Abstract
The ubiquitously expressed Calpains 1 and 2 belong to a family of calcium-dependent intracellular cysteine proteases. Both calpains are heterodimers consisting of a large subunit and a small regulatory subunit encoded by the gene Capns1. To investigate a role for the calpain small subunit in cells of the osteoblast lineage in vivo, we previously generated osteoblast-specific Capns1 knockout mice and characterized their bone phenotype. In this study, we further examined effects of low calcium and high fat diets on their bone, fat, and glucose homeostasis.
Osteoblast-specific Capns1 knockout mice showed significantly reduced serum levels of total and uncarboxylated osteocalcin, and this was presumably due to their impaired bone formation and bone resorption. The reduced bone resorptive function of the mutant mice was also significant under a low calcium diet. Thus, these results suggest that reduced uncarboxylated osteocalcin levels of mutant mice were, at least in part, due to their osteoporotic bone with impaired bone resorptive function. Interestingly, unlike osteocalcin knockout mice, mutant mice on a normal chow diet were leaner than control littermates; this was likely due to their reduced food intake and overall lower energy homeostasis. To test this hypothesis, we next provided mutant mice with a high fat diet and further examined an effect of their reduced uncarboxylated osteocalcin levels on body composition and glucose metabolism. The average mean body weight of mutant mice became indistinguishable with that of controls after two weeks on a high fat diet, and continued to show an upward trend, at least, up to 6 weeks. Moreover, mutant mice on a high fat diet exhibited a significant increase in serum levels of leptin and resistin, adipocyte-specific adipokines, and developed impaired glucose tolerance. Collectively, mice with osteoporosis and reduced bone resorptive function showed reduced serum uncarboxylated osteocalcin levels and were susceptible to increase body adiposity and develop impaired glucose tolerance under a high fat diet.
Keywords: osteoporosis, bone resorption, high fat diet, glucose metabolism
1. Introduction
Calpains 1 and 2 consist of an 80-kDa large catalytic subunit encoded by the genes Capn1 and Capn2, respectively, and a 28-kDa common small regulatory subunit encoded by the gene Capns1 [1]. Ablation of the calpain small subunit eliminates activities of both calpains and leads to embryonic lethality in mice, suggesting its critical role for their stability and activity and during embryonic development [2]. To examine a role of the calpain small subunit in cells of the osteoblast lineage in vivo, we previously generated osteoblast-specific Capns1 knockout mice [3]. Their characteristic bone phenotype was reduced trabecular and cortical bone mainly due to reduced osteoblast number and function, and consequently reduced osteoclast number.
Recently, a line of evidence has shown that osteocalcin, a bone-derived hormone and a marker for bone formation, regulates glucose homeostasis by modulating adiponectin levels [4]. Serum levels of uncarboxylated osteocalcin, an active form of osteocalcin, are regulated by insulin signaling-mediated bone resorption [5]. These results suggest that both bone formation and resorption play a critical role in modifying serum uncarboxylated osteocalcin levels, consequently, body adiposity, and glucose homeostasis in vivo. In this study, we examined whether deletion of Capns1 in osteoblasts alters uncarboxylated osteocalcin levels in mice and how dietary manipulation affects bone, fat, and glucose homeostasis in osteoblast-specific Capns1 knockout mice.
2. Materials and Methods
2.1. Animals and treatment
All experiments were performed in compliance with the guiding principles of the Guide for the Care and Use of Laboratory Animals, and approved by the subcommittee on Research Animal Care of the Massachusetts General Hospital (MGH). Mouse genotype was determined as described previously [3]. Based on our previous observations, we used Capns 1flox/flox and Osx-Cre+/- Capns1flox/flox mice as control and mutant mice, respectively [3]. To examine an effect of a low calcium diet on bone metabolism, 9-week-old male control and mutant littermates were allowed free access to either a normal chow containing 0.6% calcium and 0.4% phosphate or a low calcium diet containing 0.02% calcium and 0.4% phosphate (Harlan Teklad, Madison, WI) for 4 weeks. Other diet compositions of normal chow and low calcium diets were identical, which consisted of 18.6% protein, 66.7% carbohydrate, and 14.7% fat. Next, to examine an effect of a high fat diet on body composition and glucose metabolism, 9-week-old male control and mutant littermates were fed a high fat diet consisting of 15% protein, 43% carbohydrate, and 42% fat (Harlan Teklad). Body weight was weekly measured, and blood and bone samples were collected at the end of experiments. For measurement of food intake, mice were individually housed and food consumed for 24h was daily assessed between 11:30 AM and 12:00 PM for 4 weeks.
