Abstract
Shewanella oneidensis is a highly motile organism by virtue of a polar flagellum. Unlike most flagellated bacteria, it contains only one major chromosome segment encoding the components of the flagellum with the exception of the motor proteins. In this region, three genes encode flagellinsaccording to the original genome annotation. However, we find that only flaA and flaB encode functional filament subunits. Although these two genesare under the control of different promoters, they are actively transcribed and subsequently translated, producing a considerable number of flagellin proteins. Additionally, both flagellins are able to interact with their chaperon FliS and are subjected to feedback regulation. Furthermore, FlaA and FlaB are glycosylated by a pathwayinvolving a major glycosylating enzyme,PseB, in spite of the lack of the majority of theconsensus glycosylation sites. In conclusion, flagellar assembly in S. oneidensis has novel features despite the conservation of homologous genes across taxa.
Introduction
In environments where living conditions change constantly, microorganisms must respond to those changes rapidly. One of common approaches is that cells move away from detrimental environments and reach relatively favorable niches. Bacteria use a wide variety of cellular structures to facilitate motility, of which the flagellum is the most important and thus the best studied [1]. More than 80% of known bacterial species are motile by means of flagella [2]. In addition, flagella are indispensible in adhesion to substrates, biofilm formation, pathogenicity, and reduction of insoluble metal minerals [3]–[6]. In general, bacteria have either polar (ie. Vibrio cholera) or lateral (ie. Escherichia coli) flagella, which are alike structurally. Exceptions which have both are found [7]–[8]. Although the bacterial flagellum is one of the most complex of all prokaryotic organelles, the structure of the conventional flagellum is relatively well understood and excellent reviews are available [1], [9]–[10].
A flagellar system is tightly regulated because its synthesis and functioning is highly costly for the cell (about 2% of biosynthetic energy expenditure in E. coli) [10]–[11]. Genes encoding proteins involved in flagellum synthesis are organized into an ordered cascade in which the expression of genes at a given level is required for the expression of other genes at a lower level of assembly [10]. In Gram-negative bacteria, the regulatory cascades for lateral and polar flagella are dramatically different [12]. In bacteria with lateral flagella,at the top of the hierarchy is the flhDC operon, encoding the FlhD and FlhC proteins, which are essential for expression of downstream flagellar genes [12]. In the case of polar flagella, genes are transcribed in a four tiered hierarchy [13]. It is well established that a σ54-associated transcription activator is the master regulator at the top level among microorganisms in which such a master regulator has been identified [14]. This master regulator controls transcription of genes in the second tier, which encode components of the MS ring-switch complex as well as the regulatory factors FlrB, FlrC and FliA (σ28). FlrB and FlrC are responsible for transcription of genes in the third tier, most of which encode the basal body-hook, cap, and some of flagellins. The rest of flagellar genes encoding flagellins, the anti-sigma factor flgM, and the motor components, which make up the fourth tier, are σ28-dependent.
Owing to countless scientific efforts along with advances in glycoprotein detection and identification, it is now established that flagellins are heavily glycosylated. Studies on C. jejuni and H. pylori have illustrated two O-linked-glycosylation pathways, Pse and Leg [15]–[16]. Such modification via the O-glycan pathway is essential for flagellum assembly and bacterial motility [17]. However, C. jejuni 81–176 is the only strain for which the sites of glycosylation are known [18]. Although 19 serine or threonine residues, of which 18 reside in the D2–D3 domains, can be glycosylated only 8 are required for motility and flagellar assembly [19]–[20]. The D2–D3 domains are heavily glycosylated because they form the projections on the filament surface [21].
