Abstract
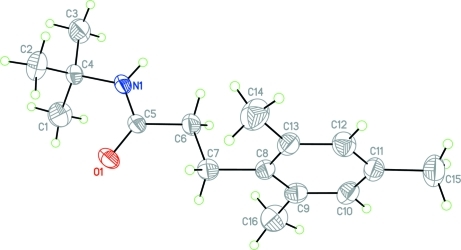
In the title compound, C16H25NO, the N-tert-butylpropanamide fragment is essentially planar, with the exception of two C atoms of the tert-butyl group (r.m.s. deviation = 0.005 Å), forming a dihedral angle of 84.09 (10)° with the plane of the mesityl fragment (r.m.s. deviation = 0.002 Å). The crystal packing is stabilized by an intermolecular N—H⋯O hydrogen bond, which links the molecules into chains with graph-set notation C(4) running parallel to the c axis.
Related literature
For graph-set notation, see: Bernstein et al. (1995 ▶).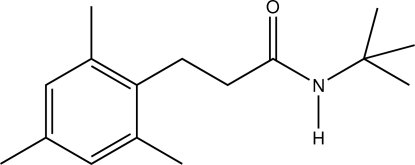
Experimental
Crystal data
C16H25NO
M r = 247.37
Monoclinic,
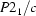
a = 12.8851 (11) Å
b = 13.3441 (11) Å
c = 9.4741 (8) Å
β = 106.540 (2)°
V = 1561.6 (2) Å3
Z = 4
Mo Kα radiation
μ = 0.06 mm−1
T = 296 K
0.20 × 0.20 × 0.20 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2003 ▶) T min = 0.987, T max = 0.987
11870 measured reflections
3870 independent reflections
1738 reflections with I > 2σ(I)
R int = 0.052
Refinement
R[F 2 > 2σ(F 2)] = 0.065
wR(F 2) = 0.173
S = 1.00
3870 reflections
169 parameters
H-atom parameters constrained
Δρmax = 0.15 e Å−3
Δρmin = −0.14 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2001 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL and PLATON (Spek, 2009) ▶; software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811015856/om2425sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811015856/om2425Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811015856/om2425Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1N⋯O1i | 0.83 | 2.17 | 2.979 (2) | 165 |
Symmetry code: (i) 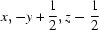 .
.
Acknowledgments
The authors are grateful to Baku State University for supporting this study. IB thanks the Spanish Research Council (CSIC) for the provision of a free-of-charge license to the Cambridge Structural Database.
supplementary crystallographic information
Comment
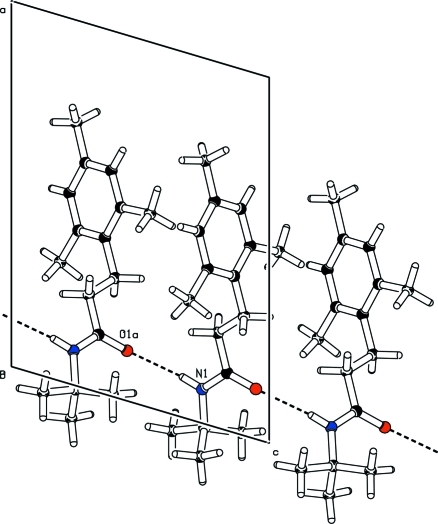
Fig. 1 shows the structure of title compound. Bond lengths and angles are unexceptional. The dihedral angle between the mesityl fragment and the C7/C6/C5/O1/N1/C4/C3 plane is 84.09 (10)°. Methyl groups of the benzene ring are into the same plane (r.m.s. deviation = 0.002 Å). In the crystal, molecules are linked by N— H···O interactions into chains with graph-set notation C(4) along [001], Figure 2, Table 1 (Bernstein et al., 1995).