2.2. Assessment of fat mass
The total body fat mass of the living mice was measured using dual-energy x-ray absorptiometry (DXA) (Lunar PIXImus densitometer; GE Lunar, Fitchburg, WI). Bilateral epididymal fat pads were carefully isolated and weighed at the end point of experiments [6, 7].
2.3. μCT analysis
Bone microarchitecture was assessed by μCT analysis ex vivo on femoral samples isolated from mice as we described previously [3].
2.4. Serology
Serum levels of osteocalcin (total and uncarboxylated) (Biomedical Technologies, Inc., Stoughton, MA), tartrate-resistant acid phosphatase (TRAP) 5b (Immunodiagnostic Systems Ltd., Tyne & Wear, UK), osteoprotegerin, insulin (ALPCO Diagnostics, Salem, NH), and adiponectin (R & D Systems Inc., Minneapolis, MN) were determined by enzyme-liked immunosorbent assay as described by the manufacture's instructions and previously [3, 7]. Blood glucose levels were measured using FreeStyle Lite glucose meter (Abbott, Alameda, CA). Mouse adipokine array analysis was performed using 100 μl serum samples as described by the manufacture's instruction (R & D Systems Inc.) The average spot density of each adipokine was semi-quantified using FluorChem SP (Alpha Innotech Corp., San Leandro, CA).
2.5. Sample preparation and histological analysis
For histological analysis, hind limbs isolated from mutant and control littermates were fixed and stored. In selected cases, samples were decalcified, and paraffin blocks were prepared by standard histological procedures. In situ hybridization analysis was performed to detect mRNA expression of TRAP as we described previously [3].
2.6. Glucose and Insulin tolerance tests
Mice were given free access to water but fasted for 4h from 10:00 AM to 2:00 PM prior to and during the experiments. In glucose tolerance test, D-glucose was intraperitoneally (i.p.) administered to mice at the concentration of 1.5g/kg, and blood glucose levels were monitored at various time points, 0, 15, 30, 60, and 120 min. For insulin tolerance test, we injected 0.75U/kg of bovine insulin (Sigma-Aldrich, St. Louis, MO) into mice i.p. and measured blood glucose levels at various time points, 0, 15, 30, 60, and 120 min.
2.7. Statistics
Data were calculated from three to five independent experiments and expressed as mean±SE of either duplicate or triplicate determinations. Statistical analysis was performed using Analysis of Variance or unpaired Student's T-test. p values less than 0.05 were accepted as significant.
3. Results
3.1. Mutant mice had reduced serum uncarboxylated osteocalcin levels, but were lean
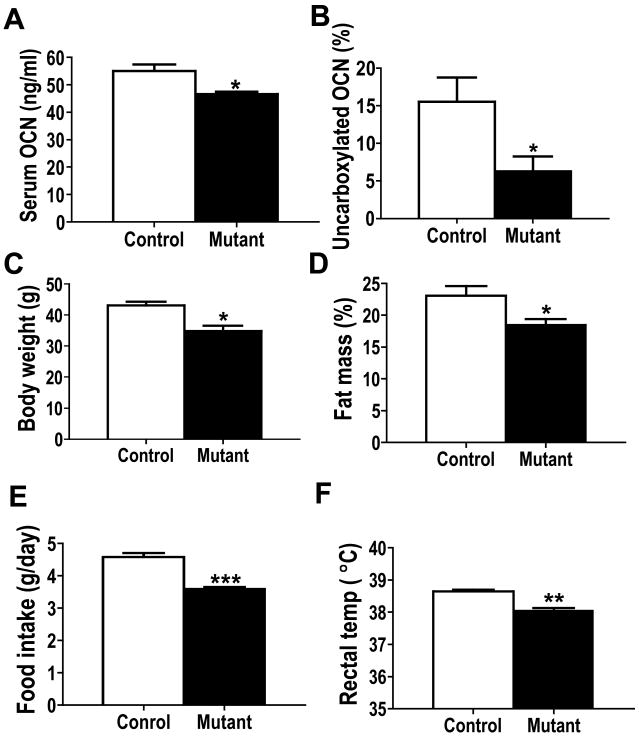
