Abstract
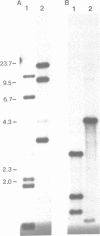
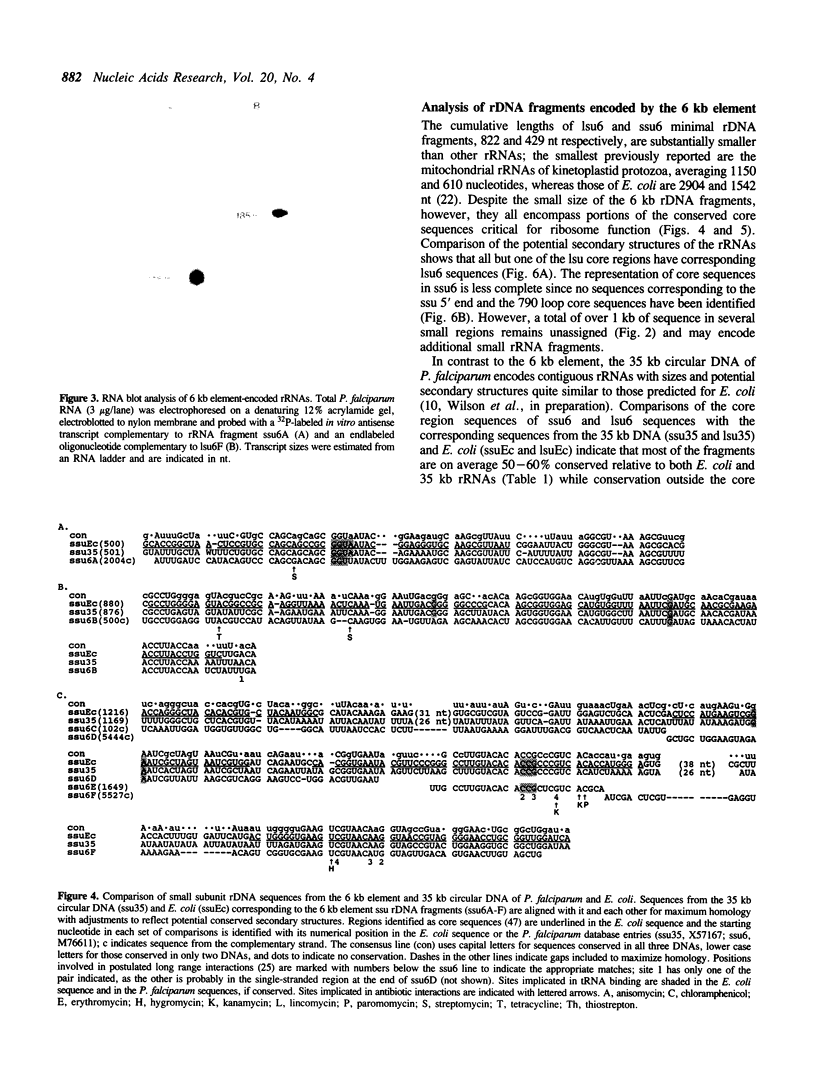
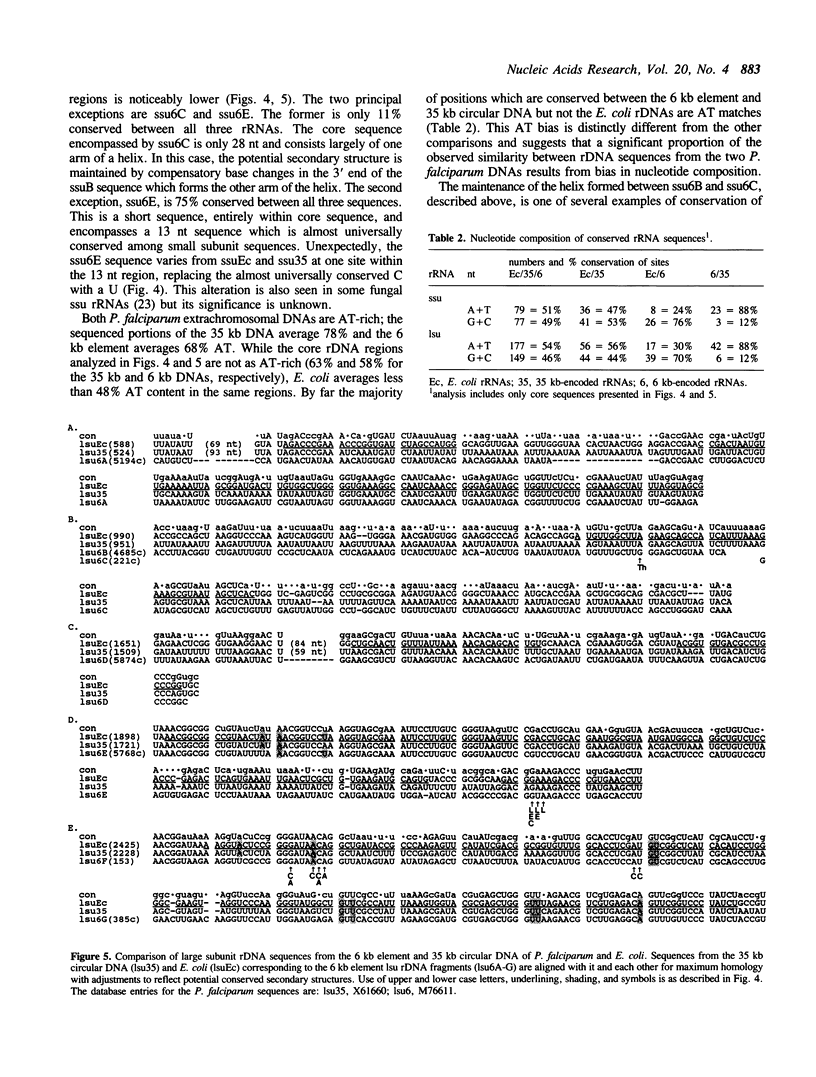
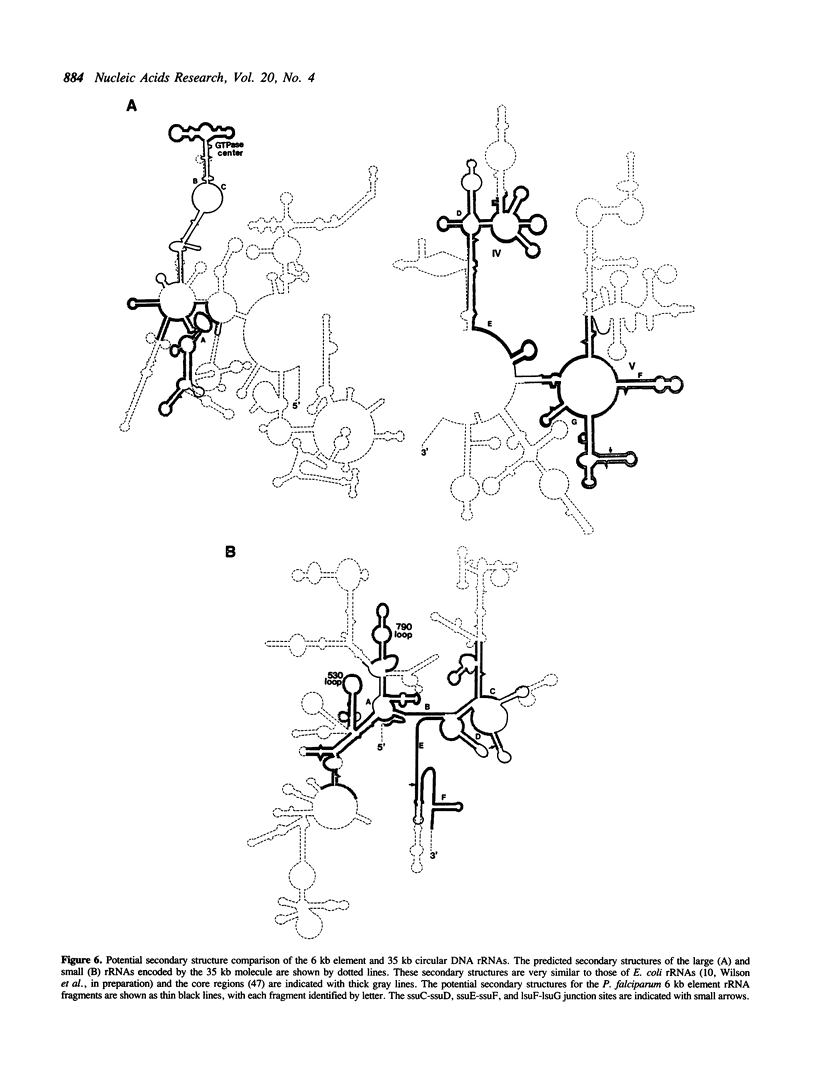
Plasmodium falciparum contains two extrachromosomal DNAs, a 6 kb linear element and a 35 kb circular DNA; both encode rDNA sequences. The 6 kb element rDNAs comprise fragments of both large and small subunit rRNAs. Comparison of these with corresponding rDNA sequences from the 35 kb DNA and E. coli show that sequences conserved between the three are largely confined to highly conserved core regions; in fact, most of the 6 kb rDNA sequences correspond to core regions. Both the 6 kb element and 35 kb rDNAs show less conservation to each other than to E. coli sequences, suggesting that the two extrachromosomal DNAs of P. falciparum are not closely related. The characteristics of the fragmented rRNAs from the 6 kb element suggest they are functional, possibly in mitochondrial ribosomes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldritt S. M., Joseph J. T., Wirth D. F. Sequence identification of cytochrome b in Plasmodium gallinaceum. Mol Cell Biol. 1989 Sep;9(9):3614–3620. doi: 10.1128/mcb.9.9.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Sloof P. Evolution of the mitochondrial protein synthetic machinery. Biosystems. 1987;21(1):51–68. doi: 10.1016/0303-2647(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Boer P. H., Gray M. W. Scrambled ribosomal RNA gene pieces in Chlamydomonas reinhardtii mitochondrial DNA. Cell. 1988 Nov 4;55(3):399–411. doi: 10.1016/0092-8674(88)90026-8. [DOI] [PubMed] [Google Scholar]
- Cedergren R., Gray M. W., Abel Y., Sankoff D. The evolutionary relationships among known life forms. J Mol Evol. 1988 Dec;28(1-2):98–112. doi: 10.1007/BF02143501. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. Mouse RNAase MRP RNA is encoded by a nuclear gene and contains a decamer sequence complementary to a conserved region of mitochondrial RNA substrate. Cell. 1989 Jan 13;56(1):131–139. doi: 10.1016/0092-8674(89)90991-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E. The functional role of ribosomal RNA in protein synthesis. Cell. 1989 May 19;57(4):525–529. doi: 10.1016/0092-8674(89)90122-0. [DOI] [PubMed] [Google Scholar]