The Shewanella species have expanded during the last two decades as an important family of facultative Gram-negative anaerobes [21]. Initially, studies on this group of microorganisms were mostly aimed at exploring the ability for the bioremediation of metal/radionuclide contaminants in the environment. In recent years, S. oneidensis, the representative species of Shewanellae, has become a research model for respiration diversity, metabolic network, and biofilm formation [21]. For motility,all Shewanella species produce a polar flagellum. Since flagellar structures and gene organization are highly conserved, it is reasonable to assume that the synthesis of the flagella in S. oneidensis is similar to that in V. cholera, the paradigm for the polar flagellum and the only organism sharing extensive regions of similar gene order [9], [22]. Nevertheless, extensive diversity among flagellated bacteria exists in the content and organization of these flagellar genes and their regulation [23]–[25]. This may be especially true in the case of S. oneidensis, which has two sets of stator systems to drive flagellar rotation [26].
Shewanella species are extremely diverse in phenotypic or ecological features, making it difficult to accurately define the core characteristics of the genus [21], [27]. As one of the most genetically conserved organelles composed of a large number of components,flagella may perfectly serve that purpose [24]. Additionally, flagella of Shewanella species have been reported to be involved in formation of biofilms and pellicles [28]–[29]. In spite of the increasing importance placed on the organelle, it is surprising that flagella of Shewanella species have not been investigated in detail. In the present study we firstelucidated the content and organization of flagellar genes in S. oneidensis and compared these features to all sequenced Shewanella strains. We then systematically examined components with uncertain functions. We found that of the two major flagellin genesa major one was under the direct control of σ28and a minor one was also actively transcribed. A bacterial two-hybrid assay revealed that FliD was unable to interact with SO3234, the predicted chaperon of FliD, suggesting the lack of such a chaperon in the polarly flagellated bacteria. Furthermore, PseBwas identified to be essential forflagellinglycosylation although the entire glycosylation pathway remains elusive in S. oneidensis.
Results
In silico analysis of flagellar genesin Shewanellae
22 Shewanella genomes at Integrated Microbial Genomes at DOE Joint Genome Institute (http://img.jgi.doe.gov) were used for sequence comparison analyses. A preliminary analysis of flagellar genes revealed a high level of similarity, enabling us to use the best studied S. oneidensis as the representative species. Differences in other Shewanella genomes arediscussed in the text to provide a more comprehensive view of diversity.
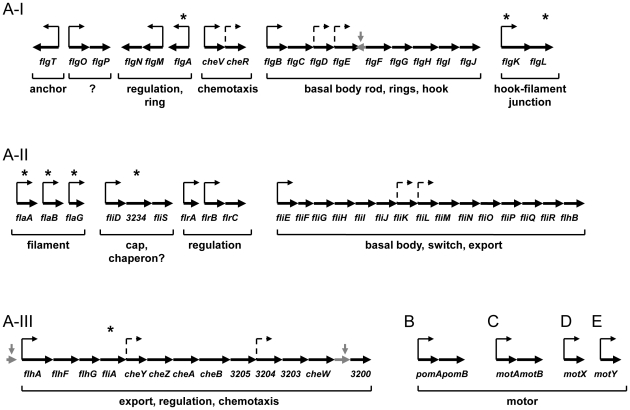
The S. oneidensis genome contains up to70 geneson its chromosome encoding the flagellar and chemotaxis proteins that are homologous to those of other polar flagellar and chemotaxis systems [22]. These include the three sets of chemotaxis genes at different locations, of which only the third is likely to be functional since a mutation in cheA-3(SO3207) eliminated bacterial chemotactic response [30]. To elucidate organization of flagellar genes in S. oneidensis, flagellar gene systems from E. coli and V. cholerae were chosen as general references, representing the best understood and the closest in phylogeny. Unlike E. coli and V. cholerae, S. oneidensis allocates only one major location(SO3200-58) on the chromosome for flagellar genes and the third set of chemotaxis genes. This 59-gene fragment (approximately 155KB in length) can be divided into three clusters, resembling three regions of the polar flagellar genes of V. cholerae (Fig. 1). The structure of operons was derived from in silico analyses (http://www.microbesonline.org, http://biocyc.org/SONE211586/) with slight adjustments in reference to the well-defined counterparts of V. cholerae [13], [31]–[33]. In this 59-gene fragment, threemisidentified genes in the original annotation (SO3246, SO3214, and SO3201 between flgE and flgF, flhB and flhA, and cheW3 and SO3200, respectively) were removed when the genome was re-annotated, as presented at http://img.jgi.doe.gov.
