Abstract
With the expansion of the potential applications of carbon nanotubes (CNT) in biomedical fields, the toxicity and biocompatibility of CNT have become issues of growing concern. Since the immune system often mediates tissue damage during pathogenesis, it is important to explore whether CNT can trigger cytotoxicity through affecting the immune functions. In the current study, we evaluated the influence of CNT on the cytotoxicity mediated by human lymphocytes in vitro. The results showed that while CNT at low concentrations (0.001 to 0.1 µg/ml) did not cause obvious cell death or apoptosis directly, it enhanced lymphocyte-mediated cytotoxicity against multiple human cell lines. In addition, CNT increased the secretion of IFN-γ and TNF-α by the lymphocytes. CNT also upregulated the NF-κB expression in lymphocytes, and the blockage of the NF-κB pathway reduced the lymphocyte-mediated cytotoxicity triggered by CNT. These results suggest that CNT at lower concentrations may prospectively initiate an indirect cytotoxicity through affecting the function of lymphocytes.
Introduction
Carbon nanotubes (CNTs) have been shown to have potential applications in multiple biomedical fields[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], especially as effective transporters for delivery of various bioactive molecules such as peptides[11], proteins[12], [13], [14], DNAs[15], [16], [17], RNAs[18], or drugs[19], [20]. Our previous study revealed that CNT conjugated tumor protein could enhance the uptake of tumor antigen by human dendritic cell (DC) and the capability of DC to induce anticancer response in vitro. The results suggest that CNT-based nanotechnology may have a prospective role in the development of more efficacious DC-based anticancer immunotherapy.[21] With the expansion of the scope of prospective CNT applications, the toxicity of CNT to mammalian cells becomes an issue of great concern. Some literatures reported that exposing cells to CNT led to cell death[22], [23], apoptosis[24], or inhibition of proliferation[25], [26], while others showed that CNT at lower concentrations had minimal toxicity[27], [28], [29]and did not significantly affect the function and viability of cells[27]. So far however, most studies on CNT toxicity have focused on the changes in cells following direct CNT exposure. The indirect toxicity mediated by the immune modulation effects of CNT has not been well explored. Because the immune system often plays a major role in tissue damage during pathogenesis[30], in addition to studying the direct CNT toxicity on cells, it is also important to evaluate whether CNT can trigger cytotoxicity through affecting the function of lymphocytes. Here in this study, the effects of CNT on lymphocyte-mediated cytotoxicity on multiple human cell lines were assessed in vitro. Moreover, we evaluated whether CNT would influence the proliferation of lymphocytes, the production of IFN-γ and TNF-α by lymphocyte, and the activation of NF-κB in an attempt to explore the mechanisms of the prospective CNT-induced immune cytotoxicity.
Results
1. Characterization of CNT
Functionalized CNT used in this study was prepared using a method similar to that described in our prior report[31]. Briefly, an oxidation/sonication procedure was utilized to introduce carboxyl groups to CNT surface for solubilization enhancement. The characterization of the functionalized CNT was carried out with standard methodology[32], [33]. Scanning electronic microscopy revealed that the tube-like structure of CNT was well maintained, with an average length of 500–800 nm and an average diameter of 20–30 nm. X-ray photoelectron spectroscopy analysis detected carboxyl groups on the surface of CNT. The resultant CNT solution had a concentration of 0.2 mg/ml (data not shown, the results have been describled in the paper we published before).[31]
2. CNT promoted lymphocyte-mediated cytotoxicity
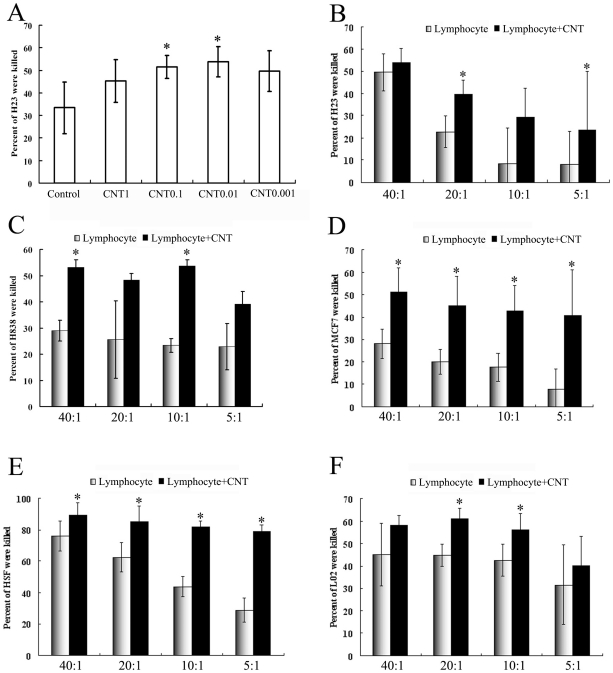
The in vitro cytotoxiciy mediated by the lymphocytes were measured using a standard methodology that had been employed in ex vivo immune studies[34], [35]. To explore the effective working concentration of CNT, various concentrations of CNT (from 0.001 to 1 µg/ml) were tested, with the H23 cell line as the target cells and the effector to target cell raito (E: T ratio) of 10∶1. After incubation with various concentrations of CNT, the lymphocytes (effector cells) were thoroughly washed, and co-incubated with the target cells for 3 more days. The percentage of viable target cells was then evaluated with an MTS assay[34], [35]. The results showed that the effective working concentration of CNT was between 0.01 to 0.1 µg/ml, with the growth inhibition rates of 53.84±6.77% and 51.53±5.17%, respectively (Fig.1A, p<0.05, compare to control group). The control group used lymphocytes that had not been exposed to CNT, and generated a growth inhibition rate of 33.41±11.44%. The CNT groups of 1 µg/ml and 0.001 µg/ml did not enhance the growth inhibition to a statistically significant level compared to the control.