- Dams E., Hendriks L., Van de Peer Y., Neefs J. M., Smits G., Vandenbempt I., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1988;16 (Suppl):r87–173. doi: 10.1093/nar/16.suppl.r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divo A. A., Geary T. G., Jensen J. B. Oxygen- and time-dependent effects of antibiotics and selected mitochondrial inhibitors on Plasmodium falciparum in culture. Antimicrob Agents Chemother. 1985 Jan;27(1):21–27. doi: 10.1128/aac.27.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagin J. E., Gardner M. J., Williamson D. H., Wilson R. J. The putative mitochondrial genome of Plasmodium falciparum. J Protozool. 1991 May-Jun;38(3):243–245. doi: 10.1111/j.1550-7408.1991.tb04436.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fox T. D. Mitochondrial genes in the nucleus. Nature. 1983 Feb 3;301(5899):371–372. doi: 10.1038/301371a0. [DOI] [PubMed] [Google Scholar]
- Fry M., Beesley J. E. Mitochondria of mammalian Plasmodium spp. Parasitology. 1991 Feb;102(Pt 1):17–26. doi: 10.1017/s0031182000060297. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Bates P. A., Ling I. T., Moore D. J., McCready S., Gunasekera M. B., Wilson R. J., Williamson D. H. Mitochondrial DNA of the human malarial parasite Plasmodium falciparum. Mol Biochem Parasitol. 1988 Oct;31(1):11–17. doi: 10.1016/0166-6851(88)90140-5. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Feagin J. E., Moore D. J., Spencer D. F., Gray M. W., Williamson D. H., Wilson R. J. Organisation and expression of small subunit ribosomal RNA genes encoded by a 35-kilobase circular DNA in Plasmodium falciparum. Mol Biochem Parasitol. 1991 Sep;48(1):77–88. doi: 10.1016/0166-6851(91)90166-4. [DOI] [PubMed] [Google Scholar]
