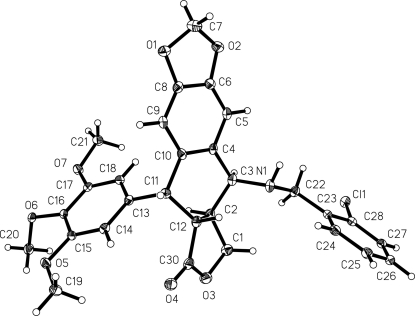
Abstract
In the title compound, C29H28ClNO7, the tetrahydrofuran ring and the six-membered ring fused to it both display envelope conformations. The dihedral angles between the plane of the benzene ring of the benzo[d][1,3]dioxole system and the planes of the other two benzene rings are 80.59 (3) and 63.60 (2)°.
Related literature
For bond-length and angle data for similar structures, see: Feng et al. (2008 ▶); Zhang et al. (1994 ▶); Zuo et al. (2009 ▶).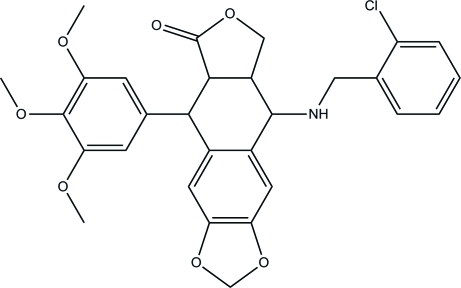
Experimental
Crystal data
C29H28ClNO7
M r = 537.97
Orthorhombic,
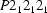
a = 10.0971 (14) Å
b = 15.264 (2) Å
c = 16.220 (2) Å
V = 2499.9 (6) Å3
Z = 4
Mo Kα radiation
μ = 0.20 mm−1
T = 113 K
0.20 × 0.18 × 0.12 mm
Data collection
Rigaku Saturn CCD area-detector diffractometer
Absorption correction: multi-scan (CrystalClear; Rigaku, 2007 ▶) T min = 0.960, T max = 0.976
26247 measured reflections
5968 independent reflections
5580 reflections with I > 2σ(I)
R int = 0.044
Refinement
R[F 2 > 2σ(F 2)] = 0.031
wR(F 2) = 0.066
S = 1.03
5968 reflections
350 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.18 e Å−3
Δρmin = −0.25 e Å−3
Absolute structure: Flack (1983), 2615 Friedel pairs
Flack parameter: 0.00 (4)
Data collection: CrystalClear (Rigaku, 2007 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811018289/hg5035sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811018289/hg5035Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811018289/hg5035Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C26—H26⋯O7i | 0.95 | 2.56 | 3.2130 (18) | 126 |
Symmetry code: (i) 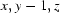 .
.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30873363), the Program of the Science Foundation of Tianjin (08JCYBJC070000) and the Major Program of the Science Foundation of Tianjin (09ZCKFSH01700).
supplementary crystallographic information
Comment
Podophyllotoxin and their derivatives are well known as substances with anti-cancer activity. In recent years, our study are paying attention to synthesize different kinds of Podophyllotoxin compounds and aim at the discovery of new derivatives with improved bioactivities. In this paper, we reported the crystal structure of title compound.
In title compound, C29H28ClNO7, bond lengths and angles are normal and in good agreement with those reported previously (Feng et al., 2008; Zhang, et al., 1994; Zuo, et al., 2009). The tetrahydrofuran ring (C1/C2/C12/C30/O3) and the six-membered ring (C2—C4/C10—C12)fused to it both display envelope conformations. The dihedral angles between the benzene ring (C4—C10) of the benzo[d]-[1,3]dioxole and the other two benzene ring (C13—C18 and C23—C28) are 80.59 (3) and 63.60 (2)°, respectively. There are weaker C—H···O intermolecular interactions, which stabilized the structure (Table 1).
Experimental
The target compound was synthesized by two steps. 2-chlorobenzaldehyde, 4β-amino podophyllotoxin, two drops of acetic acid in 95% ethanol was stirred for 6 h. Appropriate amount of NaBH4 was added into the reaction mixture to stirred for 1 h at 273 K. Then add 5% HCl to end off the reaction, the reaction mixture was concentrated in vacuo. Add saturated NaHCO3 to adjust PH>7. The reaction mixture was extracted with CH2Cl2 and dried over MgSO4 and concentrated in vacuo. The residue was resolved in a methanol solution and slow evaporation over two weeks at room temperature gave transparent crystals suitable for X-ray analysis.
