Abstract
Leptin deficiency results in a complex obesity phenotype comprising both hyperphagia and lowered metabolism. The hyperphagia results, at least in part, from the absence of induction by leptin of melanocyte stimulating hormone (MSH) secretion in the hypothalamus; the MSH normally then binds to melanocortin-4 receptor expressing neurons and inhibits food intake. The basis for the reduced metabolic rate has been unknown. Here we show that leptin administered to leptin-deficient (ob/ob) mice results in a large increase in peripheral MSH levels; further, peripheral administration of an MSH analogue results in a reversal of their abnormally low metabolic rate, in an acceleration of weight loss during a fast, in partial restoration of thermoregulation in a cold challenge, and in inducing serum free fatty acid levels. These results support an important peripheral role for MSH in the integration of metabolism with appetite in response to perceived fat stores indicated by leptin levels.
Recent research has outlined a pathway for control of body weight (1–4): leptin, the product of the ob gene in mouse, is produced by adipocytes (5). It circulates to the hypothalamus where it binds to cells expressing the leptin receptor, the product of the db gene in mouse (6–9). Proopiomelanocortin (POMC) neurons are among the hypothalamic neurons expressing the leptin receptor (10). This leptin binding leads to the secretion of melanocyte stimulating hormone (MSH), which in turn binds to neurons expressing the melanocortin-4 receptor (MC4-R) (11); these neurons then suppress appetite (12–14). This outline is based on the phenotypes of spontaneous and induced mouse mutants (5, 9, 13, 15–19) as well as on the phenotype of homologous mutations in humans (20–24). These interpretations are in agreement that leptin is the signal from the fat stores (adipocytes) to the center, and further that MSH regulates appetite. However, there are significant aspects of the mutant phenotypes that suggest both a greater complexity of body weight homeostasis, specifically the integration of appetite and metabolism, and a factor from the central nervous system (CNS) to the periphery mediating this integration.
First, pomc/pomc mutants that completely lack POMC peptides, including MSH, show a phenotype of altered lipid metabolism in addition to hyperphagia. As the fat content of the diet increases, the mice gain weight out of proportion to their food intake (17). This shows a particular inability to use dietary fat for sustaining metabolic rate. And when these pomc/pomc mutants are treated by peripheral administration of an α-MSH analog the mice lose weight and eat less, but the weight loss is much greater than the decrease in appetite (17). Again, this result is consistent with a role for MSH in mobilizing peripheral fat stores.
Second, leptin-deficient mice (ob/ob) show decreased metabolic rate (increased metabolic efficiency; ref. 25), which precedes the onset of obesity. Notably these mutants show: (i) weight gain when pair-fed with normal controls (25); (ii) longer survival in a fast than normal mice of equal initial weight (26); and (iii) decreased ability to maintain body temperature at 4°C (27). Taken together these data show that the ob/ob mice have adjusted their metabolism to conform to their perceived fat stores; specifically, in the absence of leptin, they sense no fat stores and decrease metabolism accordingly. The mechanism for such an adjustment has remained unelucidated.
To refine this model of body weight homeostasis, especially the integration of appetite and metabolism, we asked the following questions: Does leptin injection of ob/ob mutants result in increased levels of circulating MSH; and does peripheral administration of an MSH analog in ob/ob mice (i) affect weight gain and/or food intake, (ii) influence weight in the absence of food intake, i.e., during a fast, (iii) increase the ability of ob/ob mutants to regulate their temperature at 4°C, and (iv) regulate serum free fatty acid (FFA) levels?
Experimental Procedures
Mice.
Ob/ob mutant mice (C57BL/6J-Lepob) and congenic controls were purchased from The Jackson Laboratory. Mice were housed with a 12-h light-dark cycle, with food and water ad libitum (unless indicated otherwise). Blood was collected retroorbitally following approved procedures. Body core temperatures were measured by using a digital thermometer fitted with a rectal probe (Atkins Technical, Gainesville, FL). All procedures were approved by the Animal Care and Use Committees of the University of Colorado Health Sciences Center or the Oklahoma Medical Research Foundation.
Hormones.