Experimental
A mixture of 0.001 mol of 1-chloro-3-(2,4,6-trimethylphenyl)propan-2-one and 0.001 mol of tert-butylamine was stirred in water in presence of sodium hydroxide (0.003 mol) for 35–40 minutes. The crystals were recrystallized from ethanol solution (Yield 86%, melting point 143°C).1H NMRspectrum, DMSO-d6, δ, p.p.m..: 1.25 (s, 9H, 3CH3), 2.15 (s, 2H, CH2CO),2.25 (s, 9H, 3CH3), 2.75 (t, 2H, CH2Ar), 6.75 (s, 2H, 2CHAr),7.45 (s, 1H, NHCO). 13C NMR spectrum, DMSO-d6, δ, p.p.m..: 19 (2CH3), 21 (CH3), 23(CH2CO), 25 [(CH3)3], 37 (CH2Ar),50 (Ci), 129 (CHAr), 136 (Ci), 137 (Ci),162 (CONH). IR spectrum, ν (cm-1): 3360,3170, 3005, 2928, 2878, 1645,1615, 1470, 1430, 715, 605.
Refinement
All H-atoms were placed in calculated positions [C—H = 0.93 to 0.97 Å, Uiso(H) =1.2 to 1.5 Ueq(C) and N—H = 0.83 Å, Uiso(H)=1.5 Ueq(N)] and were included in the refinement in the riding model approximation. Due to weak diffracting ability of the crystal the ratio observed/unique reflections is low (45%).
Figures
Fig. 1.
The molecular structure of the title compound, showing the atom labelling scheme. Displacement ellipsoids are drawn at the 30% probability level. H atoms are shown as circles of arbitrary radius.
Fig. 2.
Part of the crystal structure showing the formation of a C(4) chain along [001]. Hydrogen bond shown as dashed lines. Symmetry code: (a) x, 1/2 - y, -1/2 + z.
Crystal data
| C16H25NO | F(000) = 544 |
| Mr = 247.37 | Dx = 1.052 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 1996 reflections |
| a = 12.8851 (11) Å | θ = 2.3–28.0° |
| b = 13.3441 (11) Å | µ = 0.06 mm−1 |
| c = 9.4741 (8) Å | T = 296 K |
| β = 106.540 (2)° | Prism, colourless |
| V = 1561.6 (2) Å3 | 0.20 × 0.20 × 0.20 mm |
| Z = 4 |
Data collection
| Bruker APEXII CCD diffractometer | 3870 independent reflections |
| Radiation source: fine-focus sealed tube | 1738 reflections with I > 2σ(I) |
| graphite | Rint = 0.052 |
| φ and ω scans | θmax = 28.3°, θmin = 2.3° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2003) | h = −17→17 |
| Tmin = 0.987, Tmax = 0.987 | k = −15→17 |
| 11870 measured reflections | l = −12→12 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.065 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.173 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.0732P)2 + 0.0483P] where P = (Fo2 + 2Fc2)/3 |
| 3870 reflections | (Δ/σ)max = 0.001 |
| 169 parameters | Δρmax = 0.15 e Å−3 |
| 0 restraints | Δρmin = −0.14 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.13712 (13) | 0.29736 (13) | 0.94818 (15) | 0.0737 (5) | |
| N1 | 0.09333 (13) | 0.20507 (13) | 0.74037 (17) | 0.0506 (5) | |
| H1N | 0.1102 | 0.1935 | 0.6635 | 0.076* | |
