Abstract
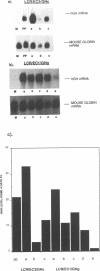
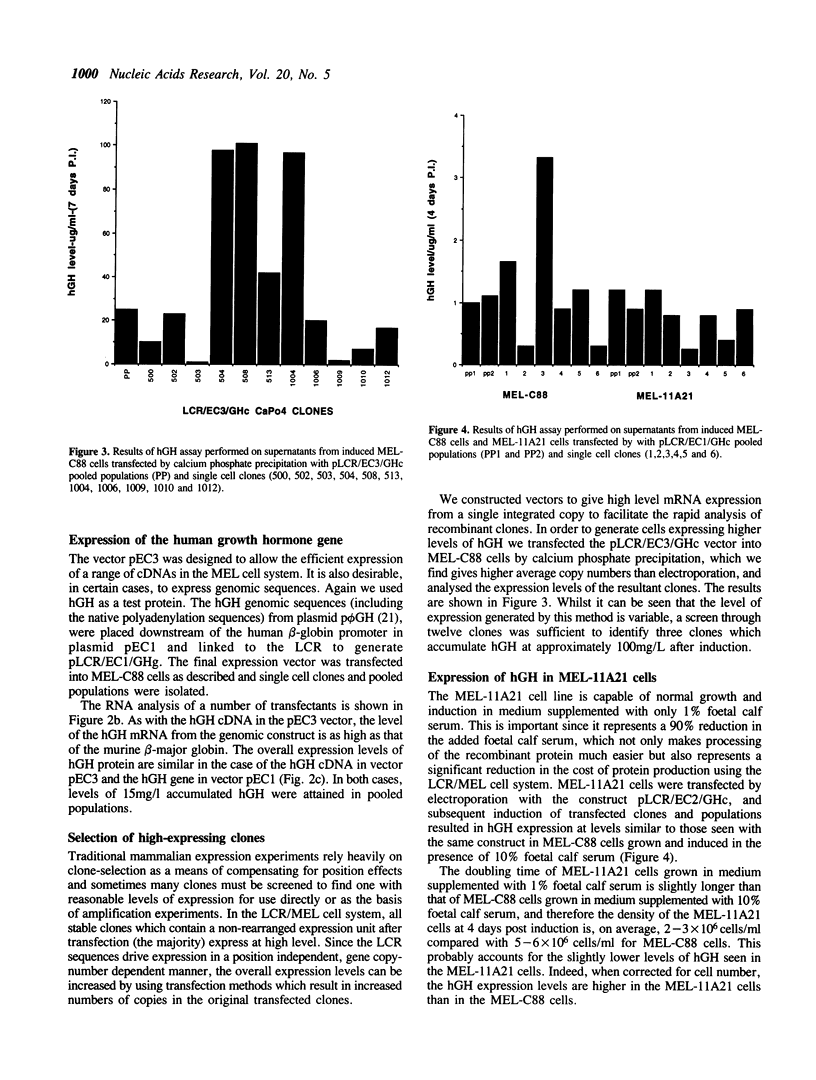
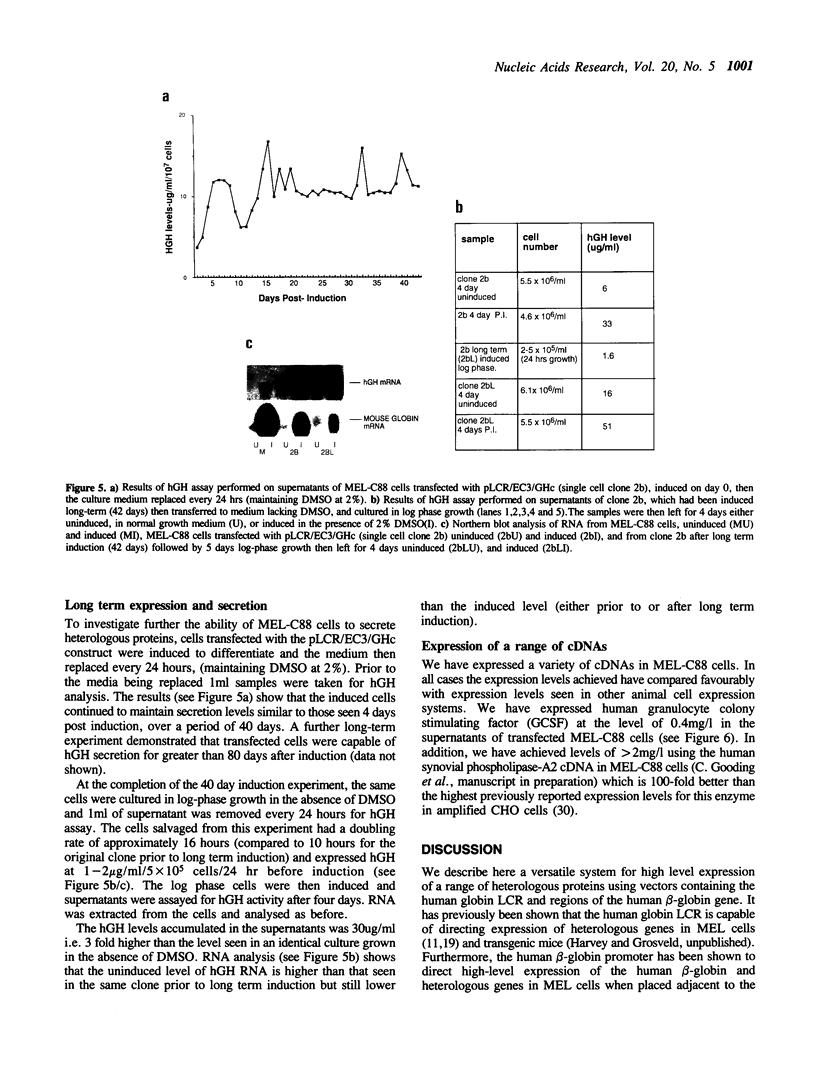
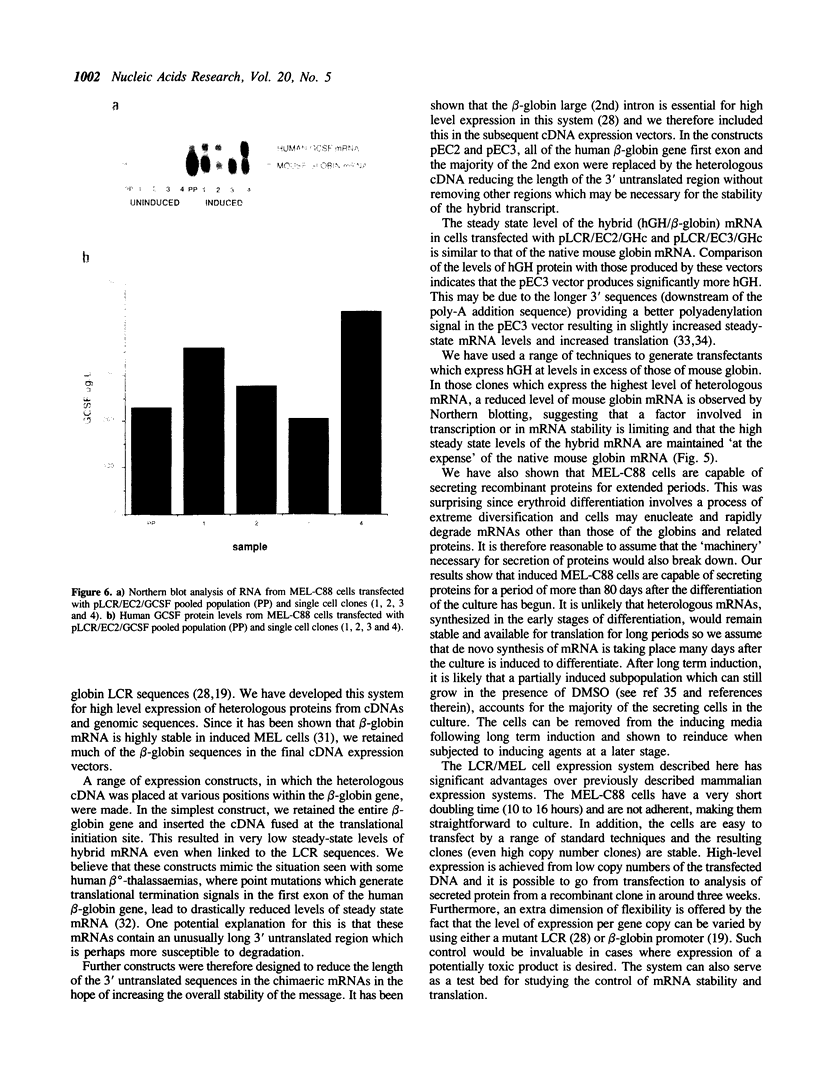
We have used the human globin locus control region (LCR) to assemble an expression system capable of high-level, integration position-independent expression of heterologous genes and cDNAs in murine erythroleukaemia (MEL) cells. The cDNAs are inserted between the human beta-globin promoter and the second intron of the human beta-globin gene, and this expression cassette is then placed downstream of the LCR and transfected into MEL cells. The cDNAs are expressed at levels similar to those of the murine beta-globin in the induced MEL cells. Heterologous genomic sequences can also be expressed at similar levels when linked to to the LCR and beta-globin promoter. In addition we demonstrate that, after induction of differentiation, MEL cells are capable of secreting heterologous proteins over a prolonged time period, making this system suitable for use in continuous production systems such as hollow fibre bioreactors. The utility of the LCR/MEL cell system is demonstrated by the expression of growth hormone at high levels (greater than 100 mg/l) 7 days after induction. Since the expression levels seen do not depend upon gene amplification and are independent of the integration position of the expression cassette, it is possible to obtain clones with stable high-level expression within 3-4 weeks after transfection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniou M., Grosveld F. beta-globin dominant control region interacts differently with distal and proximal promoter elements. Genes Dev. 1990 Jun;4(6):1007–1013. doi: 10.1101/gad.4.6.1007. [DOI] [PubMed] [Google Scholar]
- Blom van Assendelft G., Hanscombe O., Grosveld F., Greaves D. R. The beta-globin dominant control region activates homologous and heterologous promoters in a tissue-specific manner. Cell. 1989 Mar 24;56(6):969–977. doi: 10.1016/0092-8674(89)90630-2. [DOI] [PubMed] [Google Scholar]