Refinement
All C H atoms were found on difference maps, with C—H = 0.95–1.00 Å and included in the final cycles of refinement using a riding model, with Uiso(H) = 1.2Ueq(C) for aryl and methylene H atoms and 1.5Ueq(C) for the methyl H atoms. H atoms bonded N were refined freely with N—H = 0.96 (2) Å.
Figures
Fig. 1.
View of the title compound, with displacement ellipsoids drawn at the 40% probability level.
Crystal data
| C29H28ClNO7 | F(000) = 1128 |
| Mr = 537.97 | Dx = 1.429 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 8535 reflections |
| a = 10.0971 (14) Å | θ = 1.3–27.9° |
| b = 15.264 (2) Å | µ = 0.20 mm−1 |
| c = 16.220 (2) Å | T = 113 K |
| V = 2499.9 (6) Å3 | Prism, colorless |
| Z = 4 | 0.20 × 0.18 × 0.12 mm |
Data collection
| Rigaku Saturn CCD area-detector diffractometer | 5968 independent reflections |
| Radiation source: rotating anode | 5580 reflections with I > 2σ(I) |
| multilayer | Rint = 0.044 |
| Detector resolution: 14.63 pixels mm-1 | θmax = 27.9°, θmin = 1.8° |
| ω and φ scans | h = −13→13 |
| Absorption correction: multi-scan (CrystalClear; Rigaku, 2007) | k = −20→20 |
| Tmin = 0.960, Tmax = 0.976 | l = −20→21 |
| 26247 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.031 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.066 | w = 1/[σ2(Fo2) + (0.0324P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.03 | (Δ/σ)max = 0.001 |
| 5968 reflections | Δρmax = 0.18 e Å−3 |
| 350 parameters | Δρmin = −0.25 e Å−3 |
| 0 restraints | Absolute structure: Flack (1983), 2615 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.00 (4) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | −0.19083 (4) | −0.19218 (2) | 0.81504 (2) | 0.02120 (9) | |
| O1 | 0.56079 (11) | 0.23021 (7) | 0.72078 (6) | 0.0247 (3) | |
| O2 | 0.41961 (12) | 0.14072 (7) | 0.64668 (6) | 0.0240 (3) | |
| O3 | 0.09055 (11) | 0.06096 (7) | 1.11792 (6) | 0.0253 (3) | |
| O4 | 0.28209 (11) | 0.10425 (6) | 1.17321 (6) | 0.0261 (3) | |
| O5 | 0.31178 (11) | 0.40151 (6) | 1.20050 (6) | 0.0209 (2) | |
| O6 | 0.18781 (11) | 0.51133 (6) | 1.09222 (6) | 0.0189 (2) | |
| O7 | 0.12116 (11) | 0.45705 (6) | 0.94280 (6) | 0.0209 (2) | |
| N1 | 0.16797 (13) | −0.03792 (7) | 0.88773 (8) | 0.0199 (3) | |
| C1 | 0.03959 (16) | 0.03821 (10) | 1.03598 (9) | 0.0229 (3) | |
| H1A | −0.0550 | 0.0545 | 1.0307 | 0.027* | |
| H1B | 0.0491 | −0.0253 | 1.0253 | 0.027* | |
| C2 | 0.12467 (15) | 0.09136 (9) | 0.97679 (9) | 0.0178 (3) | |