The MSH analog [Ac-Cys4, D-Phe7, Cys10] α-MSH (4–13) (28) was purchased from Peninsula Laboratories. Murine recombinant leptin was kindly provided by A. F. Parlow through the National Hormone and Pituitary Program, Harbor-UCLA Medical Center, Torrance, CA. Hormones were diluted to 70 μg per ml in PBS.
Serum Analyses.
EDTA-plasma was analyzed for MSH by RIA following the manufacturer's instructions (Euro-Diagnostica Kit, IBL, Hamburg, Germany). FFA levels in serum samples were determined by Anilytics (Gaithersburg, MD).
Results
Leptin Induction of Circulating MSH.
An inductive role for leptin in the secretion of MSH is suggested by the expression of leptin receptors on POMC-expressing neurons of the hypothalmus (10) and by the correlation of hypothalamic POMC mRNA levels with circulating leptin levels (29–31). To test the inductive ability of serum leptin on circulating MSH levels, we treated leptin-deficient (ob/ob) mutants and age-matched controls with leptin (7 μg i.p.). Blood samples were drawn 1 h later from the retroorbital sinus, and plasma were analyzed for MSH levels by RIA. MSH levels were lower in ob/ob mutants compared with controls (226 pmol/liter versus 400 pmol/liter; P < 0.02; Fig. 1). Leptin injection induced a 2-fold increase of circulating MSH levels in ob/ob mutants (from 226 pmol/liter to 474 pmol/liter; P < 0.002), thereby demonstrating that leptin can in fact increase circulating MSH levels.
Figure 1.
Leptin induces circulating MSH in ob/ob mutants. Ob/ob mutant mice (ob/ob) and age-matched controls (wild type and heterozygotes, +/?), 10 per group, were injected i.p. with 0.1 ml of either 7 μg leptin (lep) or vehicle (PBS) alone (veh). One hour after the leptin injection blood was collected retroorbitally into tubes containing EDTA. Plasma was assayed for MSH by RIA.
Peripheral MSH Effects Weight Gain More than Food Intake.
Ob/ob mutants show both hyperphagia and decreased metabolic rate as witnessed by their ability to gain weight even when pair-fed with normal, congenic controls (25). To determine the effects of leptin and MSH on food intake and weight gain, we treated four sets of ob/ob mutants with leptin, an MSH analog, both, or vehicle alone. Weight measurements and daily injections (0.1 ml i.p.) were both done within a 1-h window at 1 p.m. each day. Food was weighed on a daily basis at the same time the mice were weighed. The results are shown in Fig. 2. In vehicle-treated ob/ob mice the per-mouse weight gain in 10 days was 5.72 ± 0.55 g, whereas under MSH treatment weight gain slowed to 3.88 ± 1.06 g; leptin treatment alone slowed weight gain to 2.78 ± 0.62 g, similar to the 2.88 ± 0.74 g with the combined treatment of leptin and MSH analog (Fig. 2A). Whereas both leptin treatment and MSH analog treatment effectively slowed weight gain, surprisingly, the effect of the MSH analog was greater on weight gain than on food intake (Fig. 2B). Compared with the food intake of untreated ob/ob controls, ob/ob mice receiving MSH analog decreased their daily food intake by only 0.37 g (Fig. 2B), whereas leptin treatment decreased daily food intake by 1.17 g. The greater effect of MSH on weight gain than on food intake is obvious when the ratio of decreased weight gain to decreased food intake is calculated (Δweight/Δate of 0.5 versus 0.25; Fig. 2C). These results indicate a reversal of the decreased metabolic rate of the ob/ob mutant mice (25) by the MSH analog.
Figure 2.
MSH slows weight gain in ob/ob mutants. Ob/ob mutant mice (ob/ob), 10 per group, were injected i.p. with 0.1 ml of either 7 μg leptin (Leptin), 7 μg MSH analog (MSH), both (MSH + Leptin), or vehicle alone (Control), once per day for 10 days. Weight and food intake were measured daily. (A) The graph shows the weight gain, in g, per mouse, after 10 days. (B) Total grams of food consumed per 10 mice in 10 days are listed next to the weight gain for each group of mice. (C) The differences in weight gain divided by the differences in food intake for comparisons of controls and treated mice are listed.