Figure 1. CNT promoted lymphocyte-mediated cytotoxicity.
(A) Percentage of lived H23 cells was killed by Lymphocyte alone or co-incubation with CNT of various concentrations (n = 6, ± SD). The star indicates a statistically significant difference between this group and the Lymphocyte alone groups (p<0.05). (B–F) Percentage of other cell lines (B: H23; C: H838; D: MCF7; E: HSF; F: L02) were killed by Lymphocyte alone or co-incubation with 0.01 µg/ml CNT on difference E: T ratios (n = 6, ± SD). The star indicates a statistically significant difference between this group and the Lymphocyte alone groups (p<0.05).
To confirm the immune modulatory effect of CNT, we next tested whether CNT of 0.01 µg/ml would also induce a lymphocyte-mediated cytotoxicity against other human cell lines, across a wider range of E: T ratios (from 40∶1 to 5∶1). As shown in Fig 1, CNT of 0.01 µg/ml induced cell growth inhibition consistently across various human cell lines, including three tumor cell lines (MCF7, H838 and H23) and two non-tumor cell lines (HSF and L02). When the MCF7 was used as the target cells, the CNT induced growth inhibitions were 51.23±10.72%, 45.08±12.58%, 42.65±11.3% and 40.73±20.26%, for E/T ratios of 40∶1, 20∶1, 10∶1 and 5∶1, respectively (p<0.05, compared to control); whereas the control group generated much lower inhibitions of 28.15±6.42%, 20.1±5.63%, 17.66±6.21% and 8.08±9.01% respectively. Similar trends were observed in experiments with other cell lines (Fig 1), suggesting that a low concentration of CNT could indeed enhance the lymphocyte-mediated cytotoxicity in vitro.
3. CNT did not influence cell Viability
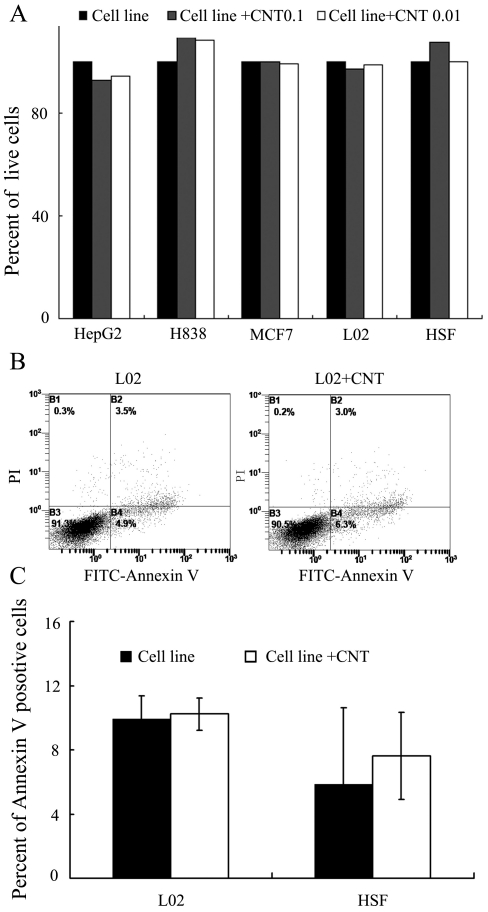
It is important to investigate whether a prospective CNT toxicity on the target cells was responsible for the higher tumor inhibition observed in Fig. 1. To address this issue, the target cells were incubated for 3 days in either normal medium or mediums containing CNT of 0.01 or 0.1 µg/ml. The viability of the MCF7 cells was then evaluated by the MTS assay. The results showed no significant difference in the number of live cells among the groups (Fig 2A) further explore the issue, we also tested whether CNT of 0.01 µg/ml would induce an apoptosis effect in L02 and HSF cell lines. As shown in Fig 2B, C CNT failed to induce an obvious apoptosis in either cell lines at this concentration. These results suggested that the direct cytotoxicity of CNT was not the major mechanism of the enhanced growth inhibition observed in Fig 1.