| C1 | 0.0057 (2) | 0.1074 (2) | 0.8929 (3) | 0.0898 (9) | |
| H1A | 0.0617 | 0.0580 | 0.9039 | 0.135* | |
| H1B | −0.0606 | 0.0750 | 0.8940 | 0.135* | |
| H1C | 0.0263 | 0.1545 | 0.9727 | 0.135* | |
| C2 | −0.0933 (2) | 0.2444 (2) | 0.7318 (3) | 0.0853 (8) | |
| H2A | −0.0668 | 0.2942 | 0.8065 | 0.128* | |
| H2B | −0.1597 | 0.2168 | 0.7417 | 0.128* | |
| H2C | −0.1062 | 0.2746 | 0.6364 | 0.128* | |
| C3 | −0.0456 (2) | 0.0878 (2) | 0.6233 (3) | 0.0851 (8) | |
| H3A | 0.0075 | 0.0357 | 0.6355 | 0.128* | |
| H3B | −0.0529 | 0.1218 | 0.5316 | 0.128* | |
| H3C | −0.1140 | 0.0592 | 0.6231 | 0.128* | |
| C4 | −0.01024 (17) | 0.16181 (16) | 0.7488 (2) | 0.0521 (6) | |
| C5 | 0.15762 (17) | 0.26650 (16) | 0.8371 (2) | 0.0510 (5) | |
| C6 | 0.25971 (18) | 0.29603 (19) | 0.8001 (2) | 0.0676 (7) | |
| H6A | 0.2405 | 0.3213 | 0.7000 | 0.081* | |
| H6B | 0.3040 | 0.2368 | 0.8039 | 0.081* | |
| C7 | 0.32616 (18) | 0.37485 (17) | 0.9021 (2) | 0.0607 (6) | |
| H7A | 0.2826 | 0.4347 | 0.8977 | 0.073* | |
| H7B | 0.3454 | 0.3500 | 1.0025 | 0.073* | |
| C8 | 0.42772 (17) | 0.40167 (16) | 0.8626 (2) | 0.0505 (5) | |
| C9 | 0.5242 (2) | 0.35047 (15) | 0.9257 (2) | 0.0570 (6) | |
| C10 | 0.61689 (19) | 0.37656 (17) | 0.8878 (2) | 0.0611 (6) | |
| H10 | 0.6810 | 0.3426 | 0.9316 | 0.073* | |
| C11 | 0.61718 (18) | 0.45079 (17) | 0.7879 (3) | 0.0587 (6) | |
| C12 | 0.52139 (19) | 0.49934 (17) | 0.7257 (2) | 0.0630 (6) | |
| H12 | 0.5199 | 0.5495 | 0.6570 | 0.076* | |
| C13 | 0.42684 (17) | 0.47697 (17) | 0.7607 (2) | 0.0572 (6) | |
| C14 | 0.3253 (2) | 0.5357 (2) | 0.6890 (3) | 0.0936 (9) | |
| H14A | 0.3410 | 0.5858 | 0.6252 | 0.140* | |
| H14B | 0.2998 | 0.5676 | 0.7636 | 0.140* | |
| H14C | 0.2706 | 0.4912 | 0.6326 | 0.140* | |
| C15 | 0.7201 (2) | 0.4793 (2) | 0.7516 (3) | 0.0929 (9) | |
| H15A | 0.7026 | 0.5186 | 0.6631 | 0.139* | |
| H15B | 0.7576 | 0.4197 | 0.7374 | 0.139* | |
| H15C | 0.7655 | 0.5177 | 0.8311 | 0.139* | |
| C16 | 0.5308 (2) | 0.26788 (19) | 1.0379 (3) | 0.0885 (9) | |
| H16A | 0.6026 | 0.2403 | 1.0664 | 0.133* | |
| H16B | 0.4797 | 0.2162 | 0.9954 | 0.133* | |
| H16C | 0.5144 | 0.2949 | 1.1229 | 0.133* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0738 (12) | 0.1073 (13) | 0.0501 (8) | −0.0259 (10) | 0.0340 (8) | −0.0230 (9) |
| N1 | 0.0494 (11) | 0.0637 (11) | 0.0448 (9) | −0.0096 (9) | 0.0234 (8) | −0.0049 (9) |
| C1 | 0.088 (2) | 0.106 (2) | 0.0798 (17) | −0.0271 (17) | 0.0309 (15) | 0.0263 (16) |
| C2 | 0.0554 (16) | 0.092 (2) | 0.112 (2) | 0.0023 (14) | 0.0289 (15) | −0.0023 (16) |
| C3 | 0.0772 (18) | 0.098 (2) | 0.0874 (18) | −0.0387 (16) | 0.0342 (15) | −0.0283 (16) |