| H2 | 0.0883 | 0.1522 | 0.9740 | 0.021* | |
| C3 | 0.14712 (15) | 0.05866 (8) | 0.88890 (9) | 0.0180 (3) | |
| H3 | 0.0667 | 0.0725 | 0.8554 | 0.022* | |
| C4 | 0.26551 (15) | 0.10614 (8) | 0.85124 (9) | 0.0162 (3) | |
| C5 | 0.28349 (15) | 0.09545 (9) | 0.76558 (9) | 0.0193 (3) | |
| H5 | 0.2256 | 0.0594 | 0.7343 | 0.023* | |
| C6 | 0.38597 (16) | 0.13821 (9) | 0.72912 (9) | 0.0189 (3) | |
| C7 | 0.54559 (17) | 0.18360 (12) | 0.64454 (10) | 0.0287 (4) | |
| H7A | 0.5496 | 0.2248 | 0.5975 | 0.034* | |
| H7B | 0.6174 | 0.1399 | 0.6382 | 0.034* | |
| C8 | 0.47041 (15) | 0.19194 (10) | 0.77350 (9) | 0.0185 (3) | |
| C9 | 0.45689 (15) | 0.20311 (9) | 0.85659 (9) | 0.0180 (3) | |
| H9 | 0.5156 | 0.2400 | 0.8865 | 0.022* | |
| C10 | 0.35316 (15) | 0.15831 (8) | 0.89679 (9) | 0.0163 (3) | |
| C11 | 0.34335 (15) | 0.16871 (8) | 0.99015 (8) | 0.0161 (3) | |
| H11 | 0.4342 | 0.1599 | 1.0133 | 0.019* | |
| C12 | 0.25620 (15) | 0.09411 (9) | 1.02231 (9) | 0.0179 (3) | |
| H12 | 0.3033 | 0.0382 | 1.0093 | 0.021* | |
| C13 | 0.29684 (15) | 0.25985 (8) | 1.01666 (8) | 0.0158 (3) | |
| C14 | 0.32622 (15) | 0.28769 (8) | 1.09600 (9) | 0.0168 (3) | |
| H14 | 0.3742 | 0.2503 | 1.1320 | 0.020* | |
| C15 | 0.28577 (15) | 0.37031 (9) | 1.12340 (8) | 0.0158 (3) | |
| C16 | 0.21789 (15) | 0.42648 (8) | 1.07030 (8) | 0.0158 (3) | |
| C17 | 0.18852 (15) | 0.39801 (8) | 0.99061 (8) | 0.0157 (3) | |
| C18 | 0.22750 (14) | 0.31519 (9) | 0.96379 (9) | 0.0165 (3) | |
| H18 | 0.2068 | 0.2965 | 0.9094 | 0.020* | |
| C19 | 0.37066 (17) | 0.34058 (10) | 1.25669 (9) | 0.0248 (4) | |
| H19A | 0.3180 | 0.2867 | 1.2580 | 0.037* | |
| H19B | 0.3734 | 0.3664 | 1.3120 | 0.037* | |
| H19C | 0.4609 | 0.3270 | 1.2386 | 0.037* | |
| C20 | 0.08614 (17) | 0.52134 (10) | 1.15354 (10) | 0.0233 (4) | |
| H20A | 0.0070 | 0.4889 | 1.1365 | 0.035* | |
| H20B | 0.0642 | 0.5836 | 1.1596 | 0.035* | |
| H20C | 0.1179 | 0.4984 | 1.2064 | 0.035* | |
| C21 | 0.10712 (18) | 0.43615 (10) | 0.85726 (9) | 0.0254 (4) | |
| H21A | 0.1949 | 0.4285 | 0.8325 | 0.038* | |
| H21B | 0.0604 | 0.4838 | 0.8291 | 0.038* | |
| H21C | 0.0564 | 0.3818 | 0.8515 | 0.038* | |
| C22 | 0.04768 (15) | −0.08553 (9) | 0.86421 (9) | 0.0193 (3) | |
| H22A | 0.0434 | −0.0891 | 0.8033 | 0.023* | |
| H22B | −0.0305 | −0.0519 | 0.8832 | 0.023* | |
| C23 | 0.04024 (15) | −0.17743 (9) | 0.89942 (8) | 0.0157 (3) | |
| C24 | 0.13828 (16) | −0.21272 (9) | 0.94947 (9) | 0.0196 (3) | |
| H24 | 0.2148 | −0.1789 | 0.9617 | 0.024* | |