We previously reported that osteoblast-specific Capns1 mutant knockout mice showed reduced trabecular and cortical bone mainly due to reduced osteoblast number and function determined by histomorphometric analysis. Mutant mice also had lower serum levels of osteocalcin and TRAP5b, markers for bone formation and resorption, respectively [3]. Thus, to further examine which fraction of osteocalcin levels was reduced, either carboxylated or uncarboxylated, we measured total and uncarboxylated osteocalcin levels in control and osteoblast-specific Capns1 knockout mice [4]. Consistent with our previous report, serum total osteocalcin levels were significantly lower in mutant than control mice (Fig. 1 A), and this reduction was mainly due to reduced uncarboxylated osteocalcin levels (Fig. 1B). Because osteocalcin knockout mice showed an increase in visceral fat mass [4], we next examined body composition of mutant mice. Interestingly, unlike osteocalcin knockouts, we found that mutant mice were leaner than controls on a normal chow diet (Fig. 1C and 1D). DXA analysis showed that total body fat mass (% fat) of mutant mice was significantly lower than controls on a normal chow diet at 12 weeks of age (Fig. 1D). Moreover, mutant mice consumed a lesser amount of food (Fig. 1E) than control littermates, and their average rectal temperature (Fig. 1F), an indicator of metabolic rate, was also lower than that of controls. Collectively, osteoblast-specific Capns1 knockout mice were leaner than control littermates despite their significantly reduced serum uncarboxylated levels; this was likely due to their overall lower energy balance than control mice mainly mediated by their reduced food intake of a normal chow diet.
Figure. 1.
Osteoblast-specific Capns1 knockout mutant mice showed reduced serum total osteocalcin (OCN) levels (A), which was mainly due to a significant reduction of uncarboxylated osteocalcin levels (B). Mutant mice had lower body weight (C) and were leaner (D) than control mice. The average food intake (E) and rectal temperature (F) of mutant mice were lower than those of control mice. *, p<0.05. **, p<0.005, ***, p<0.001.
3.2. Osteoblast-specific Capns1 knockout mice showed reduced bone resorptive function in response to a low calcium diet
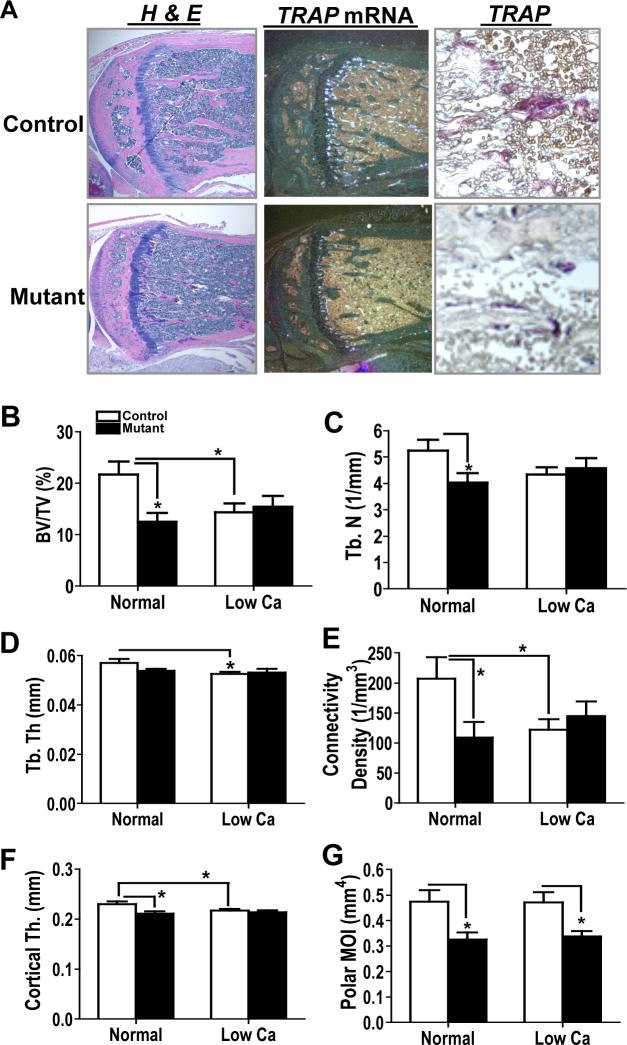