Figure 2. Influence of CNT on cell Viability.
(A) Percentage of different cell lines after co-incubation with CNT of various concentrations (n = 6, ± SD). (B, C) Effect of CNT on L02 cell apoptosis. The test was conducted by Annexin-V and PI double staining and analyzed by flow cytometry. Apoptosis of L02 cells was analyzed in L02 cells alone (L02), CNT co-cultured with L02 (L02+CNT), Annexin V + means the cells were PI negative and Annexin V positive. Data are shown as means ± SD of three independent experiments.
4. Influence of CNTs on lymphocyte proliferation
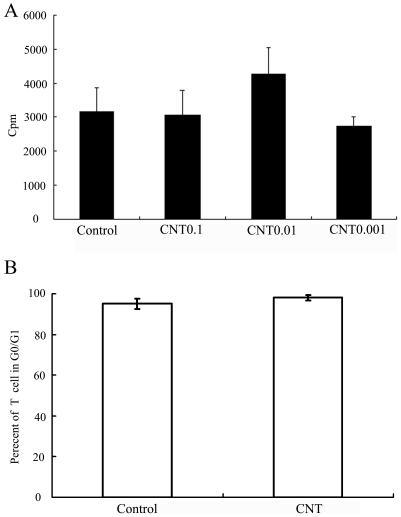
To explore the mechanism by which CNT enhanced lymphocytes' cytotoxicity, we next evaluated the influence of CNT on lymphocyte proliferation. CNT of various concentrations (0.001, 0.01, and 0.1 µm/ml) were added to the culture medium, and lymphocyte proliferation was subsequently evaluated with the standard technique of [3 H]-thymidine uptake. The results showed no significant difference among the groups, though CNT of 0.01 µm/ml tended to raise the uptake of [3 H]-thymidine. (Fig 3A) Moreover, there was no difference in the percentage of lymphocytes that stayed in G0/G1 phases (Fig 3B).
Figure 3. Influence of CNT on lymphocyte proliferation.
(A) Influence of CNT on lymphocyte proliferation, lymphocytes were cultured alone or co-cultured with CNT of various concentrations for 3 days. [3 H]-thymidine were added at the last 18 hour. (B) Influence of CNT on cell cycle of lymphocyte. Lymphocytes were cultured alone or co-cultured with CNT for 3 days, then the cell were collected and detected by PI.
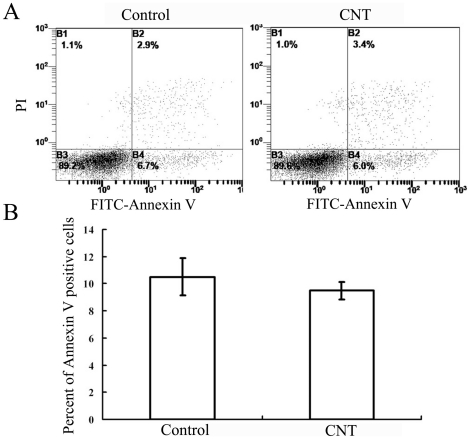
We also investigated whether CNT would promote the apoptosis of the lymphocytes. As shown in Fig 4, CNT did not induce an obvious apoptosis in lymphocytes either. These results showed that CNT did not significantly affect the lymphocytes proliferation or apoptosis, suggesting that the enhanced lymphocyte-mediated cytotoxicity was probably mediated through other mechanisms.
Figure 4. Influence of CNT on lymphocyte apoptosis.
The test was conducted by Annexin-V and PI double staining and analyzed by flow cytometry. Apoptosis of lymphocyte cells was analyzed in lymphocyte cells alone (Lymphocyte), CNT co-cultured with lymphocyte (Lymphocyte+CNT), Annexin V + means the cells were PI negative and Annexin V positive. Data are shown as means ± SD of five independent experiments.
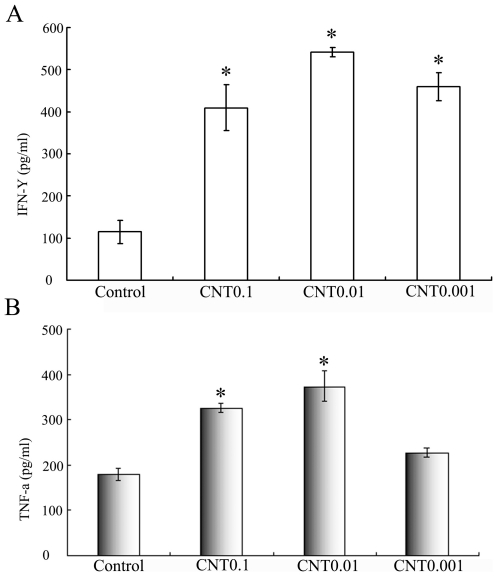
We next evaluated if CNT would affect the secretion of proinflammatory cytokines IFN-γ and TNF-α, which are generally regarded as the key signs of lymphocyte activation. Lymphocytes were cultured in medium containing CNT of either 0.001, 0.01, or 0.1 µg/ml for 24 hours. The levels of secreted proinflammatory cytokines IFN-γ and TNF-α were measured in culture supernatants using a standard double-sandwich ELISA protocol. As presented in Fig 5, lymphocyte cultured with CNT of 0.01 µg/ml or 0.1 µg/ml produced significant higher amounts of both cytokines compared with the untreated cells (p<0.05). The results suggested that CNT at proper concentrations might promote lymphocyte activation and the secretion of proinflammatory cytokines.