| C4 | 0.0465 (13) | 0.0615 (13) | 0.0528 (12) | −0.0089 (11) | 0.0211 (10) | 0.0006 (11) |
| C5 | 0.0506 (13) | 0.0637 (14) | 0.0426 (11) | −0.0100 (11) | 0.0193 (10) | −0.0045 (11) |
| C6 | 0.0587 (15) | 0.0892 (17) | 0.0620 (13) | −0.0250 (13) | 0.0287 (12) | −0.0247 (13) |
| C7 | 0.0588 (15) | 0.0677 (14) | 0.0581 (13) | −0.0112 (12) | 0.0204 (11) | −0.0143 (12) |
| C8 | 0.0481 (13) | 0.0538 (12) | 0.0503 (11) | −0.0108 (11) | 0.0151 (10) | −0.0124 (11) |
| C9 | 0.0636 (16) | 0.0493 (13) | 0.0551 (12) | −0.0051 (12) | 0.0120 (11) | −0.0082 (11) |
| C10 | 0.0482 (14) | 0.0597 (14) | 0.0708 (15) | 0.0019 (11) | 0.0097 (12) | −0.0091 (13) |
| C11 | 0.0501 (14) | 0.0606 (14) | 0.0681 (14) | −0.0077 (12) | 0.0211 (11) | −0.0114 (12) |
| C12 | 0.0674 (17) | 0.0595 (14) | 0.0639 (14) | −0.0038 (13) | 0.0216 (12) | 0.0052 (12) |
| C13 | 0.0487 (13) | 0.0610 (14) | 0.0592 (13) | −0.0004 (11) | 0.0113 (11) | −0.0035 (12) |
| C14 | 0.0691 (19) | 0.103 (2) | 0.101 (2) | 0.0138 (16) | 0.0129 (16) | 0.0256 (18) |
| C15 | 0.0692 (18) | 0.105 (2) | 0.116 (2) | −0.0129 (16) | 0.0455 (16) | −0.0076 (18) |
| C16 | 0.095 (2) | 0.0770 (18) | 0.0887 (18) | −0.0019 (16) | 0.0175 (16) | 0.0204 (15) |
Geometric parameters (Å, °)
| O1—C5 | 1.226 (2) | C7—H7B | 0.9700 |
| N1—C5 | 1.329 (2) | C8—C13 | 1.391 (3) |
| N1—C4 | 1.477 (2) | C8—C9 | 1.395 (3) |
| N1—H1N | 0.8310 | C9—C10 | 1.386 (3) |
| C1—C4 | 1.508 (3) | C9—C16 | 1.517 (3) |
| C1—H1A | 0.9600 | C10—C11 | 1.371 (3) |
| C1—H1B | 0.9600 | C10—H10 | 0.9300 |
| C1—H1C | 0.9600 | C11—C12 | 1.370 (3) |
| C2—C4 | 1.513 (3) | C11—C15 | 1.510 (3) |
| C2—H2A | 0.9600 | C12—C13 | 1.383 (3) |
| C2—H2B | 0.9600 | C12—H12 | 0.9300 |
| C2—H2C | 0.9600 | C13—C14 | 1.511 (3) |
| C3—C4 | 1.512 (3) | C14—H14A | 0.9600 |
| C3—H3A | 0.9600 | C14—H14B | 0.9600 |
| C3—H3B | 0.9600 | C14—H14C | 0.9600 |
| C3—H3C | 0.9600 | C15—H15A | 0.9600 |
| C5—C6 | 1.507 (3) | C15—H15B | 0.9600 |
| C6—C7 | 1.517 (3) | C15—H15C | 0.9600 |
| C6—H6A | 0.9700 | C16—H16A | 0.9600 |
| C6—H6B | 0.9700 | C16—H16B | 0.9600 |
| C7—C8 | 1.503 (3) | C16—H16C | 0.9600 |
| C7—H7A | 0.9700 | ||
| C5—N1—C4 | 126.81 (16) | C8—C7—H7B | 109.1 |
| C5—N1—H1N | 116.8 | C6—C7—H7B | 109.1 |
| C4—N1—H1N | 116.1 | H7A—C7—H7B | 107.9 |
| C4—C1—H1A | 109.5 | C13—C8—C9 | 118.8 (2) |
| C4—C1—H1B | 109.5 | C13—C8—C7 | 120.6 (2) |
| H1A—C1—H1B | 109.5 | C9—C8—C7 | 120.6 (2) |
| C4—C1—H1C | 109.5 | C10—C9—C8 | 119.6 (2) |
| H1A—C1—H1C | 109.5 | C10—C9—C16 | 118.9 (2) |
| H1B—C1—H1C | 109.5 | C8—C9—C16 | 121.4 (2) |
| C4—C2—H2A | 109.5 | C11—C10—C9 | 122.2 (2) |
| C4—C2—H2B | 109.5 | C11—C10—H10 | 118.9 |
| H2A—C2—H2B | 109.5 | C9—C10—H10 | 118.9 |
| C4—C2—H2C | 109.5 | C12—C11—C10 | 117.4 (2) |