It was recently shown that serum levels of uncarboxylated osteocalcin, an active form of osteocalcin, are positively regulated by bone resorption through insulin-mediated signaling in osteoblasts [5]. Therefore, we next examined bone resorptive function of osteoblast-specific Capns1 knockout mice to examine a mechanism by which they showed lower serum uncarboxylated osteocalcin levels than control mice. In situ hybridization and histological analyses showed that ablation of Capns1 in osteoblasts reduced the number of cells expressing TRAP mRNA (Fig. 2A middle) and stained with TRAP (Fig. 2A right) along tibial trabecular bones of primary spongiosa. Serum levels of TRAP5b (control [n=16] 4.3±0.4 vs. mutant [n=21] 2.7±0.2*** U/L. ***, p<0.001) and osteoprotegerin (control [n=11] 2513±135.5 vs. mutant [n=7] 1891 ±246.4* pg/ml. *, p<0.05) were significantly reduced in mutant mice vs. control littermates. These results again suggest that mutant osteoporotic bone may have a reduced ability for bone resorption.
Figure. 2.
A. Deletion of Capns1 in cells of the osteoblast lineage showed reduced trabecular bone in primary spongiosa of proximal tibias. Mutant trabecular bone displayed reduced TRAP mRNA expression and reduced number of cells stained with TRAP. H&E; hematoxylin and eosin staining. B-G. Osteoblast-specific Capns1 knockout mice were resistant to a low calcium diet-induced bone resorption. μCT analysis was performed on femoral bone samples isolated from 13-week old control and mutant mice either on a normal chow or a low calcium diet for 4 weeks. BV/TV, bone volume/ total volume; Tb. N., trabecular number; Tb. Th., trabecular thickness; Cortical Th., cortical thickness; and pMOI, polar moments of inertia. A normal diet; control (n=9) vs. mutant (n=9): A high fat diet; control (n=11) vs. mutant (n=12). *p<0.05.
To further examine bone resorptive function of mutant mice, 9-week-old male littermate mice were fed either a normal chow (9 control and 9 mutant mice) or a low calcium diet (11 control and 12 mutant mice) for 4 weeks and ex vivo μCT analysis was performed on femoral bones. Mutant mice exhibited reduced bone volume (BV)/ total volume (TV) (Fig. 2B), trabecular number (Tb. N.) (Fig. 2C), connectivity density (Fig. 2E), cortical thickness (Cortical Th.) (Fig. 2F), and polar moments of inertia (pMOl) (Fig. 2G), an indicator for bone strength, vs. controls on a normal chow diet. Further, a low calcium diet significantly reduced BV/TV (Fig. 2B), trabecular thickness (Tb. Th.) (Fig. 2D), connectivity density (Fig. 2E), and Cortical Th. (Fig. 2F) in control trabecular and cortical bones; however, mutant bones were resistant to a low calcium diet-induced bone resorption (Fig. 2B-2G). Collectively, deletion of Capns1 in cells of the osteoblast lineage showed limited bone resorptive function, at least, in response to a low calcium diet.