- Chen Z., Banks J., Rifkind R. A., Marks P. A. Inducer-mediated commitment of murine erythroleukemia cells to differentiation: a multistep process. Proc Natl Acad Sci U S A. 1982 Jan;79(2):471–475. doi: 10.1073/pnas.79.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis P., Antoniou M., Grosveld F. Definition of the minimal requirements within the human beta-globin gene and the dominant control region for high level expression. EMBO J. 1990 Jan;9(1):233–240. doi: 10.1002/j.1460-2075.1990.tb08100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth A., Hendrick D. Human alpha-globin gene expression following chromosomal dependent gene transfer into mouse erythroleukemia cells. Cell. 1978 Sep;15(1):55–63. doi: 10.1016/0092-8674(78)90082-x. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987 Dec 23;15(24):10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Trowsdale J., Saito I. Mulcos: a vector for amplification and simultaneous expression of two foreign genes in mammalian cells. Gene. 1988 Nov 15;71(1):19–27. doi: 10.1016/0378-1119(88)90073-x. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990 Jul 13;62(1):15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- Kabat D., Sherton C. C., Evans L. H., Bigley R., Koler R. D. Synthesis of erythrocyte-specific proteins in cultured friend leukemia cells. Cell. 1975 Jul;5(3):331–338. doi: 10.1016/0092-8674(75)90109-9. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J. Selection and coamplification of heterologous genes in mammalian cells. Methods Enzymol. 1990;185:537–566. doi: 10.1016/0076-6879(90)85044-o. [DOI] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987 Oct;7(10):3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. M., Hession C., Johansen B., Hayes G., McGray P., Chow E. P., Tizard R., Pepinsky R. B. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989 Apr 5;264(10):5768–5775. [PubMed] [Google Scholar]