Figure 1. The S. oneidensis flagellar genes.
The S. oneidensis genome containsone major regionfor flagellar genes (labelled A) as well as scattered motor genes (pomAB (SO1529-30), motAB (SO4287-6), motX (SO3936), and motY (SO2754). Region A, divided into three clusters I, II, III, encompassesgenes SO3200-58. Thick arrows denote ORFs (not drawn to scale) with corresponding genes below. Genes with an asterisk above were subjected to the physiological analyses in this study. Putative promoters are designated by thin arrows based on operon structures predicted at two sites (http://www.microbesonline.org; http://biocyc.org/SONE211586/). Solid and dash lines represent ones identified at both sites and ones found at only either site, respectively. Pseudogenes removed from the genome are in light grey.
The first cluster (A–I) includesflgT, flgOP, flgNM,flgA(SO3253), cheV3-R3, and flgBCDEFGHIJK(SO3240)L. While flgM and cheVR respectively encode an anti-σ28 factor and achemotaxis protein, the rest of the genes are annotated as structural genes for a sodium-driven motor ring (FlgT), a gene encoding a protein ofunknown function (FlgOP), one for a basal body rod, others for rings, and hook protein (FlgBCDEFGHIJKL), a chaperon (FlgN) and an assembly protein (FlgA) [34]–[35]. The flgA gene was not assigned in the original genome sequencing but it shows sufficient homology with flgA of other bacteria to be included so after validating its requirementfor motility [29], we designated it flgA. Another unassigned gene in the first annotation is SO3240 (encoding an ortholog of FlgK), which was proposed to be non-functional due to two frameshifts in its predicted gene sequence. In contrast, all other sequenced Shewanella strains contain a full-length flgK, suggesting that there may be sequencing errors in the flgK gene of S. oneidensis.
The second cluster (A-II) of S. oneidensis flagellar genes consists of flaA(SO3238), flaB(SO3237), flaG, fliD-SO3234-fliS, flrA, flrBC, and fliEFGHIJKLMNOPQR-flhB. Except for flrABC encoding regulatory proteins, products of all these genes are components of the S. oneidensis flagellum such as filament, basal body, switch, and export proteins. Among genes in this cluster, SO3237, SO3238 and SO3234 were not named in the original annotation. Sequence analysis of SO3237 and SO3238 by BLAST revealed that the best fits were to flagellins, especially those of less than 300 a.a. in length. We therefore named SO3237 and SO3238flaB and flaA, respectively. Previously, flaA, flaB, and flaG were proposed to encode flagellin subunits and removal of all three genes or flaA and flaB together resulted in a complete loss of motility [26], [36]. However, several lines of evidence suggest that FlaG is unlikely to be a flagellin subunit. These include that 1) the protein is too small (119 a.a.) to include all necessary domains of a functional flagellin [37], 2) the low sequence similarity to FlaA or FlaB (9%), 3) a Pseudomonas fluorescensflaG mutant was as motile as its parental strain [38], and 4) the flaG is the first gene of the four-gene “non-flagellin” operon flaG-fliD-flaI-fliS in V. cholerae [13].