3.3. A high fat diet induced an excess increase of body adiposity in osteoblast-specific Capns1 knockout mice
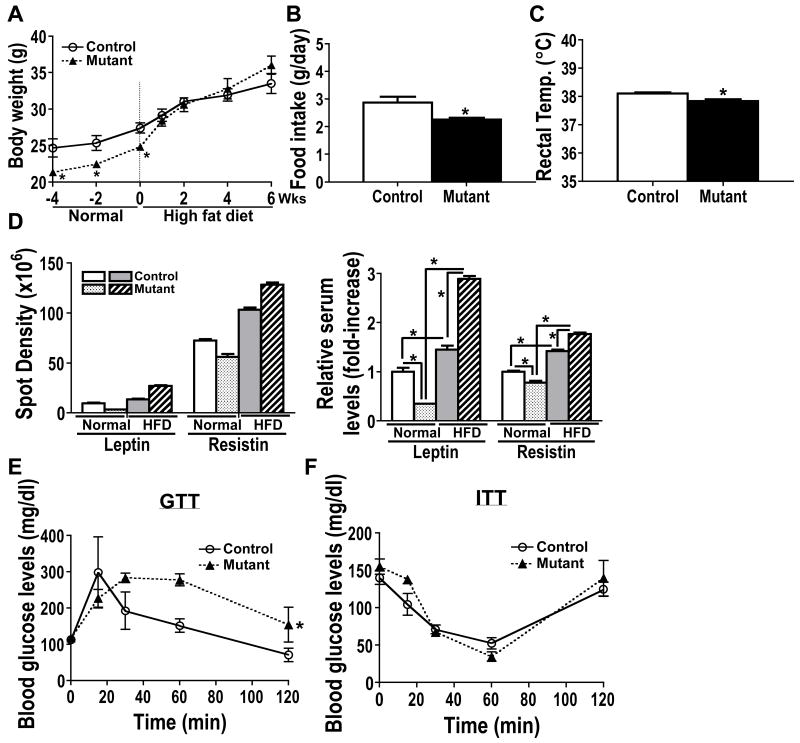
Based on their significantly reduced serum uncarboxylated osteocalcin levels, we anticipated that osteoblast-specific Capns1 knockout mice would exhibit increased body adiposity and insulin resistance; yet, they were leaner than control mice (Fig. 1). Therefore, we hypothesized that those expected phenotypes must be masked by their markedly reduced food intake of a normal chow diet. To test this hypothesis, we next provided 9-week old control and mutant mice with a high fat diet. The average mean body weight of mutant mice became indistinguishable from that of controls when fed a high fat diet for 2 weeks, and continued to show its rising trend at least up to 6 weeks (Fig. 3A). The average daily food consumption by mutant mice and their rectal temperature remained lower than those of controls under a high fat diet (Fig. 3B and 3C); this suggests that increased body weight of mutant mice on a high fat diet was not merely due to their increased food intake. To determine whether a high fat diet increased body adiposity of mutant mice, we next measured serum levels of various adipokines. A high fat diet significantly increased serum leptin and resistin levels, adipocyte-specific markers for body adiposity and insulin resistance [8], respectively, in both control and mutant mice. However, the average serum levels of leptin and resistin became significantly higher in mutant mice than controls on a high fat diet although both levels were lower in mutants vs. controls on a normal chow diet (Fig. 3D). We also found that serum adiponectin levels of mutant mice were reduced by 25% compared with those of control mice, which was consistent with reduced uncarboxylated osteocalcin levels of mutant mice. Collectively, these results suggest that the body weight gain of mutant mice on a high fat diet was likely due to their increased body adiposity, and that their significantly increased serum resistin and decreased adiponectin levels might indicate the development of impaired glucose homeostasis in mutant mice on a high fat diet.
Fig. 3.
A. Osteoblast-specific Capns1 knockout mutant mice showed reduced body weight compared with control mice on a normal chow diet. The average body weight of the mutant mice became indistinguishable with that of the control mice after a 2-week high fat diet, and continued to show its upward trend compared with that of the controls up to 6 weeks. B and C. The average food intake (B) and rectal temperature (C) of the mutant mice were lower than those of the control mice under a high fat diet. D. A high fat diet increased serum levels of leptin and resistin in both control and mutant mice, but the effect was significantly greater in mutant mice than controls. The average spot density of serum adipokine levels was semi-quantified using FluorChem SP. Left panel shows an actual average reading of spot density of an adipokine. Right panel shows an average relative spot density of an adipokine. An average spot density of an adipokine of control mice on a normal chow diet was set as 1. E. Glucose tolerance test (GTT). Mutant mice on a high fat diet for 6 weeks showed impaired glucose tolerance compared with control littermates in i.p. GTT. F. Insulin tolerance test (ITT). No difference was found between control and mutant mice in i.p. ITT. Statistical analysis of GTT and ITT were performed using repeated measurement of two-way ANOVA. *, p<0.05.