Figure 5. CNT promote lymphocytes secretion cytokines.
Lymphocyte cell were cultured alone or co-cultured with CNT of various concentrations for 24 hours. The levels of secreted cytokines IFN-γ and TNF-α were measured in culture supernatants by ELISA.
5. CNT promote lymphocyte cytotoxicity by NF-κB
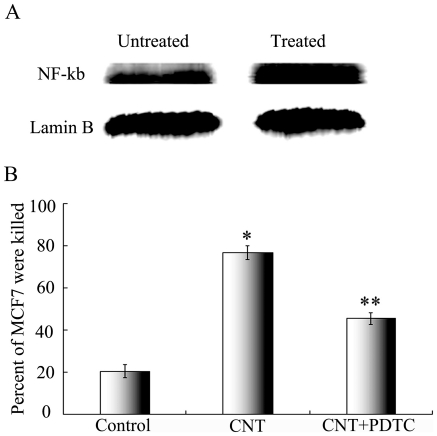
To further explore the mechanism of CNT induced lymphocyte activation, we evaluated the influence of CNT on the NF-κB pathway in lymphocyte, which is a major transcription factor that regulates genes responsible for both the innate and adaptive immune responses. Lymphocytes were incubated in either normal medium or that contains CNT of 0.01 µg/ml. Nuclear protein was extracted and the western blotting was utilized to compare the NF-κB activation in the two groups of lymphocytes. The results showed that the NF-κB was more activated in the CNT-treated group (Fig. 6A), Lamin B was used as a control. To further explore the role of NF-κB activation in CNT induced cytotoxicity, the effects of NF-κB inhibitor PDTC was evaluated during cytotoxicity experiments. As presented in Fig 6B, while CNT enhanced the cytotoxicity against MCF7 cells by the lymphocytes, PDTC decreased the toxic effect significantly (p<0.05 compared to the CNT group). These results suggested that the activation of NF-κB probably involved in the lymphocyte-mediated toxicity induced by CNT exposure.
Figure 6. CNT promote lymphocytes Cytotoxicity by NF-κB.
(A) The influence of CNT on the concentration of NF-κB p65 in the lymphocyte cell nuclei. Lymphocytes were incubated in either normal medium or that contains CNT of 0.01 µg/ml for 24 hours. Nuclear protein was extracted and the western blotting was utilized to compare the NF-κB activation. Lamin B was used as control. (B) The influence of NF-κB inhibitor PDTC on the lymphocyte-mediated cytotoxicity of CNT. MCF7 were co-cultured with Lymphocyte or Lymphocyte added CNT, or Lymphocyte added CNT and PDTC. The percent of lived MCF7 cell were detected by MTS.
Discussion
The toxicity and biocompatibility of CNT have become issues of great concern with the growth of prospective applications of CNT in biomedical fields. The aim of this study was to evaluate the influence of CNT on lymphocyte-mediated cytotoxicity in vitro, since the immune system often mediates tissue damage during pathogenesis. The results showed that CNT at low concentration (0.001 to 0.1 µg/ml) enhanced the immune-mediated cytotoxicity against multiple types of human cells in vitro (Fig 1), but did not cause obvious cell death or apoptosis directly (Fig 2). In addition, CNT increased the secretion of cytokines signaling the activation of lymphocytes, including -γ and TNF-α (Fig 5), but failed to trigger a proliferation of the lymphocytes (Fig 4). Furthermore, CNT upregulated the NF-κB expression in immune cells, and the blockage of the NF-κB pathway reduced the CNT-induced cytotoxicity by lymphocytes (Fig 6). These results suggest that CNT at lower concentrations may trigger changes in lymphocytes, which in turn may cause an indirect cytotoxicity.
Dumorter et al reported that CNTs could be uptaken by lymphocytes and macrophages in vitro without affecting the cell viability, and that CNT did not influence the functional activity of the immune cells[27]. They also noticed that CNT provoked the secretion of proinflammatory cytokines by macrophages. In agreement with their findings, the results of this study showed that CNT could enhance the secretion of IFN-γ and TNF-α by lymphocytes/PBMC. In addition, we observed that CNT also increased the lymphocyte-mediated cytotoxicity against multiple human cell lines in vitro. The enhanced cytokine secretion by the lymphocytes could be partially responsible for the enhanced immune cytotoxicity induced by CNT, since IFN-γ and TNF-α both mediate inflammatory reactions.
