diagram showing prevalence of left ventricular mass in female subjects of Type 2 DM and control patients
Figure 4.
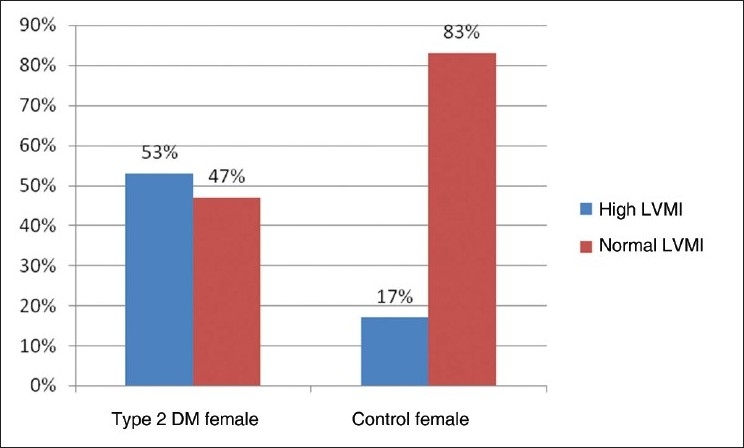
Bar diagram showing prevalence of left ventricular mass index in female subjects of Type 2 DM and control patients
Tarnow et al. reported that the prevalence of LVH indexed by height2.7 was 43% (38-50%), and was similar in men and women in normotensive, normoalbuminuric type 2 DM patients.[7] In comparison with that study, the prevalence of LVH in our study was slightly higher. But they measured LVH indexed to height2.7instead of LVMI and they used the Penn formula for the calculation of LVM.
In this research, we found that there is a significant difference in LVM between normotensive, normoalbuminuric type 2 DM patients and the control group which must be noted because increased LVM is associated with increased cardiovascular morbidity and mortality and its early diagnosis and prevention is important; drug therapy can cause improvement in left ventricular function and can decrease cardiovascular morbidity. The high prevalence of LVH in diabetic patients supports this idea that early echocardiographic screening may be beneficial to these patients.
In our study it was also observed that the means of LVPWT, IVST, and LVID(D) were statistically significantly high in diabetic patients in comparison to healthy control subjects [Table 2]. The increased thickness of the ventricular walls, in combination with the dilatation of the left ventricle, both contribute to the observed increase in LVM. This study shows that heart muscle disease complicates diabetes independently of hypertension.
The prevalence of LVH in the predominately nondiabetic population (95%) in the Framingham Heart Study assessed by echocardiography was reported to be 16% in men and 21% in women. In that study, 42 women had diabetes and were characterized by an increased left ventricular wall thickness and a 22% greater LVM than their nondiabetic peers.
In our present study, the prevalence of LVH in the nondiabetic, normotensive control population was 18% in males and 17% in females [Figures 1,2]. These findings are consistent with the Framingham Heart Study.
None of our patients fulfilled the classical Sokolow-Lyon electrocardiogram criteria for LVH. This agrees with the Framingham Heart Study which demonstrated on ECG a LVH prevalence of 0.5%, applying the same method. In contrast, a recent Italian study reported a prevalence of ECG-LVH of 17% in type 2 diabetic patients.[8] These patients were, however, characterized by old age, long known duration of diabetes, arterial hypertension, and micro- or macroalbuminuria in nearly half of the population.
The prevalence of LVH increases with the severity of hypertension, ranging from 38% to 72% in hypertensive diabetic populations.[9] In the present study, nearby 50% of patients had LVH, even though none of the included type 2 diabetic patients was receiving or had prior treatment with antihypertensive medications, and all had an arterial blood pressure below the recommended cut-off of 140/90 mmHg. While other studies have found a relationship between arterial blood pressure and LVM in diabetic patients with and without hypertension, this was not the case in the present study, where blood pressure levels were lower.
In the nondiabetic population, LVH is commonly associated with ischemic heart diseases and vice versa. However, evidence suggests that ischemic heart disease is a consequence rather than cause of LVH. Lee et al.[10] investigated a cohort of more than 5000 patients and found that the increased wall thickness of the ventricular septum or of the left ventricular posterior wall was not associated with prevalent coronary heart disease. Consequently, our findings of an increased wall thickness could not be explained by such a mechanism.
Though this study was conducted with normotensive patients, it appeared clearly that features of diabetic cardiomyopathy were associated with increasing blood pressure levels. Increasing blood pressure could either be an etiologic factor or simply part of the hemodynamic features of diabetic cardiomyopathy as reported in experimental studies. An increased heart size may reflect the increase in the circulating blood volume, and systolic dysfunction could be secondary to increased peripheral resistance, as some studies have shown increasing peripheral resistance in diabetic patients. This could therefore be attributable to early changes preceding established hypertension.
In our study, type 2 diabetes patients with common risk factors for the development of LVH, such as hypertension, albuminuria, thyroid disorder, ischemic heart diseases, and dyslipedimia, were excluded from the study. But when compared, patients with a high LVMI had a higher BMI than the BMI of those with a normal LVMI. The LVMI also increased with the longer duration of diabetes and poor glycemic control as suggested by higher HbAIcin the diabetic population with LVH. Sato et al. also reported a significant correlation between glycemic control, duration of DM, and severity of nephropathy and LVMI.[11]
That urinary albumin excretion rate is strongly associated with the degree of LVM hypertrophy has been demonstrated in several previous studies of nondiabetics[12] and type 1 and type 2 diabetic patients with micro and macroalbuminuria.[13] Furthermore, in hypertensive diabetic and nondiabetic patients with LVH, an increased urinary albumin excretion rate resulted in an increased risk for cardiovascular morbidity and mortality.[14] But in our study we selected the patients from both diabetic and healthy control groups, those having no micro- and macroalbuminuria. So in this study, albuminuria was not the cause of LVH.