3.4. A high fat diet promoted the development of impaired glucose tolerance in osteoblast-specific Capns1 knockout mice
Lastly, to examine whether a high fat diet promoted abnormal glucose homeostasis in mutant mice on a high fat diet, we then performed glucose and insulin tolerance tests. There were no significant differences in fasting glucose levels between control and mutant mice (control 113±2 vs. mutant 117±5.2 mg/dl). However, mutant mice on a 6-week high fat diet showed impaired glucose tolerance compared with control littermates (Fig. 3E). No significant difference was found between two groups in an insulin tolerance test (Fig. 3F), suggesting that the integrity of the hypothalamus-pituitary-adrenal axis was intact in mutant mice. Collectively, osteoblast-specific Capns1 knockout mice, which had osteoporotic bone with impaired bone resorptive function, developed impaired glucose tolerance under a high fat diet.
4. Discussion
Osteoporosis is a systemic skeletal disease characterized by low bone density and microarchitectural deterioration of bone tissue with a consequent increase in bone fragility [9]. Osteoporotic bone is characterized by low bone mineral density, disrupted bone microarchitecture, and abnormal amount and composition of bone matrix proteins. Osteoporosis is most common in postmenopausal women; however, it may also develop in men, children, or any individuals suffering from some hormonal or chronic diseases, or those taking particular medications such as steroids [10].
Several lines of evidence have shown an association between bone and fat mass. Obese patients often show higher bone mineral density than lean individuals [11-14]. Paradoxically, an inverse relationship between bone and fat mass has been also well documented. Clinical studies demonstrated that postmenopausal women [15] and young children particularly under a low calcium diet show significantly reduced bone mass and increased fat mass [16]. Mechanisms underlining these observations have been intensively studied by using genetically obese mouse models including ob/ob [17] and db/db mice [18], which lack expression of leptin and leptin receptor, respectively. More recently, Karsenty's group has shown that osteocalcin, a hormone secreted from bone, regulates glucose homeostasis [4]. Serum levels of uncarboxylated osteocalcin, are regulated by bone resorption mediated by insulin signaling in osteoblasts [5]. Lower serum uncarboxylated osteocalcin levels were observed in patients with osteoporosis taking medication, which inhibits bone resorption [19-21], than in controls.
We showed previously that ablation of the calpain small subunit of calcium-dependent cysteine proteases, calpains, in cells of the osteoblast lineage reduces both osteoblast and osteoclast number and activity in mice [3]. Our present study further demonstrated that osteoblast-specific Capns1 knockout mutant mice had reduced total osteocalcin levels mainly due to decreased uncarboxylated osteocalcin levels, and that osteoporotic mutant mice were resistant to a low calcium diet-induced bone resorption. However, unlike osteocalcin knockout mice, they were leaner than control mice. Interestingly, a high fat diet significantly increased body adiposity in mutant mice compared with controls assessed by levels of adipocyte-specific adipokines, leptin and resistin. An increase in body adiposity of mutant mice was not due to their increased food intake of a high fat diet; this was rather suggestive of their reduced metabolic rate. A prolonged exposure to a high fat diet further promoted the development of mild but significantly impaired glucose tolerance in mutant mice; this was consistent with their increased serum levels of resistin and reduced levels of uncarboxylated osteocalcin and adiponectin [22] compared with those of control mice on a high fat diet. Further, lower serum uncarboxylated osteocalcin levels were observed in patients with osteoporosis taking medication, which inhibits bone resorption [19-21]. Therefore, our results suggest that those patients may be also susceptible to develop obesity and impaired glucose tolerance under a high fat diet. Dietary energy restriction could play a critical role for preventing those metabolic diseases among the patients with osteoporosis in a clinical setting.
In conclusion, osteoblast-specific Capns1 knockout mice with osteoporosis and reduced bone resorptive function, exhibited reduced serum uncarboxylated osteocalcin levels and a lean phenotype under a normal chow diet. However, the mice significantly gained body adiposity and developed impaired glucose tolerance on a high fat diet.
Research highlights.