NF-κB is a major transcription factor that regulates genes responsible for both the innate and adaptive immune response, including those involved in lymphocyte development, maturation and proliferation. It has been reported that CNT could activate the NF-κB pathways in a few types non-immune cells, including keratinocytes[25], mesothelial cells[22], and lung cancer A549 cells[23]. However, it was unknown whether CNT could also activate the NF-κB pathway in lymphocytes. Here we observed for the first time that CNT could upregulate NF-κB expression in lymphocytes, and that blocking NF-κB reduced the CNT-induced immune cytotoxicity. The findings suggested that NF-κB was probably involved in the CNT-induced and lymphocyte-mediated cytotoxicity. However, it should be noted that cellular pathways other than NF-κB could not be excluded, and further studies are necessary to completely understand the transcriptional mechanism of the effects triggered by CNT.
The in vivo biological properties of CNT have also been evaluated in animal models in recent years. While CNT administered via the respiratory tract can cause severe lung damages[36], [37], [38], CNT given intravenously did not affect the long-term survival of the mice[39], [40], [41]. Prior research on the cellular toxicity of CNT mainly focused on the apoptosis or death of target cells following direct CNT exposure in vitro, while the indirect CNT toxicity mediated by the immune cells has not been reported in literature. Here we observed that CNT at low concentration (0.01 to 0.1 µg/ml) enhanced the lymphocyte-mediated cytotoxicity against multiple human cell lines in vitro (Fig 2), but failed to cause obvious cell death or apoptosis directly. Although this effect needs further evaluation with in vivo studies, the finding suggested yet another potential mechanism for CNT induced-toxicity.
In summary, while low-dose CNT failed to induce apoptosis in target cells directly, they can enhance the lymphocyte-mediated cytotoxicity in vitro. The results suggest that CNT may potentially trigger an indirect cytotoxicity through enhancing the function of lymphocytes.
Materials and Methods
1. Preparation and Characterization of Functionalized CNT
Multiwalled carbon nanotubes were purchased from Chengdu Organic Chemicals Co. Ltd., with the purity of greater than 95%, diameter of 20 to 30 nm, average length of 50 µm, amorphous carbon of less than 3%, ash (catalyst residue) of less than 1.5%, special surface area of greater than 233 m2/g, and the thermal conductivity of about 2000 W/m.k. Stable aqueous suspensions of purified and shortened CNT were prepared by oxidation and sonication of the purchased commercial product using a method described in our previous study[31]. In brief, CNT were suspended in a 3∶1 mixture of concentrated H2SO4/HNO3 and sonicated at 540 W for 45 s. The resulting mixture was filtered through a polycarbonate filter membrane of 2 µm pore (Millipore) and rinsed thoroughly till neutralized. The obtained CNT were dried completely and suspended in pure water at the concentration of 0.3 mg/ml by sonication. Centrifugation (5000 rpm for 30 min) removed the unreacted components from the solution to afford a stable suspension of CNT. The size and shape of CNT were evaluated by scanning electron microscopy. The surface element chemistry of the oxidized CNT was analyzed by X-ray photoelectric spectroscopy (XPS, VG Escalab MK II, UK). The CNT suspension was sonicated again prior to mixing with culture or reaction mediums for further application.
2. Cell Culturing
The human cell lines NCI-H23 (human lung adenocarcinoma), NCI-H838 (human lung adenocarcinoma), MCF-7 (human breast adenocarcinoma), L02(human hepatocyte) and HSF(human skin fibroblast) ,HepG2(human hepatic carcinoma) were maintained in RPMI 1640 medium (Gibco Life Technologies) supplemented with 10% fetal calf serum (FCS; Gibco), 100 U/mL penicillin, 100 µg/mL streptomycin in 5% CO2 and humidified atmosphere at 37°C. All cell lines were purchased from Cell Resource Center, IBMS, CAMS/PUMC).
3. Preparation of human peripheral blood mononuclear cells
Healthy volunteers' blood were collected as we previously described.[21] Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Paque density gradient centrifugation. All donors were required to sign an informed consent form according to procedures approved by the Ethics Committee at Chinese Academy of Medical Sciences and Peking Union Medical College. The PBMC were suspended in RPMI 1640 medium supplemented with 10% (vol/vol) FCS, 2 mM L-glutamine, 0.1 mM nonessential amino acids (Life Technologies, Grand Island, NY), 1 mM sodium pyruvate, 100 U/mL penicillin, 100 µg/mL streptomycin, 1% HEPES buffer, and 10 µM 2-mercaptoethanol. The PBMC, consisting of mainly lymphocytes[35], were used as effector cells in the cytotoxicity studies.