- Gardner M. J., Williamson D. H., Wilson R. J. A circular DNA in malaria parasites encodes an RNA polymerase like that of prokaryotes and chloroplasts. Mol Biochem Parasitol. 1991 Jan;44(1):115–123. doi: 10.1016/0166-6851(91)90227-w. [DOI] [PubMed] [Google Scholar]
- Geary T. G., Jensen J. B. Effects of antibiotics on Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1983 Mar;32(2):221–225. doi: 10.4269/ajtmh.1983.32.221. [DOI] [PubMed] [Google Scholar]
- Gray M. W. Origin and evolution of mitochondrial DNA. Annu Rev Cell Biol. 1989;5:25–50. doi: 10.1146/annurev.cb.05.110189.000325. [DOI] [PubMed] [Google Scholar]
- Haselman T., Camp D. G., Fox G. E. Phylogenetic evidence for tertiary interactions in 16S-like ribosomal RNA. Nucleic Acids Res. 1989 Mar 25;17(6):2215–2221. doi: 10.1093/nar/17.6.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempelmann E., Ling I., Wilson R. J. S-antigens and isozymes in strains of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1981;75(6):855–858. doi: 10.1016/0035-9203(81)90431-4. [DOI] [PubMed] [Google Scholar]
- Hänfler A., Kleuvers B., Göringer H. U. The involvement of base 1054 in 16S rRNA for UGA stop codon dependent translational termination. Nucleic Acids Res. 1990 Oct 11;18(19):5625–5632. doi: 10.1093/nar/18.19.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J. T., Aldritt S. M., Unnasch T., Puijalon O., Wirth D. F. Characterization of a conserved extrachromosomal element isolated from the avian malarial parasite Plasmodium gallinaceum. Mol Cell Biol. 1989 Sep;9(9):3621–3629. doi: 10.1128/mcb.9.9.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhausen M., Nelson R. G., Parsons M., Newport G., Stuart K., Agabian N. Molecular characterization of initial variants from the IsTat I serodeme of Trypanosoma brucei. Mol Biochem Parasitol. 1983 Nov;9(3):241–254. doi: 10.1016/0166-6851(83)90100-7. [DOI] [PubMed] [Google Scholar]