| C25 | 0.12665 (16) | −0.29669 (9) | 0.98209 (9) | 0.0218 (3) | |
| H25 | 0.1958 | −0.3201 | 1.0151 | 0.026* | |
| C26 | 0.01461 (16) | −0.34623 (10) | 0.96657 (9) | 0.0206 (3) | |
| H26 | 0.0055 | −0.4028 | 0.9904 | 0.025* | |
| C27 | −0.08436 (15) | −0.31306 (9) | 0.91619 (8) | 0.0184 (3) | |
| H27 | −0.1614 | −0.3467 | 0.9049 | 0.022* | |
| C28 | −0.06935 (15) | −0.23039 (9) | 0.88264 (8) | 0.0165 (3) | |
| C30 | 0.21676 (16) | 0.08957 (9) | 1.11209 (9) | 0.0211 (3) | |
| H1 | 0.2339 (18) | −0.0558 (10) | 0.8486 (10) | 0.029 (5)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.01945 (19) | 0.02013 (16) | 0.02402 (18) | −0.00103 (16) | −0.00483 (16) | 0.00179 (14) |
| O1 | 0.0245 (6) | 0.0268 (6) | 0.0227 (6) | −0.0046 (5) | 0.0060 (5) | −0.0004 (4) |
| O2 | 0.0276 (7) | 0.0272 (6) | 0.0172 (5) | −0.0013 (5) | 0.0006 (5) | −0.0011 (4) |
| O3 | 0.0264 (7) | 0.0266 (6) | 0.0229 (6) | −0.0033 (5) | 0.0008 (5) | 0.0018 (5) |
| O4 | 0.0340 (7) | 0.0244 (5) | 0.0200 (6) | −0.0020 (5) | −0.0058 (5) | 0.0028 (4) |
| O5 | 0.0264 (6) | 0.0203 (5) | 0.0160 (5) | −0.0005 (5) | −0.0052 (5) | −0.0020 (4) |
| O6 | 0.0253 (6) | 0.0112 (4) | 0.0203 (5) | −0.0021 (5) | 0.0045 (5) | −0.0013 (4) |
| O7 | 0.0305 (6) | 0.0163 (5) | 0.0159 (5) | 0.0054 (5) | −0.0063 (5) | 0.0013 (4) |
| N1 | 0.0177 (7) | 0.0138 (6) | 0.0282 (7) | 0.0003 (5) | −0.0035 (6) | −0.0016 (5) |
| C1 | 0.0202 (9) | 0.0249 (8) | 0.0234 (8) | −0.0020 (7) | −0.0009 (7) | 0.0017 (6) |
| C2 | 0.0168 (8) | 0.0154 (7) | 0.0213 (7) | 0.0017 (6) | −0.0017 (6) | 0.0016 (6) |
| C3 | 0.0163 (8) | 0.0147 (7) | 0.0230 (8) | 0.0008 (6) | −0.0051 (6) | −0.0003 (6) |
| C4 | 0.0178 (8) | 0.0104 (6) | 0.0203 (7) | 0.0030 (6) | −0.0027 (6) | 0.0012 (5) |
| C5 | 0.0213 (8) | 0.0140 (6) | 0.0227 (8) | 0.0009 (6) | −0.0054 (6) | −0.0020 (6) |
| C6 | 0.0232 (9) | 0.0165 (7) | 0.0171 (8) | 0.0050 (6) | −0.0008 (6) | 0.0017 (6) |
| C7 | 0.0255 (9) | 0.0387 (10) | 0.0219 (8) | −0.0006 (8) | 0.0017 (7) | 0.0007 (7) |
| C8 | 0.0170 (8) | 0.0152 (7) | 0.0232 (8) | 0.0026 (7) | 0.0003 (6) | 0.0023 (6) |
| C9 | 0.0176 (8) | 0.0142 (7) | 0.0221 (8) | 0.0001 (6) | −0.0023 (6) | −0.0031 (6) |
| C10 | 0.0179 (8) | 0.0126 (6) | 0.0182 (7) | 0.0059 (6) | −0.0010 (6) | −0.0014 (5) |
| C11 | 0.0169 (8) | 0.0164 (7) | 0.0151 (7) | 0.0013 (6) | −0.0033 (6) | 0.0010 (5) |
| C12 | 0.0184 (8) | 0.0139 (6) | 0.0214 (8) | 0.0027 (6) | −0.0028 (6) | 0.0012 (6) |
| C13 | 0.0142 (8) | 0.0153 (7) | 0.0179 (7) | −0.0017 (6) | 0.0000 (7) | 0.0002 (5) |