4. Cytotoxicity Studies
The cytotoxicity experiments were conducted using the standard methods for ex vivo immune studies[35]. Specifically, the MTS cell viability assay was utilized to evaluate the condition of various target cells. MTS assay is a currently well-adopted method that is superior than the MTT assay in both sensitivity and applicability[34], [35]. The MCF7, H23, H838, HSF and L02 cells were employed here as the target cells. For various experimental groups the lymphocytes/PBMC were first incubated for 3 days with either normal RPMI 1640 medium, or mediums containing 0.1, 0.01 and 0.001 µg/ml of CNT respectively. After 3 days, the lymphocytes (effector cells) were removed and washed for 4 times with PBS, before mixing with the target cells for the cytotoxicity study.
The cytotoxicity study was performed in 96-well U-bottom plates according to standard protocols [35]. The target cells were added to the wells with culture medium and incubated for 4 hours so that the cells became adherent. Effector cells were mixed in the wells according to the indicated effector:target (E:T) ratios. The total volume per well was adjusted to 200 µl. The plate was then incubated in a CO2 incubator at 37°C for 3 days. The positive control and the background control were set up according to the instruction of the manufacturer (MTS Cell Titer Kit, Promega) and each used 6 wells. The background control was taken from 6 wells containing medium and MTS solution. The positive control was taken from 6 wells containing the target cells, medium and MTS, but without the effector cells. After 3 days, the supernatants were removed, and the plates were washed thrice with PBS. One hundred µl RPMI 1640 and 20 µl MTS solution were added per well. The plates were incubated for about 90 minutes at 37°C. When the color turned to brown, the plates were measured for light absorption by an ELISA plate reader at 490 nm. Each experiment was repeated in 6 wells to ensure reliable readings. Results were compared by analysis of variance (ANOVA), using the SPSS13.0 software. P value of <0.05 was considered significant. The percentage cytotoxicity was calculated according to the following equation (A490 indicates the light absorption at 490 nm)[35]:
5. Cell Viability Assays after CNT Treatment
MCF7, H838, H23, L02, or HSF cells were washed twice with aseptic PBS, followed by a 10-minute centrifugation at 1000 rpm. The spun-down cells were re-suspended in RPMI 1640 culture medium supplemented with 10% FBS. Proper amount of CNT (0.1 µg or 0.01 µg) was added to 500 µl RPMI and thoroughly mixed, and incubated with 1×106 cells at 37°C with 5% CO2 for 72 hours. The viability of the cells was then evaluated by the MTS method described above.
6. Effects of CNT on cell apoptosis
CNT and human cell lines were prepared as described above. Human cell lines L02 and HSF were cultured in either normal medium or that containing CNT (0.01 µg/ml) for 3 days. The cells were harvested and quantified, stained with Annexin-V kit (BD, USA), and analyzed with flow cytometry (FACS Vantage).
7. Effects of CNTs on lymphocyte apoptosis
CNT and lymphocytes were prepared as described above. Lymphocytes were cultured in either normal medium or that containing CNT (0.01 ug/ml) for 3 days. The cells were harvested and quantified, stained with Annexin-V kit (BD, USA), and analyzed with flow cytometry (FACS Vantage).
8. Effects of CNT on lymphocyte proliferation
The lymphocytes were incubated in RPMI 1640 medium with 10%FBS that contained CNT of 0.1, 0.01, or 0.001 µ g/ml for 72 hours. Control wells contained lymphocytes cultured in medium without CNT. Cultures were pulsed with 1 µCi/well [3 H]-TdR (Shanghai Nucleus Research Institute, China) on day 2, and harvested 18 hours later with a Tomtec automated harvester (Wallac Inc.). Thymidine uptake by lymphocytes was quantified with a liquid scintillation & luminescence counter (Wallac MicroBeta® TriLux).
9. Effects of CNT on cell cycle phase of lymphocytes
Lymphocytes were cultured alone or co-cultured with CNT for 3 days, then harvested and quantified. One million Lymphocytes were fixed with 70% cold ethanol at 4°C for 60 min, washed with PBS twice, and stained with 50 µg/ml PI (Sigma) at room temperature for 5 min. Flow cytometry of the lymphocytes was then performed. Data were analyzed with ModFIT software
10. Effects of CNT on IFN-γ and TNF-α Production
The lymphocytes were incubated in RPMI 1640 medium with 10%FBS that contained CNT of 0.1, 0.01, or 0.001 µg/ml for 72 hours. Control wells contained lymphocytes cultured in medium without CNT. Culture supernatants were collected. The IFN-γ and TNF-α in the supernatants were measured by standard sandwich ELISA techniques using the Quantikine Immunoassay Kit (Jingmei Inc, Shenzhen, China) according to the manufacturer's instructions. The plates were measured for light absorption by an ELISA plate reader (Bio-Rad) with a 450 nm filter.
11. Western blot assay of NF-κB
The lymphocytes were incubated in RPMI 1640 medium with 10%FBS and CNT of 0.01 µg/ml for 24 hours. Control wells contained lymphocytes cultured in medium without CNT. The nuclear proteins were extracted using a nuclear protein extraction kit (Nanjing KeyGen Biotech, China). Standard western blotting procedures were carried out with specific NF-κB p65 antibody (Santa Cruz), Lamin B antibody (Santa Cruz), and secondary antibodies labeled with horseradish peroxidase (Santa Cruz). Antibody/antigen complexes were detected using the ECL reagent (Millipore).
