Abstract
The objective of this study was to elucidate the mechanisms by which nitric oxide (NO) inhibits rat aortic smooth muscle cell (RASMC) proliferation. Two products of the arginine-NO pathway interfere with cell growth by distinct mechanisms. NG-hydroxyarginine and NO appear to interfere with cell proliferation by inhibiting arginase and ornithine decarboxylase (ODC), respectively. S-nitroso-N-acetylpenicillamine, (Z)-1-[N-(2-aminoethyl)-N-(2-aminoethyl)-amino]-diazen-1-ium-1,2-diolate, and a nitroaspirin derivative (NCX 4016), each of which is a NO donor agent, inhibited RASMC growth at concentrations of 1–3 μM by cGMP-independent mechanisms. The cytostatic action of the NO donor agents as well as α-difluoromethylornithine (DFMO), a known ODC inhibitor, was prevented by addition of putrescine but not ornithine. These observations suggested that NO, like DFMO, may directly inhibit ODC. Experiments with purified, recombinant mammalian ODC revealed that NO inhibits ODC possibly by S-nitrosylation of the active site cysteine in ODC. DFMO, as well as the NO donor agents, interfered with cellular polyamine (putrescine, spermidine, spermine) production. Conversely, increasing the expression and catalytic activity of arginase I in RASMC either by transfection of cells with the arginase I gene or by induction of arginase I mRNA with IL-4 resulted in increased urea and polyamine production as well as cell proliferation. Finally, coculture of rat aortic endothelial cells, which had been pretreated with lipopolysaccharide plus a cytokine mixture to induce NO synthase and promote NO production, caused NO-dependent inhibition of target RASMC proliferation. This study confirms the inhibitory role of the arginine-NO pathway in vascular smooth muscle proliferation and indicates that one mechanism of action of NO is cGMP-independent and attributed to its capacity to inhibit ODC.
Keywords: cGMP, NG-hydroxyarginine, atherosclerosis, ornithine decarboxylase, polyamines
Nitric oxide (NO) is a physiological mediator of numerous cellular and organ functions (1), including inhibition of cell proliferation (2, 3). The mechanism by which NO inhibits cell proliferation appears to be multifaceted in that both cGMP-dependent and cGMP-independent mechanisms may be involved (2–5). After critical evaluation of the literature, we concluded that the evidence for involvement of cGMP in expression of the cytostatic effect of NO was indirect and inconsistent. Unpublished experiments in this laboratory consistently revealed cGMP-independent cytostatic effects of NO in various mammalian cell types including Caco-2 tumor cells, murine macrophages, rat aortic endothelial cells (RAEC), and rat aortic smooth muscle cells (RASMC). Therefore, our focus of attention has been on cGMP-independent mechanisms by which NO and the arginine-NO pathway inhibit cell proliferation. One such mechanism appears to be inhibition of two critical enzymes in the arginine-polyamine pathway, resulting in decreased polyamine production and consequent interference with cell proliferation (2, 3, 6, 7). NO is not the only product of NO synthase (NOS) that inhibits cell proliferation. N-hydroxyarginine (NOHA), the principal intermediate in the NOS-catalyzed conversion of arginine to NO plus citrulline, is also a potent competitive inhibitor of arginase (7, 8) and inhibitor of tumor cell proliferation (2). NOHA appears to interfere with tumor cell proliferation by cGMP-independent mechanisms involving the inhibition of arginase (2). NO has been found to be an inhibitor of ornithine decarboxylase (ODC) (2, 3, 6) and, like α-difluoromethylornithine (DFMO), can inhibit tumor cell proliferation by this cGMP-independent mechanism. Therefore, two products of NOS are capable of interfering with tumor cell proliferation by inhibiting two sequential steps in the arginine-polyamine pathway by cGMP-independent mechanisms. Arginase, which catalyzes the conversion or arginine to ornithine plus urea, is important not only in the urea cycle in the liver (arginase I isoform) but also in biochemical pathways essential to cell growth and wound healing in all cells (arginase I and II isoforms). Ornithine is, in turn, converted to putrescine by ODC, following which putrescine is converted to spermidine and spermine. The three polyamines (putrescine, spermidine, spermine) are required for mammalian cell growth (9).
Atherosclerosis is a complex inflammatory condition involving the arterial vascular bed, and early vascular lesions are characterized by increased monocyte/macrophage invasion, proliferation, and activation as well as increased vascular smooth muscle proliferation (10). Another feature of early lesions in atherogenesis is distinct vascular endothelial cell dysfunction characterized by impaired endothelium-derived NO production and impaired endothelium-dependent vasodilation (10–12). Considering the possibility that one function of the arginine-NO pathway may be to inhibit or modulate cell proliferation, a deficiency in endothelial NO production could account, at least in part, for the increased proliferation of monocytes/macrophages and vascular smooth muscle that occurs in atherosclerosis. NO as well as activation of NOS have been reported to inhibit vascular smooth muscle cell proliferation (13, 14). Therefore, the objective of the present study was to elucidate the mechanisms by which NO and the arginine-NO pathway bring about inhibition of vascular smooth muscle cell proliferation in an in vitro cell culture model using RASMC.
Materials and Methods
Chemicals and Solutions.