| C14 | 0.0157 (8) | 0.0161 (7) | 0.0187 (7) | 0.0005 (6) | −0.0041 (6) | 0.0036 (5) |
| C15 | 0.0154 (8) | 0.0179 (6) | 0.0140 (7) | −0.0047 (6) | −0.0006 (6) | −0.0013 (5) |
| C16 | 0.0181 (8) | 0.0125 (6) | 0.0169 (7) | −0.0027 (6) | 0.0024 (6) | −0.0017 (5) |
| C17 | 0.0148 (7) | 0.0146 (6) | 0.0178 (7) | −0.0023 (6) | 0.0002 (6) | 0.0030 (5) |
| C18 | 0.0174 (7) | 0.0169 (7) | 0.0152 (7) | −0.0020 (6) | 0.0010 (6) | −0.0006 (6) |
| C19 | 0.0292 (9) | 0.0276 (8) | 0.0175 (8) | −0.0019 (7) | −0.0070 (7) | 0.0028 (6) |
| C20 | 0.0239 (9) | 0.0189 (8) | 0.0272 (9) | −0.0012 (6) | 0.0056 (7) | −0.0033 (6) |
| C21 | 0.0386 (10) | 0.0215 (8) | 0.0160 (8) | −0.0003 (7) | −0.0056 (7) | 0.0025 (6) |
| C22 | 0.0196 (8) | 0.0172 (7) | 0.0213 (8) | −0.0031 (6) | −0.0033 (6) | 0.0018 (6) |
| C23 | 0.0182 (8) | 0.0151 (7) | 0.0138 (7) | 0.0006 (6) | 0.0017 (6) | −0.0017 (5) |
| C24 | 0.0202 (8) | 0.0212 (8) | 0.0174 (7) | −0.0011 (6) | −0.0004 (6) | −0.0022 (6) |
| C25 | 0.0274 (9) | 0.0211 (8) | 0.0169 (7) | 0.0059 (7) | −0.0040 (7) | 0.0001 (6) |
| C26 | 0.0284 (9) | 0.0158 (7) | 0.0174 (7) | 0.0022 (6) | 0.0046 (7) | 0.0003 (6) |
| C27 | 0.0200 (8) | 0.0183 (7) | 0.0169 (7) | −0.0029 (7) | 0.0044 (6) | −0.0025 (6) |
| C28 | 0.0168 (8) | 0.0189 (7) | 0.0139 (7) | 0.0018 (6) | 0.0002 (6) | −0.0018 (6) |
| C30 | 0.0246 (9) | 0.0143 (7) | 0.0245 (8) | 0.0018 (6) | −0.0008 (7) | 0.0032 (6) |
Geometric parameters (Å, °)
| Cl1—C28 | 1.7455 (15) | C11—C13 | 1.5301 (19) |
| O1—C8 | 1.3803 (18) | C11—C12 | 1.531 (2) |
| O1—C7 | 1.4348 (18) | C11—H11 | 1.0000 |
| O2—C6 | 1.3802 (17) | C12—C30 | 1.511 (2) |
| O2—C7 | 1.431 (2) | C12—H12 | 1.0000 |
| O3—C30 | 1.3504 (19) | C13—C14 | 1.3874 (19) |
| O3—C1 | 1.4669 (18) | C13—C18 | 1.3925 (19) |
| O4—C30 | 1.2116 (18) | C14—C15 | 1.3981 (19) |
| O5—C15 | 1.3637 (16) | C14—H14 | 0.9500 |
| O5—C19 | 1.4315 (17) | C15—C16 | 1.395 (2) |
| O6—C16 | 1.3771 (16) | C16—C17 | 1.3956 (19) |
| O6—C20 | 1.4374 (18) | C17—C18 | 1.3937 (19) |
| O7—C17 | 1.3697 (17) | C18—H18 | 0.9500 |
| O7—C21 | 1.4307 (18) | C19—H19A | 0.9800 |
| N1—C22 | 1.4659 (19) | C19—H19B | 0.9800 |
| N1—C3 | 1.4892 (17) | C19—H19C | 0.9800 |
| N1—H1 | 0.959 (17) | C20—H20A | 0.9800 |
| C1—C2 | 1.522 (2) | C20—H20B | 0.9800 |
| C1—H1A | 0.9900 | C20—H20C | 0.9800 |
| C1—H1B | 0.9900 | C21—H21A | 0.9800 |
| C2—C12 | 1.520 (2) | C21—H21B | 0.9800 |
| C2—C3 | 1.527 (2) | C21—H21C | 0.9800 |
| C2—H2 | 1.0000 | C22—C23 | 1.5164 (19) |