12. Inhibition effect of NF-κB by PDTC
To further explore the role of NF-κB activation in CNT induced cytotoxicity, the effects of NF-κB inhibitor PDTC (Pyrrolidinedithiocarbamic acid, ammonium salt) was evaluated during cytotoxicity experiments. As presented in Fig. 6, while CNT enhanced the cytotoxicity against MCF7 cells by the lymphocytes, PDTC decreased the toxic effect significantly (p<0.05 compared to the CNT group). These results suggested that the activation of NF-κB probably involved in the lymphocyte-mediated toxicity induced by CNT exposure.
13. Statistics
Statistical analysis was performed with the statistical SPSS 13.0 software. The nonparametric test was used to calculate the probability of significant differences among the groups. Statistical significance was defined as p <0.05.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by funds from the Ministry of Science and Technology of China (2011CB933504, 2011CB911003, 2011CB911004) and the Natural Science Foundation of China (81071870). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bianco A, Kostarelos K, Partidos CD, Prato M. Biomedical applications of functionalised carbon nanotubes. Chem Commun (Camb) 2005;5:571–577. doi: 10.1039/b410943k. [DOI] [PubMed] [Google Scholar]
- 2.Cai D, Mataraza JM, Qin ZH, Huang Z, Huang J, et al. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat Methods. 2005;2:449–454. doi: 10.1038/nmeth761. [DOI] [PubMed] [Google Scholar]
- 3.Heller DA, Jeng ES, Yeung TK, Martinez BM, Moll AE, et al. Optical detection of DNA conformational polymorphism on single-walled carbon nanotubes. Science. 2006;311:508–511. doi: 10.1126/science.1120792. [DOI] [PubMed] [Google Scholar]
- 4.Kam NW, O'Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Wu DC, Zhang WD, Jiang X, He CB, et al. Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angew Chem Int Ed Engl. 2005;44:4782–4785. doi: 10.1002/anie.200500042. [DOI] [PubMed] [Google Scholar]
- 6.Martin CR, Kohli P. The emerging field of nanotube biotechnology. Nat Rev Drug Discov. 2003;2:29–37. doi: 10.1038/nrd988. [DOI] [PubMed] [Google Scholar]
- 7.Ni Y, Hu H, Malarkey EB, Zhao B, Montana V, et al. Chemically functionalized water soluble single-walled carbon nanotubes modulate neurite outgrowth. J Nanosci Nanotechnol. 2005;5:1707–1712. doi: 10.1166/jnn.2005.189. [DOI] [PubMed] [Google Scholar]
- 8.Sun YP, Fu K, Lin Y, Huang W. Functionalized carbon nanotubes: properties and applications. Acc Chem Res. 2002;35:1096–1104. doi: 10.1021/ar010160v. [DOI] [PubMed] [Google Scholar]
- 9.Williams KA, Veenhuizen PT, de la Torre BG, Eritja R, Dekker C. Nanotechnology: carbon nanotubes with DNA recognition. Nature. 2002;420:761. doi: 10.1038/420761a. [DOI] [PubMed] [Google Scholar]
- 10.Zanello LP, Zhao B, Hu H, Haddon RC. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006;6:562–567. doi: 10.1021/nl051861e. [DOI] [PubMed] [Google Scholar]
- 11.Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun. 2004. pp. 16–17. [DOI] [PubMed]
- 12.Kam NW, Dai H. Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J Am Chem Soc. 2005;127:6021–6026. doi: 10.1021/ja050062v. [DOI] [PubMed] [Google Scholar]
- 13.Kam NW, Liu Z, Dai H. Carbon nanotubes as intracellular transporters for proteins and DNA: an investigation of the uptake mechanism and pathway. Angew Chem Int Ed Engl. 2006;45:577–581. doi: 10.1002/anie.200503389. [DOI] [PubMed] [Google Scholar]
- 14.Shi Kam NW, Jessop TC, Wender PA, Dai H. Nanotube molecular transporters: internalization of carbon nanotube-protein conjugates into Mammalian cells. J Am Chem Soc. 2004;126:6850–6851. doi: 10.1021/ja0486059. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Nie L, Wang T, Qin Y, Guo Z, et al. Carbon nanotube delivery of the GFP gene into mammalian cells. Chembiochem. 2006;7:239–242. doi: 10.1002/cbic.200500227. [DOI] [PubMed] [Google Scholar]
- 16.Pantarotto D, Singh R, McCarthy D, Erhardt M, Briand JP, et al. Functionalized carbon nanotubes for plasmid DNA gene delivery. Angew Chem Int Ed Engl. 2004;43:5242–5246. doi: 10.1002/anie.200460437. [DOI] [PubMed] [Google Scholar]
- 17.Singh R, Pantarotto D, McCarthy D, Chaloin O, Hoebeke J, et al. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. J Am Chem Soc. 2005;127:4388–4396. doi: 10.1021/ja0441561. [DOI] [PubMed] [Google Scholar]
- 18.Lu Q, Moore JH, Huang G, Mount AS, Rao AM, et al. RNA Polymer Translocation with Single-Walled Carbon Nanotubes. Nano Lett. 2004;4:2473–2477. [Google Scholar]