Sources of lipopolysaccharide, cytokines, cell culture media and supplements, and reagents for urea determination, arginase assay, ODC assay, and protein determination have been described (2, 6, 7). NOHA was obtained from Cayman Chemicals (Ann Arbor, MI), S-ethylisothiourea was from TCI America (Portland, OR), and zaprinast was from Sigma. NG-methylarginine and S-nitroso-N-acetylpenicillamine (SNAP) were synthesized as described (15, 16). 1H-(1,2,4)oxadiazolo[4,3-α]quinoxalin-1-one (ODQ) was purchased from Alexis Biochemicals (San Diego). (Z)-1-[N-(2-aminoethyl)-N-(2-aminoethyl)-amino]-diazen-1-ium-1,2-diolate (DETA-NO) and 1,1-diethyl-2-hydroxy-2-nitroso-hydrazine (DEA-NO) were generously provided by David A. Wink, National Institutes of Health, Bethesda, MD. NCX 4016 was provided by NicOx. All other chemicals and reagents were obtained from Sigma unless specified otherwise.
Cell Culture of RASMC and Measurement of Cell Proliferation.
RASMC were a generous gift from Steven Gross, Cornell Medical College, New York. Cells were plated, grown, subcultured, and cultured as described (17). Subcultured strains were used between passages 15 and 25. Exponentially growing RASMC were trypsinized and resuspended in fresh DMEM-Hepes medium supplemented with 10% (vol/vol) FBS, 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. The cells were seeded in 12-ml plates at a density of 3 × 103 cells/cm2 and incubated at 37°C in a humidified atmosphere of 5% CO2-95% air. After 24 h the cells were washed twice with PBS. The growth medium was replaced with 1 ml of arginine-free DMEM-Hepes supplemented with 5% FBS and 50 μM l-arginine, and any test agents and 0.5 μCi [3H]thymidine (6.7 Ci/mmol; NEN) were added. In determining the rates of DNA synthesis, a modification of the [methyl-3H]thymidine incorporation procedure described previously (2) was used. Cell proliferation data are expressed as percent of control, as described (2).
Coculture Procedures.
RAEC were plated on Falcon cell culture inserts on polyethylene terephthalate track-etched membranes with 3-mm pore size (Becton Dickinson). Cells were seeded at a density of 4.5 × 104 cells/cm2 in DMEM-Hepes containing 20% FBS, 1% endothelial growth supplement, 2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 10 units/ml heparin, and incubated at 37°C in a humidified atmosphere of 5% CO2-95% air. Cells were allowed to attach and the medium was changed the next day and then every other day. At confluence (3–4 days), some of the cells were activated by addition of lipopolysaccharide (100 μg/ml), IFN-γ (100 units/ml), IL-1β (400 units/ml), and tumor necrosis factor α (1,000 units/ml) and subsequently incubated for 6 h, followed by removal of lipopolysaccharide and cytokines by extensive washing before coculture with RASMC as follows. The medium was replaced after washing RAEC twice with arginine-free and serum-free medium (Specialty Media, Lavellette, NJ), containing 2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and SITE+3 serum supplement. The nonactivated RAEC cultured on inserts also were washed. RASMC were seeded at a density of 104 cells/cm2 in 6-well companion plates for the inserts in DMEM-Hepes containing 10% FBS, 2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 1 mM sodium pyruvate. The cells were synchronized for 48 h by washing twice with PBS and replacing the medium with arginine-free and serum-free supplemented medium as described above. The activated and nonactivated RAEC that had been plated on the inserts were added to the wells containing the synchronized RASMC. An equal number of wells contained only RASMC. To each well and each insert, 1 ml of serum-free supplemented medium containing 100 μM l-arginine was added. After 24 h of coculture, 0.5 μCi of [methyl-3H]thymidine was added to each well, inserted, and incubated for another 24 h, after which time DNA synthesis in RASMC was assessed.
Determination of Polyamine Concentrations in Cells.
The concentrations of putrescine, spermidine, and spermine in RASMC were determined by a sensitive HPLC procedure (18). RASMC were plated at a density of 106 cells per 100-mm dish and grown to 80% confluence before the start of experiments. After 24-h incubation of RASMC with the indicated test agents, the cells (≈5 × 106) in each dish were rapidly washed twice with ice-cold PBS and then lysed in 0.5 ml of 1.5 M HClO4, and the solution was neutralized by the addition of 0.25 ml K2CO3. The neutralized extracts were used for determination of polyamines.
ODC Assay.
ODC activity was determined by monitoring the formation of [14C]CO2 from l-[1-14C]ornithine exactly as described (6).
Arginase Assay.
Arginase activity was determined by methods that we have described (7). Briefly, RASMC (5 × 106 cells/sample) were washed twice with ice-cold PBS, harvested, pelleted by centrifugation, and then lysed. Supernatant fractions were assayed for arginase activity under optimal conditions of pH (9.6) and arginine concentration (20 mM) by monitoring the conversion of l-[guanido-14C]arginine to [14C]urea during 10-min incubation.
Determination of Urea Concentrations in Cell Culture Medium.
Normal RASMC, IL-4-treated RASMC, control-transfected RASMC, and arginase I-transfected RASMC were analyzed for urea production by determination of urea released into the cell culture medium. Cell culture media were collected and analyzed spectrophotometrically for urea exactly as described (7).
Determination of cGMP Levels in Cells.