| C3—C4 | 1.526 (2) | C22—H22A | 0.9900 |
| C3—H3 | 1.0000 | C22—H22B | 0.9900 |
| C4—C10 | 1.401 (2) | C23—C24 | 1.389 (2) |
| C4—C5 | 1.411 (2) | C23—C28 | 1.397 (2) |
| C5—C6 | 1.359 (2) | C24—C25 | 1.392 (2) |
| C5—H5 | 0.9500 | C24—H24 | 0.9500 |
| C6—C8 | 1.385 (2) | C25—C26 | 1.384 (2) |
| C7—H7A | 0.9900 | C25—H25 | 0.9500 |
| C7—H7B | 0.9900 | C26—C27 | 1.387 (2) |
| C8—C9 | 1.3654 (19) | C26—H26 | 0.9500 |
| C9—C10 | 1.411 (2) | C27—C28 | 1.383 (2) |
| C9—H9 | 0.9500 | C27—H27 | 0.9500 |
| C10—C11 | 1.526 (2) | ||
| C8—O1—C7 | 104.68 (11) | C14—C13—C18 | 119.54 (12) |
| C6—O2—C7 | 104.77 (12) | C14—C13—C11 | 118.26 (12) |
| C30—O3—C1 | 110.13 (12) | C18—C13—C11 | 122.19 (12) |
| C15—O5—C19 | 115.91 (11) | C13—C14—C15 | 120.58 (13) |
| C16—O6—C20 | 115.86 (10) | C13—C14—H14 | 119.7 |
| C17—O7—C21 | 116.85 (11) | C15—C14—H14 | 119.7 |
| C22—N1—C3 | 112.14 (11) | O5—C15—C16 | 116.50 (12) |
| C22—N1—H1 | 105.2 (10) | O5—C15—C14 | 123.39 (12) |
| C3—N1—H1 | 112.8 (10) | C16—C15—C14 | 120.10 (13) |
| O3—C1—C2 | 104.31 (12) | O6—C16—C15 | 121.80 (12) |
| O3—C1—H1A | 110.9 | O6—C16—C17 | 119.02 (12) |
| C2—C1—H1A | 110.9 | C15—C16—C17 | 119.00 (12) |
| O3—C1—H1B | 110.9 | O7—C17—C18 | 124.07 (13) |
| C2—C1—H1B | 110.9 | O7—C17—C16 | 115.16 (12) |
| H1A—C1—H1B | 108.9 | C18—C17—C16 | 120.77 (13) |
| C12—C2—C1 | 101.62 (11) | C13—C18—C17 | 120.00 (13) |
| C12—C2—C3 | 109.44 (12) | C13—C18—H18 | 120.0 |
| C1—C2—C3 | 119.88 (12) | C17—C18—H18 | 120.0 |
| C12—C2—H2 | 108.4 | O5—C19—H19A | 109.5 |
| C1—C2—H2 | 108.4 | O5—C19—H19B | 109.5 |
| C3—C2—H2 | 108.4 | H19A—C19—H19B | 109.5 |
| N1—C3—C4 | 110.76 (12) | O5—C19—H19C | 109.5 |
| N1—C3—C2 | 110.88 (12) | H19A—C19—H19C | 109.5 |
| C4—C3—C2 | 109.56 (11) | H19B—C19—H19C | 109.5 |
| N1—C3—H3 | 108.5 | O6—C20—H20A | 109.5 |
| C4—C3—H3 | 108.5 | O6—C20—H20B | 109.5 |
| C2—C3—H3 | 108.5 | H20A—C20—H20B | 109.5 |
| C10—C4—C5 | 120.25 (14) | O6—C20—H20C | 109.5 |
| C10—C4—C3 | 123.63 (13) | H20A—C20—H20C | 109.5 |
| C5—C4—C3 | 116.12 (13) | H20B—C20—H20C | 109.5 |
| C6—C5—C4 | 118.11 (14) | O7—C21—H21A | 109.5 |
| C6—C5—H5 | 120.9 | O7—C21—H21B | 109.5 |
| C4—C5—H5 | 120.9 | H21A—C21—H21B | 109.5 |
| C5—C6—O2 | 128.50 (14) | O7—C21—H21C | 109.5 |
| C5—C6—C8 | 121.80 (13) | H21A—C21—H21C | 109.5 |
| O2—C6—C8 | 109.61 (13) | H21B—C21—H21C | 109.5 |
| O2—C7—O1 | 107.52 (12) | N1—C22—C23 | 113.68 (12) |
| O2—C7—H7A | 110.2 | N1—C22—H22A | 108.8 |