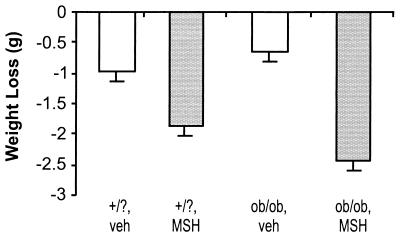
Peripheral MSH Accelerates Weight Loss During a Fast.
Further support of the effect of MSH on metabolic rate is obtained by measuring weight loss during a 5-h fast (Fig. 3). Mutant ob/ob mice and age-matched heterozygote and wild-type controls were injected with 7 μg MSH analog or vehicle and then deprived of food for 5 h. During the fast both control and ob/ob mice lost significantly more weight with MSH analog treatment than from vehicle: vehicle-treated versus MSH-treated is 0.96 ± 0.16 g versus 1.88 ± 0.13 g (P < 0.005) for controls, and 0.67 ± 0.14 g versus 2.43 ± 0.17 g (P < 0.00005) for ob/ob mice. In the absence of food intake the effect of MSH on additional weight loss has to be ascribed to metabolic effects. In heterozygote and wild-type controls the peripherally administered MSH analog may override the physiological suppression of MSH under fasting, leading to increased metabolism.
Figure 3.
MSH accelerates weight loss during a fast in ob/ob mutants. Ob/ob mutant mice (ob/ob) and age-matched controls (wild type and heterozygotes, +/?), five per group, were injected i.p. with 0.1 ml of either 7 μg MSH analog (MSH) or vehicle alone (veh). At the time of injection weights were taken and food was removed. Five hours later weights were taken again. The graph shows the weight loss in the different groups at the end of the 5-h fast.
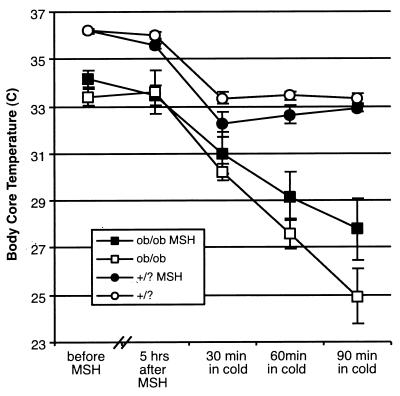
Peripheral MSH Attenuates Impairment of Thermal Regulation.
In 1977 the decreased ability of ob/ob mutants to maintain body temperature at 4°C was noted (27). Again, this impaired thermoregulation precedes the onset of obesity in these mice. We hypothesized that the lower level of circulating MSH in leptin-deficient (ob/ob) mutants was, at least partially, responsible for their impaired thermal regulation. To test this, we injected ob/ob mutants with 7 μg of MSH analog or vehicle only. The mice were fasted for 5 h at room temperature, and then exposed to 4°C for 90 min. As seen in Fig. 4, at room temperature MSH treatment did not increase core temperatures, which stayed around 36.2°C in controls and around 33°C in ob/ob mutants. However, in the cold challenge, controls initially dropped their core temperatures by an average of 3°C, regardless of MSH treatment; they then adjusted to a new core temperature of around 33°C (33.3°C for untreated and 32.9°C for MSH-treated mice; in both cases this is 2.6°C below their core temperature at room temperature). Ob/ob mice treated with the MSH analog maintained their core temperature in the cold much better than did the vehicle-treated ob/ob mutants. After 90 min in the cold, core temperatures in untreated ob/ob mice fell to an average of 24.9°C, whereas in MSH-treated ob/ob mice they only fell to 27.7°C; for untreated mutants the drop in core temperature after 90 min in the cold is 8.7°C compared with only 5.7°C in MSH analog-treated mutants. As the mice had no opportunity to eat between injection of the MSH analog and the cold challenge, food intake cannot be responsible for the altered thermal regulation in MSH-treated ob/ob mice. Further the MSH analog is not thermogenic per se as it has no effect at room temperature. The lower temperature in ob/ob mice at room temperature suggests that leptin levels, or some other metric of perceived energy stores, influence the homeostatic thermal “set point.” This might be affected by leptin, or by leptin-induced MSH, acting in the CNS. However, the ability of peripheral MSH to improve thermoregulation in ob/ob mutants during a cold challenge shows that the effect of peripheral MSH analog on thermoregulation in ob/ob mice is through improved ability to mobilize energy stores.