At 24 h before initiation of experiments, 106 RASMC were plated in 60-mm dishes. At the start of experiments, zaprinast or ODQ was added 30 min before addition of SNAP. Incubations were continued for exactly 30 sec, after which time cGMP was extracted from cells by using ice-cold 65% ethanol as follows. The cell culture medium was aspirated and discarded, and the cells were washed twice with 4 ml of ice-cold PBS. The ethanol was added to the cells in each dish and the cells were scraped and transferred into a microcentrifuge tube. The dish was washed with 0.5 ml of 65% ethanol and added to the ethanol cell extract in the corresponding tube. Samples were sonicated briefly and centrifuged at 5,000 g for 15 min at 4OC. The clear supernatants were transferred to fresh tubes, and the ethanol was evaporated under a stream of nitrogen at 60°C. The samples were then redissolved in 1.1 ml of sample buffer (0.05 M sodium acetate, pH 5.8). cGMP was assayed by RIA following the acetylation assay protocol as described by the manufacturer (Amersham Pharmacia, catalog no. RPA 525).
Vector Construction and Transfection of RASMC.
pEF1-rARGI, a mammalian expression plasmid for rat arginase I, was constructed by inserting the EcoRI–BsaAI 1,194-bp coding region fragment of pARGr-2 (19) into the EcoRI/Pmel sites of the plasmid pEF1/Myc-His C. No additional epitope sequences were fused to the arginase I coding sequence in this construct. For control transfection, the β-galactosidase expression plasmid (pEF1/Myc-His/lacZ) alone, which contains the Escherichia coli lacZ gene under control of the human EF-1α promoter (Invitrogen), was used to represent the expression of an unrelated exogenous protein. RASMC were transfected with pEF1/rARGI or pEF1/Myc-His/lacZ by using Lipofectamine (Life Technologies, Grand Island, NY) according to the manufacturer's instructions. Stably transfected cells were selected with the antibiotic G418 (500 μg/ml) in complete DMEM. Cells were maintained at 37°C in complete DMEM containing 10% FBS and 500 μg/ml G418. After approximately 3 weeks, G418-resistant clones were isolated and analyzed individually for expression of arginase I. The individual clones were grown in DMEM containing 10% FBS and 250 μg/ml G418. The G418 was omitted from the cell culture medium beginning 2 days before initiating any experiments. Stably transfected RASMC were examined for expression of arginase I by Western blot analysis as described (17).
Statistical Analyses.
Where indicated, data were analyzed statistically by using the Bonferroni t test for unpaired values. Probability values of <0.05 were taken to indicate statistical significance.
Results
We have reported previously that NOHA and NO, products of the arginine-NO pathway, inhibit tumor cell proliferation (2). Fig. 1 illustrates that NO interferes also with the proliferation of vascular smooth muscle cells from rat aorta. SNAP, an S-nitrosothiol NO donor agent, and DETA-NO, a NONOate NO donor agent, each inhibited RASMC proliferation in a concentration-dependent manner. SNAP was slightly more potent than DETA-NO, eliciting significant effects at a concentration of 1 μM. NCX 4016, a nitro analog of aspirin that can serve as a NO donor agent in vivo or in vitro in the presence of cells or tissues (20–22), inhibited RASMC proliferation with a potency equivalent to that of SNAP. Aspirin (acetylsalicylic acid) tested at 1 μM to 1 mM was completely inactive (data not shown). DFMO, a well established ODC inhibitor and cytostatic agent, was used as a positive control test agent, and it inhibited RASMC proliferation at relatively high concentrations ranging from 0.1 to 1 mM.
Figure 1.
Inhibition of RASMC proliferation by SNAP, DETA-NO, NCX 4016 (NCX), and DFMO. Cell proliferation was assessed by thymidine incorporation into DNA during the final 24 h of RASMC incubation, as described in the text. Test agents were added to cells at the time of thymidine addition. The concentrations of test agents were (from left to right for each test agent): SNAP, 1, 3, 10, and 30 μM; DETA-NO, 3, 10, 30, and 100 μM; NCX, 1, 3, 10, and 30 μM; DFMO, 100 μM, 300 μM, and 1 mM. Data were calculated as dpm per 105 cells per well and expressed as percentage of control (assigned 100%), where control represents cells grown in the absence of added test agents. Each of the points was significantly different (P < 0.05) from control (100%) except the value for 3 μM DETA-NO (P > 0.05). Data represent means ± SE of duplicate determinations from 3–4 separate experiments.
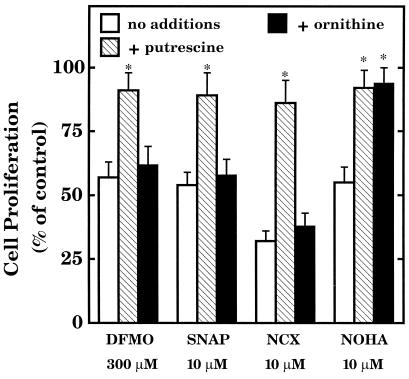
DFMO inhibits cell growth by inhibiting the ODC-catalyzed conversion of ornithine to putrescine and thereby interfering with polyamine synthesis (23). In cell culture, the cytostatic action of DFMO can be prevented or overcome by the addition of excess putrescine (product of ODC) but not excess ornithine (substrate for ODC and product of arginase) (2), as illustrated in Fig. 2. On the other hand, the cytostatic action of NOHA, a potent inhibitor of arginase (8), can be prevented by addition of either excess putrescine or excess ornithine (2), as illustrated in Fig. 2. The cytostatic actions of SNAP and NCX 4016 were prevented by addition of excess putrescine but not ornithine, thereby resembling the effects of DFMO rather than NOHA (Fig. 2). These observations support the view that NO interferes with cell proliferation by inhibiting ODC, whereas NOHA may interfere with cell proliferation by inhibiting arginase, as was found in tumor cells (2).