Osteoblast-specific Capns1 knockout mutant mice showed reduced serum uncarboxylated osteocalcin levels, but were lean on a normal chow diet.
The mutant bones were osteoporotic and resistant to a low calcium diet-induced bone resorption.
The mutant mice significantly increased body adiposity and developed impaired glucose tolerance on a high fat diet.
Acknowledgments
We are grateful to Dr. Mary L. Bouxsein and Leeann Louis for μCT analysis. We also thank Yuko Sumiyama and Kimberly Atkin for technical supports. This work was partially supported by National Institutes of Health Grants R01 DK072102, BADERC Pilot and Feasibility Grant Award (a part of NIH P30 DK057521), and MGH interim support fund (to M.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 2.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–4481. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimada M, Greer PA, McMahon AP, Bouxsein ML, Schipani E. In vivo targeted deletion of calpain small subunit, capn4, in cells of the osteoblast lineage impairs cell proliferation, differentiation, and bone formation. J Biol Chem. 2008;283:21002–21010. doi: 10.1074/jbc.M710354200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimada M, Ishibashi S, Yamamoto K, Kawamura M, Watanabe Y, Gotoda T, Harada K, Inaba T, Ohsuga J, Yazaki Y, et al. Overexpression of human lipoprotein lipase increases hormone-sensitive lipase activity in adipose tissue of mice. Biochem Biophys Res Commun. 1995;211:761–766. doi: 10.1006/bbrc.1995.1878. [DOI] [PubMed] [Google Scholar]
- 7.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 9.Consensus development conference: prophylaxis and treatment of osteoporosis. Am J Med. 1991;90:107–110. doi: 10.1016/0002-9343(91)90512-v. [DOI] [PubMed] [Google Scholar]
- 10.R. o. a. W. S. Group. Prevention and Management of Osteoporosis: Report of a WHO Scientific Group (2000: Geneva, Switzerland) WHO Technical Report Series. 2003;921 [Google Scholar]
- 11.Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8:567–573. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- 12.Riggs BL, Khosla S, Melton LJ., 3rd A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 13.Riggs BL, Melton LJ., 3rd Involutional osteoporosis. N Engl J Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 14.Tremollieres FA, Pouilles JM, Ribot C. Vertebral postmenopausal bone loss is reduced in overweight women: a longitudinal study in 155 early postmenopausal women. J Clin Endocrinol Metab. 1993;77:683–686. doi: 10.1210/jcem.77.3.8370689. [DOI] [PubMed] [Google Scholar]
- 15.Manson JE, Martin KA. Clinical practice. Postmenopausal hormone-replacement therapy. N Engl J Med. 2001;345:34–40. doi: 10.1056/NEJM200107053450106. [DOI] [PubMed] [Google Scholar]
- 16.Skinner JD, Bounds W, Carruth BR, Ziegler P. Longitudinal calcium intake is negatively related to children's body fat indexes. J Am Diet Assoc. 2003;103:1626–1631. doi: 10.1016/j.jada.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 18.Williams GA, Callon KE, Watson M, Costa JL, Ding Y, Dickinson M, Wang Y, Naot D, Reid IR, Cornish J. Skeletal phenotype of the leptin receptor-deficient db/db mouse. J Bone Miner Res. 2011 doi: 10.1002/jbmr.367. [DOI] [PubMed] [Google Scholar]
- 19.Aonuma H, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low serum levels of undercarboxylated osteocalcin in postmenopausal osteoporotic women receiving an inhibitor of bone resorption. Tohoku J Exp Med. 2009;218:201–205. doi: 10.1620/tjem.218.201. [DOI] [PubMed] [Google Scholar]
- 20.Kaji H, Hisa I, Inoue Y, Naito J, Sugimoto T, Kasuga M. Analysis of factors affecting increase in bone mineral density at lumbar spine by bisphosphonate treatment in postmenopausal osteoporosis. J Bone Miner Metab. 2009;27:76–82. doi: 10.1007/s00774-008-0005-y. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi M, Yamaguchi T, Nawata K, Takaoka S, Sugimoto T. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin Nutr. 29:761–765. doi: 10.1016/j.clnu.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med. 2002;80:696–702. doi: 10.1007/s00109-002-0378-7. [DOI] [PubMed] [Google Scholar]