- Krowczynska A., Yenofsky R., Brawerman G. Regulation of messenger RNA stability in mouse erythroleukemia cells. J Mol Biol. 1985 Jan 20;181(2):231–239. doi: 10.1016/0022-2836(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lim S., Mullins J. J., Chen C. M., Gross K. W., Maquat L. E. Novel metabolism of several beta zero-thalassemic beta-globin mRNAs in the erythroid tissues of transgenic mice. EMBO J. 1989 Sep;8(9):2613–2619. doi: 10.1002/j.1460-2075.1989.tb08401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Miller L. K. Baculoviruses as gene expression vectors. Annu Rev Microbiol. 1988;42:177–199. doi: 10.1146/annurev.mi.42.100188.001141. [DOI] [PubMed] [Google Scholar]
- Nandi A. K., Roginski R. S., Gregg R. G., Smithies O., Skoultchi A. I. Regulated expression of genes inserted at the human chromosomal beta-globin locus by homologous recombination. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3845–3849. doi: 10.1073/pnas.85.11.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipsen S., Talbot D., Fraser P., Grosveld F. The beta-globin dominant control region: hypersensitive site 2. EMBO J. 1990 Jul;9(7):2159–2167. doi: 10.1002/j.1460-2075.1990.tb07385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzina S., Hanscombe O., Whyatt D., Grosveld F., Philipsen S. Hypersensitive site 4 of the human beta globin locus control region. Nucleic Acids Res. 1991 Apr 11;19(7):1413–1419. doi: 10.1093/nar/19.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatzman A. R. Gene expression using gram-negative bacteria. Curr Opin Biotechnol. 1990 Oct;1(1):5–11. doi: 10.1016/0958-1669(90)90004-5. [DOI] [PubMed] [Google Scholar]
- Talbot D., Collis P., Antoniou M., Vidal M., Grosveld F., Greaves D. R. A dominant control region from the human beta-globin locus conferring integration site-independent gene expression. Nature. 1989 Mar 23;338(6213):352–355. doi: 10.1038/338352a0. [DOI] [PubMed] [Google Scholar]
- Talbot D., Philipsen S., Fraser P., Grosveld F. Detailed analysis of the site 3 region of the human beta-globin dominant control region. EMBO J. 1990 Jul;9(7):2169–2177. doi: 10.1002/j.1460-2075.1990.tb07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]