Figure 2.
Influence of added putrescine or ornithine on the cytostatic actions of DFMO, SNAP, NCX 4016 (NCX), and NOHA in RASMC. Cell proliferation was assessed by thymidine incorporation into DNA during the final 24 h of RASMC incubation, as described in the text. Test agents were added to cells at the time of thymidine addition. Putrescine (100 μM) or ornithine (100 μM) was added to cells at the same time as the test agents. Data were calculated as dpm per 105 cells per well and expressed as percentage of control (assigned 100%), where control represents cells grown in the absence of added test agents. * signifies statistically significant difference (P < 0.05) from corresponding control (no additions). Data represent means ± SE of duplicate determinations from four separate experiments.
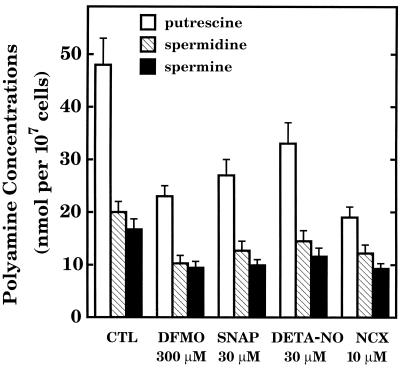
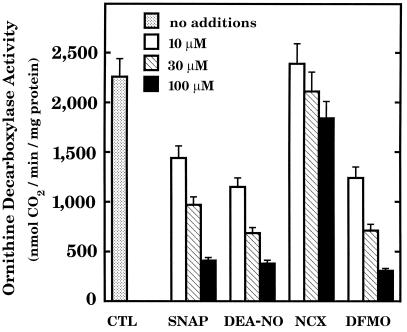
The next step was to verify that NO, like DFMO, interferes with polyamine production, as might be expected for chemical agents that inhibit ODC. DFMO was tested as a positive control and inhibited the formation and accumulation of putrescine, spermidine, and spermine (Fig. 3). Polyamine production was inhibited also by SNAP, DETA-NO, and NCX 4016 at concentrations that interfered with RASMC proliferation. These observations are consistent with the finding that NO directly inhibits ODC (2, 3, 6). Indeed, SNAP and DEA-NO inhibited mammalian recombinant ODC in a concentration-dependent manner (Fig. 4). DFMO was tested as a positive control. NCX 4016 was not active, presumably because this nitro derivative of aspirin must first be metabolized to aspirin and NO before the effects of NO can be observed (20). Metabolic conversion of NCX 4016 would be expected to occur in vivo and in vitro in the presence of cells or tissues but not in an isolated, purified ODC reaction mixture.
Figure 3.
Inhibition of polyamine production in RASMC by DFMO, SNAP, DETA-NO, and NCX 4016 (NCX). Test agents were added to RASMC 24 h before determination of polyamine concentrations. Cells were washed, lysed, and extracted as described in the text. Concentrations of spermidine and spermine are expressed as nmol per 107 cells. Putrescine concentrations are actually 100-fold lower than indicated and, therefore, should be multiplied by 10−2 to obtain the true values. All values for polyamine concentrations in the presence of added test agents were significantly different (P < 0.05) from values for control (CTL). Data represent means ± SE of duplicate determinations from 4–5 separate experiments.
Figure 4.
Inhibition of purified ODC by SNAP, DEA-NO, NCX 4016 (NCX) and DFMO. DTT was separated from the enzyme by gel filtration through a Sephadex G-25 column. ODC was preincubated with the test agents, as indicated, at 37°C for 15 min. Enzyme mixture was then added to assay buffer containing 0.4 mM ornithine (labeled with 0.1 μCi l-[1-14C]ornithine; 55 Ci/mmol) and 40 μM pyridoxal 5′-phosphate. ODC activity was measured after 30-min incubation at 37°C as described (6). All concentrations of SNAP, DEA-NO, and DFMO tested inhibited ODC significantly (P < 0.05). NCX was not active (P > 0.05). CTL, control. Data represent means ± SE of duplicate determinations from four separate experiments.