- Powers T., Noller H. F. A functional pseudoknot in 16S ribosomal RNA. EMBO J. 1991 Aug;10(8):2203–2214. doi: 10.1002/j.1460-2075.1991.tb07756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott C. D., Dahlberg A. E. A single base change at 726 in 16S rRNA radically alters the pattern of proteins synthesized in vivo. EMBO J. 1990 Jan;9(1):289–294. doi: 10.1002/j.1460-2075.1990.tb08107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott C. D., Göringer H. U. A single mutation in 16S rRNA that affects mRNA binding and translation-termination. Nucleic Acids Res. 1990 Sep 25;18(18):5381–5386. doi: 10.1093/nar/18.18.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer M., Bennett-Guerrero E., Byahatti S., Czarnecki S., O'Connell D., Meyer M., Khoury J., Cheng X., Schwartz I., McLaughlin J. Base changes at position 792 of Escherichia coli 16S rRNA affect assembly of 70S ribosomes. Proc Natl Acad Sci U S A. 1990 May;87(10):3700–3704. doi: 10.1073/pnas.87.10.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel L. W., Miller J. Cytochrome oxidase activity in platelet-free preparations of Plasmodium knowlesi. J Parasitol. 1969 Aug;55(4):825–829. [PubMed] [Google Scholar]
- Scheibel L. W., Pflaum W. K. Cytochrome oxidase activity in platelet-free preparations of Plasmodium falciparum. J Parasitol. 1970 Dec;56(6):1054–1054. [PubMed] [Google Scholar]
- Schnare M. N., Cook J. R., Gray M. W. Fourteen internal transcribed spacers in the circular ribosomal DNA of Euglena gracilis. J Mol Biol. 1990 Sep 5;215(1):85–91. doi: 10.1016/S0022-2836(05)80097-X. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Gray M. W. Sixteen discrete RNA components in the cytoplasmic ribosome of Euglena gracilis. J Mol Biol. 1990 Sep 5;215(1):73–83. doi: 10.1016/S0022-2836(05)80096-8. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Heinonen T. Y., Young P. G., Gray M. W. A discontinuous small subunit ribosomal RNA in Tetrahymena pyriformis mitochondria. J Biol Chem. 1986 Apr 15;261(11):5187–5193. [PubMed] [Google Scholar]