| H2A—C2—H2C | 109.5 | C12—C11—C15 | 121.7 (2) |
| H2B—C2—H2C | 109.5 | C10—C11—C15 | 121.0 (2) |
| C4—C3—H3A | 109.5 | C11—C12—C13 | 122.8 (2) |
| C4—C3—H3B | 109.5 | C11—C12—H12 | 118.6 |
| H3A—C3—H3B | 109.5 | C13—C12—H12 | 118.6 |
| C4—C3—H3C | 109.5 | C12—C13—C8 | 119.2 (2) |
| H3A—C3—H3C | 109.5 | C12—C13—C14 | 119.3 (2) |
| H3B—C3—H3C | 109.5 | C8—C13—C14 | 121.5 (2) |
| N1—C4—C1 | 110.16 (18) | C13—C14—H14A | 109.5 |
| N1—C4—C3 | 106.71 (16) | C13—C14—H14B | 109.5 |
| C1—C4—C3 | 109.3 (2) | H14A—C14—H14B | 109.5 |
| N1—C4—C2 | 109.41 (18) | C13—C14—H14C | 109.5 |
| C1—C4—C2 | 110.9 (2) | H14A—C14—H14C | 109.5 |
| C3—C4—C2 | 110.2 (2) | H14B—C14—H14C | 109.5 |
| O1—C5—N1 | 123.68 (19) | C11—C15—H15A | 109.5 |
| O1—C5—C6 | 121.82 (19) | C11—C15—H15B | 109.5 |
| N1—C5—C6 | 114.49 (17) | H15A—C15—H15B | 109.5 |
| C5—C6—C7 | 113.87 (17) | C11—C15—H15C | 109.5 |
| C5—C6—H6A | 108.8 | H15A—C15—H15C | 109.5 |
| C7—C6—H6A | 108.8 | H15B—C15—H15C | 109.5 |
| C5—C6—H6B | 108.8 | C9—C16—H16A | 109.5 |
| C7—C6—H6B | 108.8 | C9—C16—H16B | 109.5 |
| H6A—C6—H6B | 107.7 | H16A—C16—H16B | 109.5 |
| C8—C7—C6 | 112.32 (17) | C9—C16—H16C | 109.5 |
| C8—C7—H7A | 109.1 | H16A—C16—H16C | 109.5 |
| C6—C7—H7A | 109.1 | H16B—C16—H16C | 109.5 |
| C5—N1—C4—C1 | −54.9 (3) | C7—C8—C9—C16 | −1.2 (3) |
| C5—N1—C4—C3 | −173.5 (2) | C8—C9—C10—C11 | −0.9 (3) |
| C5—N1—C4—C2 | 67.3 (3) | C16—C9—C10—C11 | −179.6 (2) |
| C4—N1—C5—O1 | −1.4 (3) | C9—C10—C11—C12 | 0.1 (3) |
| C4—N1—C5—C6 | 178.5 (2) | C9—C10—C11—C15 | 178.5 (2) |
| O1—C5—C6—C7 | −6.5 (3) | C10—C11—C12—C13 | 0.6 (3) |
| N1—C5—C6—C7 | 173.65 (19) | C15—C11—C12—C13 | −177.8 (2) |
| C5—C6—C7—C8 | 179.5 (2) | C11—C12—C13—C8 | −0.4 (3) |
| C6—C7—C8—C13 | 88.1 (2) | C11—C12—C13—C14 | 178.8 (2) |
| C6—C7—C8—C9 | −90.9 (2) | C9—C8—C13—C12 | −0.5 (3) |
| C13—C8—C9—C10 | 1.1 (3) | C7—C8—C13—C12 | −179.47 (19) |
| C7—C8—C9—C10 | −179.89 (18) | C9—C8—C13—C14 | −179.6 (2) |
| C13—C8—C9—C16 | 179.8 (2) | C7—C8—C13—C14 | 1.4 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1N···O1i | 0.83 | 2.17 | 2.979 (2) | 165 |
Symmetry codes: (i) x, −y+1/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: OM2425).
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Bruker (2001). SAINT . Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2005). APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
- Sheldrick, G. M. (2003). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811015856/om2425sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811015856/om2425Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811015856/om2425Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report