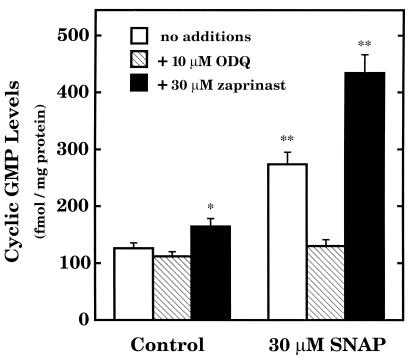
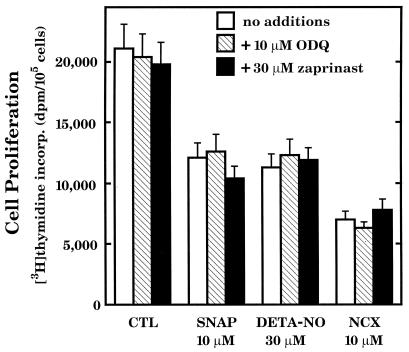
Some of the biological actions of NO are known to be mediated by the second messenger actions of cGMP (24). These actions include vascular smooth muscle relaxation and inhibition of platelet aggregation. The evidence that cGMP is involved in the cytostatic action of NO is inconsistent and controversial (2–5). Experiments were conducted here to ascertain whether or not cGMP can account for the inhibitory action of NO on RASMC proliferation. First, it was necessary to conduct experiments to determine whether NO can stimulate cGMP production in RASMC under the defined experimental conditions. SNAP was used as the NO donor agent and concentrations ranging from 1 μM to 1 mM were added to cells for various time intervals ranging from 0 to 5 min before ice-cold 65% ethanol was added to cells and assayed for cGMP. A 30-sec time interval was found to be the most consistent time interval for peak cGMP accumulation in response to added SNAP (data not shown) and was used in the experiments. Fig. 5 illustrates that 30 μM SNAP caused a greater than 2-fold accumulation of cGMP in RASMC and this effect was abolished by addition of 10 μM ODQ (guanylyl cyclase inhibitor) (25) and enhanced by addition of 30 μM zaprinast (cGMP phosphodiesterase inhibitor) (26). Thus, the NO-cGMP signal transduction pathway is present and functional in RASMC under the present assay conditions. However, the NO-cGMP signal transduction system does not appear to be involved in the cytostatic action of NO in RASMC. For example, neither ODQ nor zaprinast, at concentrations that influenced cellular cGMP levels (Fig. 5), affected the cytostatic action of SNAP, DETA-NO, and NCX 4016 in RASMC (Fig. 6). Moreover, neither ODQ nor zaprinast influenced control cell proliferation. These observations indicate that NO inhibits RASMC growth largely by cGMP-independent mechanisms.
Figure 5.
Stimulation of cGMP production in RASMC by SNAP. RASMC were grown to 80% confluence and used in experiments. Cells were pretreated with ODQ or zaprinast as indicated for 15 min. SNAP was added for 30 sec, after which time cell incubations were terminated by addition of 4 ml of ice-cold 65% ethanol. Cells were extracted with ethanol, and extracts then were assayed for cGMP as described in the text. * signifies that values were significantly different (P < 0.05) from control (no additions). ** signifies that values were significantly different (P < 0.05) from corresponding control values (absence of SNAP). Data represent means ± SE of triplicate determinations from three separate experiments.
Figure 6.
Influence of added ODQ or zaprinast on the cytostatic actions of SNAP, DETA-NO, and NCX 4016 (NCX). Cell proliferation was assessed by thymidine incorporation into DNA during the final 24 h of RASMC incubation, as described in the text. Test agents were added to cells at the time of thymidine addition. ODQ (10 μM) or zaprinast (30 μM) was added to cells at the same time as the test agents. Data were calculated as dpm per 105 cells per well and expressed as such. Values for SNAP, DETA-NO, and NCX were not significantly different (P > 0.05) from corresponding values in the presence of either ODQ or zaprinast, but were significantly different (P < 0.05) from control (CTL) values. Values for ODQ or zaprinast alone were not significantly different (P > 0.05) from control values. Data represent means ± SE of duplicate determinations from four separate experiments.
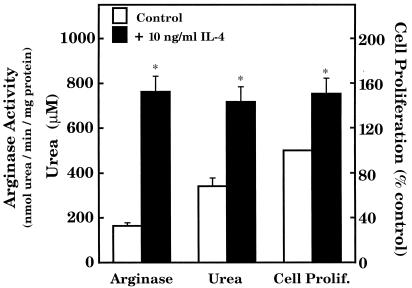
The present study and a previous study from this laboratory (2) indicate that NO and NOHA interfere with cell proliferation by mechanisms associated with the inhibition of ODC and arginase, respectively. One likely mechanism that may account for inhibition of RASMC proliferation is decreased polyamine production, as shown for NO and NCX 4016 above. The next experiment was designed to determine whether the opposite is true, that is, if an increase in arginase activity in RASMC results in an increase in cell proliferation. To obtain RASMC with elevated expression of arginase, cells were either treated with IL-4, which causes induction of arginase I (17), or stably transfected with an expression plasmid containing rat arginase I cDNA. RASMC stably transfected with the expression plasmid vector alone represented control transfected cells. Fig. 7 illustrates that the induction of arginase I in RASMC by IL-4 (10 ng/ml) was associated with a 50% increase in urea production and cell proliferation. IL-4 also caused a 50% increase in polyamine (spermidine and spermine) production after 24 h incubation with RASMC (data not shown). Similarly, increased arginase I expression in RASMC afforded by transfection of cells with arginase I cDNA was associated with comparable increases in urea production and cell proliferation (Fig. 8) and polyamine (spermidine and spermine) production (data not shown).
Figure 7.
Influence of IL-4 on arginase activity, urea production, and cell proliferation in RASMC. RASMC were plated at a density of 106 cells per 100-mm dish and grown to 80% confluence before the start of experiments. After 24-h incubation of RASMC with 10 ng/ml IL-4, the cells (≈5 × 106) in each dish were ready for analysis. Some dishes were used for assessment of cell proliferation, whereas other dishes were used for determination of both urea and arginase activity. Cell proliferation was assessed by thymidine incorporation into DNA during the final 24 h of RASMC incubation, as described in the text. In separate dishes, cells were harvested and washed with ice-cold PBS, and the suspensions were centrifuged to sediment the cells. Sediments were lysed, and supernatants were assayed for arginase activity as described in the text. Cell culture media from the cells used for determination of arginase activity were collected for determination of urea levels, as described in the text. * signifies that values were significantly different (P < 0.05) from corresponding control values. Data represent means ± SE of duplicate determinations from 4–5 separate experiments.
Figure 8.