Figure 4.
MSH attenuates the thermogenic defect in ob/ob mutants. Individually housed ob/ob mutant mice (ob/ob) and age-matched controls (wild type and heterozygotes, +/?), five per group, were injected with 7 μg MSH analog or vehicle alone i.p. Rectal temperatures were taken and food was removed at that time. Five hours later temperatures were taken again and mice were then placed in a cold room (4°C) for 90 min; rectal temperatures were taken in the cold after 30, 60, and 90 min.
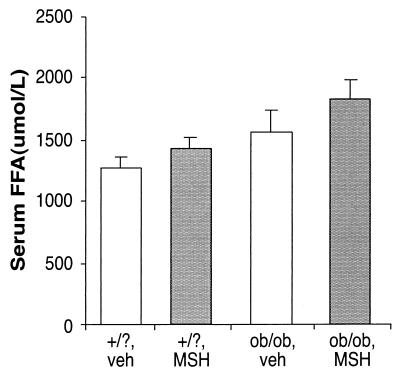
Peripheral MSH Stimulates FFA Levels.
Our previous results (17) and the above results strongly suggest that MSH acts on fat stores when slowing weight gain with unlimited food supply, when accelerating weight loss in a fast, and when partially reversing the ob/ob thermoregulatory defect in a cold challenge. One parameter indicating changes in fat metabolism is the level of FFAs in serum. Lipoprotein lipases on the surface of fat cells hydrolyze incoming triacylglycerols to yield FFAs, which are then imported into the fat cell. Hence, an increase in lipid metabolism may be reflected in fewer FFAs crossing into adipocytes and higher serum levels.
Accordingly, we measured the levels of serum FFAs in ob/ob and control mice, treated with an MSH analog or with vehicle alone. The results are shown in Fig. 5. Control mice show an increase in FFAs with MSH analog treatment by 11.4% (1,277 ± 95 μmol/liter versus 1,423 ± 92 μmol/liter) (Fig. 5); here again, the peripherally administered MSH analog overrides the normal physiological decrease in MSH levels during fasting. In ob/ob mice FFA levels increase by a statistically significant 18% with MSH analog treatment (from 1,561 ± 180 μmol/liter to 1,834 ± 150 μmol/liter; Fig. 5). Our findings that an MSH analog stimulates serum FFA levels suggest that the altered metabolism in ob/ob mice and lipid metabolism are related.
Figure 5.
MSH affects serum FFA levels in ob/ob mutants. Ob/ob mutant mice (ob/ob) and age-matched controls (wild type and heterozygotes, +/?), 10 per group, were injected i.p. with 0.1 ml of either 7 μg MSH analog (MSH) or vehicle alone (veh). One hour later blood was collected through retroorbital puncture. Serum was assayed for FFAs.
Discussion
In summary, we have shown: (i) that injection of leptin increases levels of circulating MSH in ob/ob mutants; (ii) that peripherally administered MSH analog slows weight gain in ob/ob mutants through altered metabolism; (iii) that peripherally administered MSH analog accelerates weight loss during a fast in ob/ob mutants; (iv) that peripherally administered MSH analog increases thermal homeostasis in ob/ob mutants in a cold challenge; and (v) that peripherally administered MSH analog induces serum FFA levels in ob/ob mutants.
From these results we propose that there are two distinct effects of leptin-induced MSH (see Fig. 6A): (i) MSH produced in the hypothalamus in response to leptin inhibits appetite by interaction with MC4-R expressing neurons in the arcuate nucleus, and (ii) MSH produced in the CNS diffuses to the periphery, where it alters lipid metabolism, discouraging sequestration in adipocytes and encouraging catabolism. Because the central release of MSH is partly regulated by leptin, we propose an integrated control of appetite and fat metabolism by the leptin-POMC pathway.
Figure 6.