| O1—C7—H7A | 110.2 | C23—C22—H22A | 108.8 |
| O2—C7—H7B | 110.2 | N1—C22—H22B | 108.8 |
| O1—C7—H7B | 110.2 | C23—C22—H22B | 108.8 |
| H7A—C7—H7B | 108.5 | H22A—C22—H22B | 107.7 |
| C9—C8—O1 | 128.67 (14) | C24—C23—C28 | 117.00 (13) |
| C9—C8—C6 | 121.71 (14) | C24—C23—C22 | 122.93 (13) |
| O1—C8—C6 | 109.61 (12) | C28—C23—C22 | 120.07 (13) |
| C8—C9—C10 | 118.03 (14) | C23—C24—C25 | 121.28 (14) |
| C8—C9—H9 | 121.0 | C23—C24—H24 | 119.4 |
| C10—C9—H9 | 121.0 | C25—C24—H24 | 119.4 |
| C4—C10—C9 | 120.05 (13) | C26—C25—C24 | 120.20 (14) |
| C4—C10—C11 | 122.78 (13) | C26—C25—H25 | 119.9 |
| C9—C10—C11 | 117.17 (13) | C24—C25—H25 | 119.9 |
| C10—C11—C13 | 113.17 (11) | C25—C26—C27 | 119.80 (14) |
| C10—C11—C12 | 107.35 (11) | C25—C26—H26 | 120.1 |
| C13—C11—C12 | 113.84 (12) | C27—C26—H26 | 120.1 |
| C10—C11—H11 | 107.4 | C28—C27—C26 | 119.09 (14) |
| C13—C11—H11 | 107.4 | C28—C27—H27 | 120.5 |
| C12—C11—H11 | 107.4 | C26—C27—H27 | 120.5 |
| C30—C12—C2 | 103.68 (12) | C27—C28—C23 | 122.56 (14) |
| C30—C12—C11 | 120.93 (12) | C27—C28—Cl1 | 118.38 (12) |
| C2—C12—C11 | 110.93 (11) | C23—C28—Cl1 | 119.05 (11) |
| C30—C12—H12 | 106.8 | O4—C30—O3 | 121.09 (14) |
| C2—C12—H12 | 106.8 | O4—C30—C12 | 129.53 (15) |
| C11—C12—H12 | 106.8 | O3—C30—C12 | 109.32 (13) |
| C30—O3—C1—C2 | −23.55 (15) | C10—C11—C13—C14 | −158.29 (13) |
| O3—C1—C2—C12 | 32.23 (14) | C12—C11—C13—C14 | 78.78 (17) |
| O3—C1—C2—C3 | 152.89 (12) | C10—C11—C13—C18 | 21.3 (2) |
| C22—N1—C3—C4 | −138.33 (13) | C12—C11—C13—C18 | −101.68 (15) |
| C22—N1—C3—C2 | 99.82 (14) | C18—C13—C14—C15 | 0.6 (2) |
| C12—C2—C3—N1 | 76.31 (15) | C11—C13—C14—C15 | −179.85 (13) |
| C1—C2—C3—N1 | −40.37 (18) | C19—O5—C15—C16 | 174.55 (13) |
| C12—C2—C3—C4 | −46.24 (14) | C19—O5—C15—C14 | −7.0 (2) |
| C1—C2—C3—C4 | −162.92 (13) | C13—C14—C15—O5 | −179.86 (13) |
| N1—C3—C4—C10 | −111.37 (14) | C13—C14—C15—C16 | −1.5 (2) |
| C2—C3—C4—C10 | 11.25 (18) | C20—O6—C16—C15 | −71.65 (18) |
| N1—C3—C4—C5 | 68.97 (15) | C20—O6—C16—C17 | 113.25 (15) |
| C2—C3—C4—C5 | −168.41 (12) | O5—C15—C16—O6 | 4.9 (2) |
| C10—C4—C5—C6 | −1.1 (2) | C14—C15—C16—O6 | −173.55 (13) |
| C3—C4—C5—C6 | 178.52 (12) | O5—C15—C16—C17 | −179.98 (12) |
| C4—C5—C6—O2 | −177.04 (14) | C14—C15—C16—C17 | 1.5 (2) |
| C4—C5—C6—C8 | −0.8 (2) | C21—O7—C17—C18 | −9.0 (2) |
| C7—O2—C6—C5 | −171.48 (15) | C21—O7—C17—C16 | 170.60 (13) |
| C7—O2—C6—C8 | 11.94 (16) | O6—C16—C17—O7 | −5.1 (2) |
| C6—O2—C7—O1 | −19.05 (16) | C15—C16—C17—O7 | 179.68 (13) |