Influence of arginase I transfection on arginase activity, urea production, and cell proliferation in RASMC. Transfection procedures are described in the text. RASMC were grown in 100-mm dishes and reached ≈5 × 106 cells per dish just before analysis. Some dishes were used for assessment of cell proliferation, whereas other dishes were used for determination of both urea and arginase activity. Cell proliferation was assessed by thymidine incorporation into DNA during the final 24 h of RASMC incubation, as described in the text. In separate dishes, cells were harvested and washed with ice-cold PBS, and the suspensions were centrifuged to sediment the cells. Sediments were lysed and supernatants were assayed for arginase activity as described in the text. Cell culture media from the cells used for determination of arginase activity were collected for determination of urea levels, as described in the text. * signifies that values were significantly different (P < 0.05) from corresponding control values. Data represent means ± SE of duplicate determinations from 3–5 separate experiments.
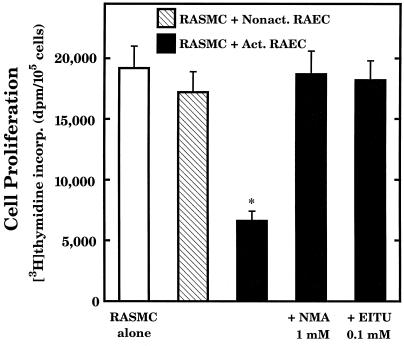
The final experiment in this study was designed to determine whether coculture of RAEC, after induction of inducible NOS to promote NO production, with RASMC could influence RASMC proliferation. Untreated RAEC caused a slight inhibition of RASMC proliferation but this cytostatic effect was markedly increased when RASMC were cocultured with RAEC that had been activated (pretreated) with lipopolysaccharide plus a cytokine mixture as described in Materials and Methods (Fig. 9). This enhanced cytostatic effect appears to be attributed to increased production of NO, and perhaps NOHA, because addition of either NG-methylarginine or S-ethylisothiourea (inhibitors of inducible NOS) to cell cocultures essentially abolished the cytostatic action of activated RAEC. These observations reveal that NO and/or NOHA generated by RAEC can diffuse into nearby RASMC and inhibit RASMC proliferation.
Figure 9.
Influence of NO-producing, activated RAEC on RASMC proliferation. Coculture, RAEC activation, and cell proliferation procedures are described in the text. As indicated, either 1 mM NG-methylarginine (NMA) or 0.1 mM S-ethylisothiourea (EITU) was added to cocultures at the start of coculture. After 24 h of coculture, 0.1 μCi of [methyl-3H]thymidine was added to each well and to each insert and incubated for another 24 h, after which time DNA synthesis in RASMC was assessed. * signifies that values were significantly different (P < 0.05) from values for RASMC alone. Data represent means ± SE of duplicate determinations from 3–4 separate experiments.
Discussion
The principal objective of the present study was to develop a better understanding of the mechanisms by which NO and the arginine-NO pathway interfere with the proliferation of vascular smooth muscle. An in vitro cell culture model was used in which the proliferation of RASMC was monitored by [3H]thymidine incorporation into cellular DNA. NO, in the form of three chemically distinct NO donor agents, inhibited RASMC proliferation at concentrations down to 1 to 3 μM. The potency of NO in inhibiting RASMC proliferation may have been much greater than was apparent in this study. The actual concentrations of NO generated from the NO donor agents used were probably much lower than the concentrations of the NO donor agents themselves. For example, 1 mM DETA-NO in aqueous solution liberates ≈3 μM NO under steady-state conditions (27). This means that NO was an effective cytostatic agent at low nanomolar concentrations in the present study. We reported previously that NOHA and NO interfere with human Caco-2 tumor cell growth by inhibiting arginase and ODC, respectively (2). The same mechanisms appear to be operational in RASMC, as shown in the present study. For example, the cytostatic effect of NOHA was prevented by addition of excess ornithine or putrescine to cell cultures, whereas the cytostatic effect of NO was prevented by addition of excess putrescine but not ornithine. These observations support the views not only that NOHA and NO interfere with two distinct enzymatic steps in the arginine-polyamine pathway (2), but also that interruption of polyamine production can cause inhibition of cell proliferation (19). Indeed, the present study reveals that NO inhibits polyamine production in RASMC.
We believe that one important mechanism by which NO interferes with cell proliferation in general is by direct inhibition of ODC (2, 3, 6) and consequent inhibition of polyamine production. This mechanism applies also to RASMC, as demonstrated in this report. Preliminary experiments revealed that NO (SNAP) inhibited crude ODC extracted from RASMC, and more extensive experiments showed the inhibitory effects of NO-donor agents on purified, mammalian, recombinant ODC. The mechanism of inhibition of ODC by NO appears to be S-nitrosylation of the active site cysteine 360 residue, thereby inactivating the enzyme because the cysteine 360 sulfhydryl is essential for the expression of catalytic activity (28, 29). As we have noted previously, S-nitrosylation of ODC by various chemical classes of NO donor agents may occur by slightly different chemical mechanisms (6). For example, S-nitrosothiols may covalently modify the cysteine 360 sulfhydryl by S-transnitrosation reactions, where the NO is readily transferred from the sulfur atom of the S-nitrosothiol to the sulfur atom of the cysteine 360 sulfhydryl. On the other hand, the NONOates generate pure NO, which then likely reacts with O2 to form N2O3, which in turn is a potent nitrating agent. We have suggested that one physiological function of endogenous S-nitrosothiols might be to modulate cell proliferation by slowing it down (6).