Proposed scheme of integrated control of appetite and fat metabolism by the leptin-POMC pathway. (A) In a situation of homeostasis, intake (feeding) and usage (metabolism) of fuel is balanced. Adipocytes release leptin, which binds to leptin receptors on central melanocortin-producing neurons. Leptin-induced release of MSH allows binding of MSH to central MC-R bearing neurons and a decrease in food intake, and it allows diffusion of central MSH to the periphery, where it binds to MC-Rs on adipocytes, causing a mobilization of fat stores. In obese mutants this homeostasis is out of balance: In the pomc/pomc mutant (B) adipocytes release large quantities of leptin; however, no MSH is released in response to it. Both central and peripheral lack of signaling of MSH leads to hyperphagia and sequestration of fuel into storage. In the ob/ob mutant (C) no functional leptin is available, causing a less than sufficient amount of MSH to be released, hence contributing to both hyperphagia and sequestration of fuel into storage. Peripheral administration of MSH leads to mobilization of fat stores and to weight loss or slowing weight gain by reconstituting the MSH lacking in the pomc/pomc mutant or by boosting the insufficient amount of MSH in the ob/ob mutant (D).
It is unlikely that peripherally administered MSH analog is acting through the CNS, especially through a direct decrease in appetite. First, autoradiographic studies of the organ distribution of radioactively labeled α-MSH injected intravenously into mice showed no detectable radioactivity in the CNS (32). This was recently confirmed in rats by using radiolabeled MSH-analog MT-II (33), which revealed specific labeling that occurred mainly at the circumventricular organs (Lex Van der Ploeg and Xiaoming Guan, personal communication). A formal, but unlikely, possibility remains that peripherally administered MSH analogs activate neurons in regions with defective blood brain barrier, which in turn project directly or indirectly to the hypothalmus. Second, our experiments were carried out in the absence of food intake.
The differences in weight gain between leptin-treated and MSH analog-treated ob/ob mice are consistent with two effects of MSH, one central (appetite suppressing), triggered by leptin injection, and one peripheral (metabolic), triggered either by leptin injection or peripheral MSH injection. In addition, the inability of peripheral MSH analog to supplement the weight-reducing effect in ob/ob mice of a leptin dose sufficient to induce normal MSH levels is consistent with the ability of leptin to induce levels of central and peripheral MSH balanced for appetite and metabolism. Whenever this is not the case (e.g., ob/ob, and pomc/pomc mutants) peripheral administration of an MSH analog permits increased metabolic rate by mobilization of fat stores.
The enhanced fasting weight loss, thermoregulation, and serum FFA levels—all without food intake—of ob/ob mutants treated with the MSH analog are consistent with an increased accessibility of fat stores in the absence of feeding differences.
The defect in thermal regulation in ob/ob mutants appears to have two components. First, the set point of temperature is lower under all conditions. Second, the ability to maintain this set point under a cold challenge is decreased. The decrease in set point may be hypothalamic, mediated either directly by leptin receptor expressing neurons or by secondary neurotransmitters. The inability to maintain even this lower set point in a cold challenge reflects the organism's inability to mobilize fat without sufficient signal (MSH) from the center to the periphery; this MSH signal depends on a leptin signal indicating sufficient fat stores. Deficient thermoregulation can be alleviated either by exogenous leptin, which in turn increases circulating MSH, or by direct peripheral administration of exogenous MSH.
We previously found that pomc/pomc null mutant mice had a specific defect in lipid metabolism, storing rather than using dietary lipids despite obesity (17). The presence of MC-Rs 1, 2, 3, and 5 on adipocytes (11, 12, 34) and the inductive effect of MSH on serum FFA levels, are consistent with a direct effect of MSH on lipid metabolism in adipocytes. It is not clear at this point through which MC-R MSH is signaling; it is not likely to signal through MC5-R, because the MC5-R null mutant is not obese (35). It is also not likely to signal through MC2-R [the corticotropin (ACTH) receptor], because the MSH analog administered after dexamethasone suppression of the hypothalamo-pituitary-adrenal axis has, unlike ACTH, no effect on stimulation of corticosterone production mediated through MC2-R expressed in the adrenal gland (data not shown). A likely candidate is MC3-R, targeted mutation of which leads to increased fat mass despite hypophagia, consistent with the peripheral role we proposed of the melanocortin system in partitioning fuel stores into fat (36, 37).