- Simpson A. M., Suyama Y., Dewes H., Campbell D. A., Simpson L. Kinetoplastid mitochondria contain functional tRNAs which are encoded in nuclear DNA and also contain small minicircle and maxicircle transcripts of unknown function. Nucleic Acids Res. 1989 Jul 25;17(14):5427–5445. doi: 10.1093/nar/17.14.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D. F., Collings J. C., Schnare M. N., Gray M. W. Multiple spacer sequences in the nuclear large subunit ribosomal RNA gene of Crithidia fasciculata. EMBO J. 1987 Apr;6(4):1063–1071. doi: 10.1002/j.1460-2075.1987.tb04859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suplick K., Morrisey J., Vaidya A. B. Complex transcription from the extrachromosomal DNA encoding mitochondrial functions of Plasmodium yoelii. Mol Cell Biol. 1990 Dec;10(12):6381–6388. doi: 10.1128/mcb.10.12.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapprich W. E., Dahlberg A. E. A single base mutation at position 2661 in E. coli 23S ribosomal RNA affects the binding of ternary complex to the ribosome. EMBO J. 1990 Aug;9(8):2649–2655. doi: 10.1002/j.1460-2075.1990.tb07447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsness P. E., Fox T. D. Escape of DNA from mitochondria to the nucleus in Saccharomyces cerevisiae. Nature. 1990 Jul 26;346(6282):376–379. doi: 10.1038/346376a0. [DOI] [PubMed] [Google Scholar]
- Trager W., Jenson J. B. Cultivation of malarial parasites. Nature. 1978 Jun 22;273(5664):621–622. doi: 10.1038/273621a0. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Wilson R. J., Bates P. A., McCready S., Perler F., Qiang B. U. Nuclear and mitochondrial DNA of the primate malarial parasite Plasmodium knowlesi. Mol Biochem Parasitol. 1985 Feb;14(2):199–209. doi: 10.1016/0166-6851(85)90038-6. [DOI] [PubMed] [Google Scholar]
- Wilson R. J., Gardner M. J., Feagin J. E., Williamson D. H. Have malaria parasites three genomes? Parasitol Today. 1991 Jun;7(6):134–136. doi: 10.1016/0169-4758(91)90276-t. [DOI] [PubMed] [Google Scholar]