Clearly, NO inhibits ODC by cGMP-independent mechanisms, and this mechanism is likely to represent at least one of the cGMP-independent components of the overall physiological process by which endogenous NO modulates cell proliferation. NO itself, NO donor agents, and NOS activation have been reported to inhibit the proliferation of various cell types from diverse species by either or both cGMP-dependent and independent mechanisms (2–5). Whether or not cGMP represents a signal transduction mechanism in the expression of the antiproliferative action of NO has been a point of controversy. In the present study, cGMP was found not to be involved in the antiproliferative action of NO in RASMC. Likewise, in a previous study, NO and NOHA inhibited human Caco-2 tumor cell proliferation by cGMP-independent mechanisms (2). Therefore, NO and the arginine-NO pathway can cause inhibition of cell proliferation largely by cGMP-independent mechanisms. It seems reasonable to consider, however, that the arginine-NO pathway functions to inhibit cell proliferation by more than one mechanism, and one mechanism might be cGMP-dependent. Two or more mechanisms might serve as back up, alternate, or complementary mechanisms.
One important physiological role of the arginine-NO pathway is to protect the cardiovascular system against pathophysiological insults that can lead to chronic disease such as hypertension, stroke injury, and atherosclerosis (30). A deficiency in the production of NO by altered vascular endothelial cells is likely to be involved in the development or progression of atherosclerosis (10, 11, 30). Coronary artery disease and atherosclerosis in general are considered to be chronic inflammatory processes characterized by invasion of vascular tissue by certain circulating blood cells, which then proliferate and elaborate factors that cause the underlying vascular smooth muscle to proliferate, eventually resulting in plaque formation, lumen occlusion, and plaque rupture. In view of the known chemical and biological properties of NO and the arginine-NO pathway, it is not unreasonable to speculate that this pathway functions in normal healthy vascular endothelial cells in multiple ways to prevent adhesion and migration of monocytes and leukocytes at the intimal surface, to act as an antioxidant to prevent oxidative stress-related oxidation of lipoproteins, and to prevent proliferation of white blood cells and vascular smooth muscle cells. The findings in the present report that the arginine-NO pathway is capable of interfering with vascular smooth muscle proliferation support this view.
A new class of NO donor agent has emerged in which the class members are clinically used nonsteroidal antiinflammatory drugs that are chemically linked to a readily hydrolyzable nitrate ester moiety (20). These nitrated drugs are hydrolyzed in vivo with the liberation of both NO and the nonsteroidal antiinflammatory drug itself. The representative compound used in the present study is nitroaspirin (NCX 4016). Nitroaspirin inhibited RASMC proliferation with a potency equivalent to that of NO and by mechanisms that appear to involve NO. Aspirin alone did not affect cell proliferation. The cytostatic effect of nitroaspirin, like that of NO, was prevented by added putrescine but not ornithine, was cGMP-independent, and was associated with concomitant inhibition of polyamine production. Nitroaspirin differed from the other NO donor agents, however, in that nitroaspirin failed to cause direct inhibition of ODC. A likely explanation for this lack of effect is that nitroaspirin is a stable compound that requires enzymatic hydrolysis to liberate NO (20) and this does not appear to occur in buffer containing purified ODC in the absence of added esterases or other factors. Interestingly, nitroaspirin was reported to reduce infarct size in a rat model of myocardial infarction without causing hypotension or a negative inotropic effect, as was the case with NO donor agents such as DETA-NO (31). Nitroaspirin releases NO in vivo without causing any apparent changes in systemic blood pressure (32). Whether this is attributed to the slow enzymatic release of NO from nitroaspirin (33) remains to be determined. Clinical trials of nitroaspirin and other NO-releasing analogs of nonsteroidal antiinflammatory drugs in cardiovascular disease such as atherosclerosis will be of interest. The combined antiinflammatory actions and other actions of aspirin and NO along with the antiproliferative action of NO might result in a clinically effective drug for the early treatment of atherosclerosis.
Acknowledgments
We thank Dr. Anthony E. Pegg for his generous supply of purified recombinant mammalian ODC and Dr. Sidney M. Morris, Jr. for providing the mammalian expression plasmid for rat arginase I. This work was supported by National Institutes of Health Grants HL35014 and HL58433.
Abbreviations
- NOS
NO synthase
- SNAP
S-nitroso-N-acetylpenicillamine
- DETA-NO
(Z)-1-[N-(2-aminoethyl)-N-(2-aminoethyl)-amino]-diazen-1-ium-1,2-diolate
- DEA-NO
1,1-diethyl-2-hydroxy-2-nitroso-hydrazine
- ODQ
1H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one
- NOHA
NG-hydroxyarginine
- DFMO
α-difluoromethylornithine
- ODC
ornithine decarboxylase
- RASMC
rat aortic smooth muscle cells
- RAEC
rat aortic endothelial cells
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 27, 1999.