Twenty-five years ago Kastin et al. (38) suggested a modifying role for hypothalamic and pituitary hormones on lipolysis, i.e., lipid mobilization. They asked whether factors produced in the hypothalamus could exert effects by a direct action on fat cells, proposing a lipid mobilizing factor. When 50 peptides and hormones from the hypophysis, hypothalamus, gastrointestinal tract, and other origins were tested for lipolytic activity in isolated rabbit fat cells, eight peptides derived from POMC stimulated glycerol release whereas all of the other peptides and hormones showed no lipolytic activity (39). The most potent lipolytic peptide was α-MSH, which also had the lowest minimal effective dose. However, when this effect was not observed in rat adipocytes in culture, the lipolytic efficacy of MSH in rabbits was considered a species anomaly (40).
The lipolytic activity we observed of MSH in mice in vivo is similar to the high lipolytic activity of MSH seen in rabbits in vivo (41). In the rabbit experiments i.v. injection of MSH at a concentration of 150 μg/kg led to a 50% increase in serum FFAs after 1 h. In our in vivo experiments in mice 7 μg of MSH (140 μg/kg) injected by the less direct i.p. route led to an increase in serum FFAs of almost 20% within 1 h. In view of our results, we concluded that MSH has a potent lipolytic activity in mice in vivo and that the absence of lipolytic activity in rat cells in vitro was a peculiarity of the tissue culture environment.
In view of our results, we propose the following modifications to the leptin-POMC scheme presented above (see Introduction and Fig. 6). First, MSH contributes to both the decrease in appetite and the increase in metabolic rate (Fig. 6A). These are complementary responses to sufficient fat stores. Lacking sufficient fat stores, an organism may be expected both to eat more and to expend less energy. Like the pomc/pomc mutants (Fig. 6B), the ob/ob mutants (Fig. 6C) evidence a decreased metabolic rate, which can be reversed by peripherally administered MSH analog (Fig. 6D). A direct effect of MSH on appetite suppression is mediated through MC4-R expressing neurons, whereas the increased metabolic rate is mediated by peripheral cells, especially adipocytes, expressing MC-Rs.
Acknowledgments
We thank Mary Flynn for art work and manuscript preparation. The work was supported by a generous grant from the Board of Trustees of the Eleanor Roosevelt Institute, Denver (M.B.B.) and by the Oklahoma Center for the Advancement of Science and Technology (OCAST), Oklahoma City (U.H.).
Abbreviations
- MSH
melanocyte stimulating hormone
- MC-R
melanocortin receptor
- POMC
proopiomelanocortin
- FFA
free fatty acid
- CNS
central nervous system
References
- 1.Elmquist J K, Maratos-Flier E, Saper C B, Flier J S. Nat Neurosci. 1998;1:445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- 2.Friedman J M, Halaas J L. Nature (London) 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 3.Fisher S L, Yagaloff K A, Burn P. J Recept Signal Transduct Res. 1999;19:203–216. doi: 10.3109/10799899909036646. [DOI] [PubMed] [Google Scholar]
- 4.Loftus T M. Semin Cell Dev Biol. 1999;10:11–18. doi: 10.1006/scdb.1998.0274. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Charlat O, Tartaglia L A, Woolf E A, Weng X, Ellis S J, Lakey N D, Culpepper J, Moore K J, Breitbart R E, et al. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 7.Chua S C, Jr, Chung W K, Wu-Peng X S, Zhang Y, Liu S M, Tartaglia L, Leibel R L. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 8.Lee G H, Proenca R, Montez J M, Carroll K M, Darvishzadeh J G, Lee J I, Friedman J M. Nature (London) 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 9.Tartaglia L A, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards G J, Campfield L A, Clark F T, Deeds J, et al. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 10.Cheung C C, Clifton D K, Steiner R A. Endocrinology. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 11.Mountjoy K G, Mortrud M T, Low M J, Simerly R B, Cone R D. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 12.Fan W, Boston B A, Kesterson R A, Hruby V J, Cone R D. Nature (London) 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 13.Huszar D, Lynch C A, Fairchild-Huntress V, Dunmore J H, Fang Q, Berkemeier L R, Gu W, Kesterson R A, Boston B A, Cone R D, et al. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 14.Seeley R J, Yagaloff K A, Fisher S L, Burn P, Thiele T E, van Dijk G, Baskin D G, Schwartz M W. Nature (London) 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 15.Bultman S J, Michaud E J, Woychik R P. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 16.Lu D, Willard D, Patel I R, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik R P, Wilkison W O, et al. Nature (London) 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 17.Yaswen L, Diehl N, Brennan M B, Hochgeschwender U. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 18.Cool D R, Normant E, Shen F, Chen H C, Pannell L, Zhang Y, Loh Y P. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- 19.Naggert J K, Fricker L D, Varlamov O, Nishina P M, Rouille Y, Steiner D F, Carroll R J, Paigen B J, Leiter E H. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 20.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte J M, et al. Nature (London) 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 21.Montague C T, Farooqi I S, Whitehead J P, Soos M A, Rau H, Wareham N J, Sewter C P, Digby J E, Mohammed S N, Hurst J A, et al. Nature (London) 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 22.Vaisse C, Clement K, Guy-Grand B, Froguel P. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 23.Yeo G S, Farooqi I S, Aminian S, Halsall D J, Stanhope R G, O'Rahilly S. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 24.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 25.Thurlby P L, Trayhurn P. Br J Nutr. 1979;42:377–385. doi: 10.1079/bjn19790127. [DOI] [PubMed] [Google Scholar]
- 26.Cuendet G S, Loten E G, Cameron D P, Renold A E, Marliss E B. Am J Physiol. 1975;228:276–283. doi: 10.1152/ajplegacy.1975.228.1.276. [DOI] [PubMed] [Google Scholar]
- 27.Trayhurn P, Thurlby P L, James W P. Nature (London) 1977;266:60–62. doi: 10.1038/266060a0. [DOI] [PubMed] [Google Scholar]
- 28.Cody W L, Mahoney M, Knittel J J, Hruby V J, Castrucci A M, Hadley M E. J Med Chem. 1985;28:583–588. doi: 10.1021/jm50001a008. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz M W, Seeley R J, Woods S C, Weigle D S, Campfield L A, Burn P, Baskin D G. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 30.Thornton J E, Cheung C C, Clifton D K, Steiner R A. Endocrinology. 1997;138:5063–5066. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- 31.Mizuno T M, Kleopoulos S P, Bergen H T, Roberts J L, Priest C A, Mobbs C V. Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- 32.Dupont A, Kastin A J, Labrie F, Pelletier G, Puviani R, Schally A V. J Endocrinol. 1975;64:237–241. doi: 10.1677/joe.0.0640237. [DOI] [PubMed] [Google Scholar]
- 33.Hruby V J, Lu D, Sharma S D, Castrucci A L, Kesterson R A, al-Obeidi F A, Hadley M E, Cone R D. J Med Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 34.Chagnon Y C, Chen W J, Perusse L, Chagnon M, Nadeau A, Wilkison W O, Bouchard C. Mol Med. 1997;3:663–673. [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Kelly M A, Opitz-Araya X, Thomas R E, Low M J, Cone R D. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 36.Chen A S, Marsh D J, Trumbauer M E, Frazier E G, Guan X M, Yu H, Rosenblum C I, Vongs A, Feng Y, Cao L, et al. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 37.Butler A A, Kesterson R A, Khong K, Cullen M J, Pelleymounter M A, Dekoning J, Baetscher M, Cone R D. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 38.Kastin A J, Redding T W, Hall R, Besser G M, Schally A V. Pharmacol Biochem Behav. 1975;3:121–126. [PubMed] [Google Scholar]
- 39.Richter W O, Schwandt P. Horm Metab Res. 1985;17:127–130. doi: 10.1055/s-2007-1013471. [DOI] [PubMed] [Google Scholar]
- 40.Ramachandran J, Lee V. Biochim Biophys Acta. 1976;428:339–346. doi: 10.1016/0304-4165(76)90041-6. [DOI] [PubMed] [Google Scholar]
- 41.Drouhault R, Valero D, Baghdiantz A, Blanquet P. J Endocrinol. 1983;97:447–452. doi: 10.1677/joe.0.0970447. [DOI] [PubMed] [Google Scholar]