| C8—O1—C7—O2 | 18.94 (16) | O6—C16—C17—C18 | 174.50 (13) |
| C7—O1—C8—C9 | 169.91 (16) | C15—C16—C17—C18 | −0.7 (2) |
| C7—O1—C8—C6 | −11.63 (15) | C14—C13—C18—C17 | 0.2 (2) |
| C5—C6—C8—C9 | 1.6 (2) | C11—C13—C18—C17 | −179.31 (13) |
| O2—C6—C8—C9 | 178.41 (13) | O7—C17—C18—C13 | 179.40 (13) |
| C5—C6—C8—O1 | −177.03 (13) | C16—C17—C18—C13 | −0.1 (2) |
| O2—C6—C8—O1 | −0.17 (16) | C3—N1—C22—C23 | −152.51 (12) |
| O1—C8—C9—C10 | 178.05 (13) | N1—C22—C23—C24 | 0.2 (2) |
| C6—C8—C9—C10 | −0.2 (2) | N1—C22—C23—C28 | 179.56 (13) |
| C5—C4—C10—C9 | 2.4 (2) | C28—C23—C24—C25 | −0.7 (2) |
| C3—C4—C10—C9 | −177.21 (13) | C22—C23—C24—C25 | 178.61 (14) |
| C5—C4—C10—C11 | −176.76 (13) | C23—C24—C25—C26 | −1.5 (2) |
| C3—C4—C10—C11 | 3.6 (2) | C24—C25—C26—C27 | 2.1 (2) |
| C8—C9—C10—C4 | −1.7 (2) | C25—C26—C27—C28 | −0.4 (2) |
| C8—C9—C10—C11 | 177.53 (13) | C26—C27—C28—C23 | −2.0 (2) |
| C4—C10—C11—C13 | −109.51 (15) | C26—C27—C28—Cl1 | 176.65 (11) |
| C9—C10—C11—C13 | 71.28 (17) | C24—C23—C28—C27 | 2.5 (2) |
| C4—C10—C11—C12 | 16.95 (18) | C22—C23—C28—C27 | −176.88 (13) |
| C9—C10—C11—C12 | −162.27 (12) | C24—C23—C28—Cl1 | −176.10 (11) |
| C1—C2—C12—C30 | −29.50 (14) | C22—C23—C28—Cl1 | 4.52 (18) |
| C3—C2—C12—C30 | −157.22 (11) | C1—O3—C30—O4 | −173.15 (13) |
| C1—C2—C12—C11 | −160.76 (12) | C1—O3—C30—C12 | 4.19 (15) |
| C3—C2—C12—C11 | 71.52 (14) | C2—C12—C30—O4 | −166.14 (15) |
| C10—C11—C12—C30 | −174.77 (13) | C11—C12—C30—O4 | −41.1 (2) |
| C13—C11—C12—C30 | −48.71 (18) | C2—C12—C30—O3 | 16.81 (14) |
| C10—C11—C12—C2 | −53.14 (15) | C11—C12—C30—O3 | 141.88 (13) |
| C13—C11—C12—C2 | 72.92 (15) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C26—H26···O7i | 0.95 | 2.56 | 3.2130 (18) | 126. |
Symmetry codes: (i) x, y−1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG5035).
References
- Feng, M., Zhao, M., Zhang, J., Yang, Z. & Chen, H. (2008). Acta Cryst. E64, o2339. [DOI] [PMC free article] [PubMed]
- Rigaku. (2007). CrystalClear Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Zhang, Y. L., Tropsha, A., McPhail, A. T. & Lee, K. H. (1994). J. Med. Chem. 37, 1460–1464. [DOI] [PubMed]
- Zuo, S., Chen, H., Lu, Y., Cao, B. & Liu, D. (2009). Acta Cryst. E65, o3257. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536811018289/hg5035sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536811018289/hg5035Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811018289/hg5035Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report