References
- 1.Moncada S, Palmer R M J, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 2.Buga G M, Wei L H, Bauer P M, Fukuto J M, Ignarro L J. Am J Physiol. 1998;275:R1256–R1264. doi: 10.1152/ajpregu.1998.275.4.R1256. [DOI] [PubMed] [Google Scholar]
- 3.Blachier F, Robert V, Selamnia M, Mayeur C, Duee P H. FEBS Lett. 1996;396:315–318. doi: 10.1016/0014-5793(96)01122-2. [DOI] [PubMed] [Google Scholar]
- 4.Garg U C, Hassid A. Am J Physiol. 1989;257:F60–F66. doi: 10.1152/ajprenal.1989.257.1.F60. [DOI] [PubMed] [Google Scholar]
- 5.Garg U C, Hassid A. Biochem Biophys Res Commun. 1990;171:474–479. doi: 10.1016/0006-291x(90)91417-q. [DOI] [PubMed] [Google Scholar]
- 6.Bauer P M, Fukuto J M, Buga G M, Pegg A E, Ignarro L J. Biochem Biophys Res Commun. 1999;262:355–358. doi: 10.1006/bbrc.1999.1210. [DOI] [PubMed] [Google Scholar]
- 7.Buga G M, Singh R, Pervin S, Rogers N E, Schmitz D A, Jenkinson C P, Cederbaum S D, Ignarro L J. Am J Physiol. 1996;271:H1988–H1998. doi: 10.1152/ajpheart.1996.271.5.H1988. [DOI] [PubMed] [Google Scholar]
- 8.Daghigh F, Fukuto J M, Ash D E. Biochem Biophys Res Commun. 1994;202:174–180. doi: 10.1006/bbrc.1994.1909. [DOI] [PubMed] [Google Scholar]
- 9.Pegg A E, Shantz L M, Coleman C S. J Cell Biochem. 1995;Suppl. 22:132–138. doi: 10.1002/jcb.240590817. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 11.Cooke J P. J Invest Med. 1998;46:377–380. [PubMed] [Google Scholar]
- 12.Drexler H, Zeiher A M, Meinzer K, Just H. Lancet. 1991;338:1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- 13.Nakaki T, Nakayama M, Kato R. Eur J Pharmacol. 1990;189:347–353. doi: 10.1016/0922-4106(90)90031-r. [DOI] [PubMed] [Google Scholar]
- 14.Stein C S, Fabry Z, Murphy S, Hart M N. Mol Immunol. 1995;32:965–973. doi: 10.1016/0161-5890(95)00062-j. [DOI] [PubMed] [Google Scholar]
- 15.Gold M E, Wood K S, Byrns R E, Fukuto J M, Ignarro L J. Proc Natl Acad Sci USA. 1990;87:4430–4434. doi: 10.1073/pnas.87.12.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ignarro L J, Lippton H, Edwards J C, Baricos W H, Hyman A L, Kadowitz P J, Gruetter C A. J Pharmacol Exp Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- 17.Wei L H, Jacobs A T, Morris S M, Jr, Ignarro L J. Am J Physiol. 2000;279:C248–C256. doi: 10.1152/ajpcell.2000.279.1.C248. [DOI] [PubMed] [Google Scholar]
- 18.Wu G, Pond W G, Flynn S P, Ott T C, Bazer F W. J Nutr. 1998;128:2395–2402. doi: 10.1093/jn/128.12.2395. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Meininger C J, Hawker J R, Jr, Haynes T E, Kepka-Lenhart D, Mistry S K, Morris S M, Jr, Wu G. Am J Physiol. 2001;280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- 20.Del Soldato P, Sorrentino R, Pinto A. Trends Pharmacol Sci. 1999;20:319–323. doi: 10.1016/s0165-6147(99)01353-x. [DOI] [PubMed] [Google Scholar]
- 21.Wallace J L, McNight W, Del Soldato P, Baydoun A, Cirino G. J Clin Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiorucci S, Antonelli E, Santucci L, Morelli O, Miglietti M, Federici B, Mannucci R, Del Soldato P, Morelli A. Gastroenterology. 1999;116:1089–1106. doi: 10.1016/s0016-5085(99)70012-0. [DOI] [PubMed] [Google Scholar]
- 23.McCann P P, Pegg A E. Pharmacol Ther. 1992;54:195–215. doi: 10.1016/0163-7258(92)90032-u. [DOI] [PubMed] [Google Scholar]
- 24.Ignarro L J. Circ Res. 1989;65:1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Garthwaite J, Southam E, Boulton C L, Nielsen E B, Schmidt K, Mayer B. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 26.Ignarro L J, Byrns R E, Wood K S. Circ Res. 1987;60:82–92. doi: 10.1161/01.res.60.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Keefer L K, Nims R W, Davies K M, Wink D A. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 28.Coleman C S, Stanley B A, Pegg A E. J Biol Chem. 1993;268:24572–24579. [PubMed] [Google Scholar]
- 29.Lu L, Stanley B A, Pegg A E. Biochem J. 1991;277:671–675. doi: 10.1042/bj2770671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Meyer G R Y, Herman A G. In: Nitric Oxide Biology and Pathobiology. Ignarro L J, editor. San Diego: Academic; 2000. pp. 547–567. [Google Scholar]
- 31.Rossoni, G., Manfredi, B., De Gennaro Colonna, V., Bernareggi, M. & Berti, F. (2001) J. Pharmacol. Exp. Ther., in press. [PubMed]
- 32.Yamamoto T, Kakar N R, Vina E R, Johnson P E, Bing R J. Life Sci. 2000;67:839–846. doi: 10.1016/s0024-3205(00)00678-0. [DOI] [PubMed] [Google Scholar]
- 33.Fiorucci S, Santucci L, Cirino G, Mencarelli A, Familiari L, Del Soldato P, Morelli A. J Immunol. 2000;165:5245–5254. doi: 10.4049/jimmunol.165.9.5245. [DOI] [PubMed] [Google Scholar]