In addition to blood pressure, urinary albumin excretion rate, BMI, and blood glucose,[15] it has also been suggested that coronary microvascular dysfunction, endothelial dysfunction and chronic inflammation, and abnormalities in the tissue renin–angiotensin–aldosterone–bradykinin system or the encoding genes might play a role in the pathogenesis of LVH. The observation that some type 2 diabetic patients have asymmetrical and some have concentric hypertrophy might suggest that the underlying pathology is not homogenous, but rather reflects the interaction of several of the above-mentioned risk factors.
CONCLUSION
Our cross-sectional study demonstrated the following:
LVM is significantly higher in type 2 diabetic patients without hypertension, albuminuria, and apparent ischemic heart disease as compared to healthy controls.
The prevalence of LVH is almost similar in both male and female patients.
LVM in diabetic patients increases with BMI. So obese, type 2 DM patients have more chances of having LVH.
LVM in diabetic patients increases with the duration of diabetes. So patients with a longer duration of diabetes have more chances of having LVH.
LVM in diabetic patients also increases with the HbAIclevel. So a poor glycemic control is also associated with more chances of having LVH.
Our small but significant study has thrown light on the prevalence of increased LVM in type 2 DM patients who are not otherwise suffer from hypertension, florid ischemic heart disease, and microvessel complications. So LVM evaluation is a mandatory workup in all type 2 DM patients for the prognostication of morbidity and mortality.
A large, prospective, double-blind study will further reveal the actual prevalence of LVM in such population. So our study in that sense is a sort of eye opener work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kadiri S, Salako BL. Cardiovascular risk factors in middle aged Nigerians. East Afr Med J. 1997;74:303–6. [PubMed] [Google Scholar]
- 2.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man: anatomic validation of the method. Circulation. 1977;55:613–8. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 3.Mor-Avi V, Sugeng L, Weinert L, MacEneaney P, Caiani EG, Koch R, et al. Fast measurement of left ventricular mass with real-time three-dimensional echocardiography: Comparison with magnetic resonance imaging. Circulation. 2004;110:1814–8. doi: 10.1161/01.CIR.0000142670.65971.5F. [DOI] [PubMed] [Google Scholar]
- 4.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen FS, Ali S, Rossing P, Bang LE, Svendsen TL, Gall MA, et al. Left ventricular hypertrophy in non-insulin dependent diabetic patients with and without diabetic nephropathy. Diabetic Med. 1997;14:538–46. doi: 10.1002/(SICI)1096-9136(199707)14:7<538::AID-DIA415>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Hirayama H, Sugano M, Abe N, Yonemochi H, Makino N. Determination of left ventricular mass by echocardiography in normotensive diabetic patients. Jpn Circ J. 2000;64:921–4. doi: 10.1253/jcj.64.921. [DOI] [PubMed] [Google Scholar]
- 7.Sato A, Tarnow L, Nielsen FS, Knudsen E, Parving HH. Left ventricular hypertrophy in normoalbuminuric type 2 diabetic patients not taking antihypertensive treatment. Q J Med. 2005;98:879–84. doi: 10.1093/qjmed/hci137. [DOI] [PubMed] [Google Scholar]
- 8.Bruno G, Giunti S, Bargero G, Ferrero S, Pagano G, Cavallo-Perin P. Sex-differences in prevalence of electrocardiographic left ventricular hypertrophy in Type 2 diabetes: The Casale Monferrato Study. Diabet Med. 2004;21:823–8. doi: 10.1111/j.1464-5491.2004.01246.x. [DOI] [PubMed] [Google Scholar]
- 9.Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, et al. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects - Hypertension Genetic Epidemiology Network (HyperGEN) Study. Circulation. 2001;103:102–7. doi: 10.1161/01.cir.103.1.102. [DOI] [PubMed] [Google Scholar]
- 10.Lee M, Gardin JM, Lynch JC, Smith VE, Tracy RP, Savage PJ, et al. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: The Cardiovascular Health Study. Am Heart J. 1997;133:36–43. doi: 10.1016/s0002-8703(97)70245-x. [DOI] [PubMed] [Google Scholar]
- 11.Sato A, Tarnow L, Parving H-H. Prevalence of left ventricular hypertrophy in type 1 diabetic patients with diabetic nephropathy. Diabetologia. 1999;42:76–80. doi: 10.1007/s001250051116. [DOI] [PubMed] [Google Scholar]
- 12.Fesler P, du Cailar G, Ribstein J, Mimran A. Left ventricular remodeling and renal function in never-treated essential hypertension. J Am Soc Nephrol. 2003;14:881–7. doi: 10.1097/01.asn.0000057855.93268.9f. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen FS, Ali S, Rossing P, Bang LE, Svendsen TL, Gall MA, et al. Left ventricular hypertrophy in non-insulin dependent diabetic patients with and without diabetic nephropathy. Diabetic Med. 1997;14:538–46. doi: 10.1002/(SICI)1096-9136(199707)14:7<538::AID-DIA415>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Wachtell K, Ibsen H, Olsen MH, Borch-Johnsen K, Lindholm LH, Mogensen CE, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: The LIFE study. Ann Intern Med. 2003;139:901–6. doi: 10.7326/0003-4819-139-11-200312020-00008. [DOI] [PubMed] [Google Scholar]
- 15.Felicio JS, Ferreira SR, Plavnik FL, Moises V, Kohlmann O Jr, Ribeiro AB, et al. Effect of blood glucose on left ventricular mass in patients with hypertension and type 2 diabetes mellitus. Am J Hypertens. 2000;13:1149–54. doi: 10.1016/s0895-7061(00)01200-0. [DOI] [PubMed] [Google Scholar]