- 19.Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Wu W, Wieckowski S, Pastorin G, Benincasa M, Klumpp C, et al. Targeted delivery of amphotericin B to cells by using functionalized carbon nanotubes. Angew Chem Int Ed Engl. 2005;44:6358–6362. doi: 10.1002/anie.200501613. [DOI] [PubMed] [Google Scholar]
- 21.Sun Z, Wang W, Meng J, Chen S, Xu H, et al. Multi-walled carbon nanotubes conjugated to tumor protein enhance the uptake of tumor antigens by human dendritic cells in vitro. Cell Res. 2010;20:1170–1173. doi: 10.1038/cr.2010.133. [DOI] [PubMed] [Google Scholar]
- 22.Pacurari M, Yin XJ, Zhao J, Ding M, Leonard SS, et al. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect. 2008;116:1211–1217. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye SF, Wu YH, Hou ZQ, Zhang QQ. ROS and NF-kappaB are involved in upregulation of IL-8 in A549 cells exposed to multi-walled carbon nanotubes. Biochem Biophys Res Commun. 2009;379:643–648. doi: 10.1016/j.bbrc.2008.12.137. [DOI] [PubMed] [Google Scholar]
- 24.Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett. 2005;155:73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappaB in human keratinocytes. Nano Lett. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raja PM, Connolley J, Ganesan GP, Ci L, Ajayan PM, et al. Impact of carbon nanotube exposure, dosage and aggregation on smooth muscle cells. Toxicol Lett. 2007;169:51–63. doi: 10.1016/j.toxlet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Dumortier H, Lacotte S, Pastorin G, Marega R, Wu W, et al. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006;6:1522–1528. doi: 10.1021/nl061160x. [DOI] [PubMed] [Google Scholar]
- 28.Davoren M, Herzog E, Casey A, Cottineau B, Chambers G, et al. In vitro toxicity evaluation of single walled carbon nanotubes on human A549 lung cells. Toxicol In Vitro. 2007;21:438–448. doi: 10.1016/j.tiv.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Walker VG, Li Z, Hulderman T, Schwegler-Berry D, Kashon ML, et al. Potential in vitro effects of carbon nanotubes on human aortic endothelial cells. Toxicol Appl Pharmacol. 2009;236:319–328. doi: 10.1016/j.taap.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Mackay IR, Leskovsek NV, Rose NR. Cell damage and autoimmunity: a critical appraisal. J Autoimmun. 2008;30:5–11. doi: 10.1016/j.jaut.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng J, Meng J, Duan J, Kong H, Li L, et al. Carbon nanotubes conjugated to tumor lysate protein enhance the efficacy of an antitumor immunotherapy. Small. 2008;4:1364–1370. doi: 10.1002/smll.200701059. [DOI] [PubMed] [Google Scholar]
- 32.Kostarelos K, Lacerda L, Pastorin G, Wu W, Wieckowski S, et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nature Nanotechnology. 2007;2:108–113. doi: 10.1038/nnano.2006.209. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Cai W, He L, Nakayama N, Chen K, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 34.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 35.Haas C, Lulei M, Fournier P, Arnold A, Schirrmacher V. A tumor vaccine containing anti-CD3 and anti-CD28 bispecific antibodies triggers strong and durable antitumor activity in human lymphocytes. Int J Cancer. 2006;118:658–667. doi: 10.1002/ijc.21390. [DOI] [PubMed] [Google Scholar]
- 36.Chou CC, Hsiao HY, Hong QS, Chen CH, Peng YW, et al. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008;8:437–445. doi: 10.1021/nl0723634. [DOI] [PubMed] [Google Scholar]
- 37.Li JG, Li QN, Xu JY, Cai XQ, Liu RL, et al. The pulmonary toxicity of multi-wall carbon nanotubes in mice 30 and 60 days after inhalation exposure. J Nanosci Nanotechnol. 2009;9:1384–1387. doi: 10.1166/jnn.2009.c162. [DOI] [PubMed] [Google Scholar]
- 38.Ma-Hock L, Treumann S, Strauss V, Brill S, Luizi F, et al. Inhalation Toxicity of Multi-Wall Carbon Nanotubes in Rats Exposed for 3 Months. Toxicol Sci. 2009. [DOI] [PubMed]
- 39.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, et al. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci U S A. 2006;103:3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NW, Chu P, et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 41.Yang ST, Wang X, Jia G, Gu Y, Wang T, et al. Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol Lett. 2008;181:182–189. doi: 10.1016/j.toxlet.2008.07.020. [DOI] [PubMed] [Google Scholar]