A great diversity in flagellar filament genes among sequenced Shewanella strains was observed. Like S. oneidensis,12 other strains possess two genes encoding flagellins of 265–275 a.a., implying that flagellar filament subunits of this length represent the ancestral set of flagellins in Shewanellae. Intriguingly, S. baltica OS185 and OS195 possess four genes encoding flagellins while two other sequenced S. baltica strains (OS155 and 223) have only two (Fig. S1). The additional two flagellar filament genes reside on a fragment of 5.4 kb betweenanalogs of S. oneidensisflaA and flaB. These fragments appear to haveresulted from transposition events perhaps due to the presence of IS4 family transposase genes. The second largest group consists of 5 strains including Shewanella sp. MR-4, MR-7, S. benthica, S. violacea, and S. frigidimarina, whosegenes encode theflagellin subunits of 465–482 a.a.. It is worth noting that S. benthica KT99 not only carries transposase gene between the flagellin gene and flaG but also have two additional genes encoding 104 a.a. and 344 a.a. proteins in place of the counterpart of S. oneidensisflaB. Given that both proteins exhibit high levels of sequence similarity to known flagellins, we speculate that this unusual structure may be the result of sequencing errors. The other two strains S. pealeana and S. piezotolerans contain genes encoding flagellins of 393–394 a.a. and 434–463 a.a., respectively. It is interesting to note that S. piezotolerans is the only strain whose two flagellins differ from each other bymore than 5 a.a..
The only ORF in the A-IIcluster that could not be functionally assigned is SO3234. The geneencodes a small protein (106 a.a.)of unknown function. Although the proteins are highly conserved among sequenced Shewanella strains, its counterparts in other organisms have not been confidently identified. In V. cholerae and Vibrio parahaemolyticus, the gene at the same location is designated flaI but its product shares sequence identity of less than 20% with SO3234 [13].
The third cluster (A-III) contains the chemotaxis genes cheY3Z3A3B3W3, the export gene flhA, the regulatory genes fliA and flhFG, and the four ORFs encoding proteins ofunknown function. The fliA gene product (σ28) is a sigma factor specific for expression of some late flagellar genes encoding the filament proteins, motor proteins and other flagellar-related secreted proteins [1]. In polarly flagellated bacteria, flhF and flhG encode proteins controlling the number and location of the flagella.
The flagellar genes that are not on this 59-gene fragment include two sets of motor genes: pomAB(SO1529, SO1530) and motAB (SO4287, SO4286), and two motor auxiliary protein genes: motX(SO3936) and motY(SO2754). Interestingly, S. oneidensis is the onlystrain containingboth proton-driven PomAB and sodium-driven MotAB whereas in all other sequenced Shewanella strains MotAB is lacking. A recent study on S. oneidensis has demonstrated that bothmotor units were neededto power the flagellum simultaneously with assistance from MotXY [26], [39].
Development of suitable complementation plasmids for flagellar genes
The bacterial flagellar system is relatively stable in evolution and up to 24 core genes are found in every microorganism possessing flagella [24]. While genomic analyses allow for the direct detection of genes encoding most flagellar components, it is still necessary to carry out mutational analyses on uncertain ones for functional validation.
In our previous studies, broad host range vectors pBBR1MCS-2 and pBBR1MCS-5 were used for complementation by introduction of the intact gene and/or its promoter region into the multiple cloning site (MCS) of either plasmid [40]–[42]. However, the majority of the flagellar gene operons are unusually large (the predicted largest is 13 kb in S. oneidensis), consisting of multiple genes with different function. As a result, a vectors such as pBBR1MCS is not suitable because: 1) mutation(s) may be introduced during amplification ofthe targeted gene and its promoter; 2) the other genes within the same operon may interfere with complementation, especially those encoding regulatory proteins. To circumvent such an obstacle, we constructed two plasmids: pHG101, a promoter-less plasmidfor genes next to their promoter and pHG102,hosting the S. oneidensis arcA promoter for genes some distance from their promoter. The arcApromoterwas chosen because the gene has been found to be expressed at the considerable level under both aerobic and anaerobic conditions [40], [43]. Th is promoter with an MCS was generated by PCR (primers available upon requestunless otherwise noted).
In this study, all mutants that displayed phenotypes distinguishable from their parental strains were subjected to complementation. Although motility of each strain on both swimming and swarming plates was examined in comparison with the wild type only swimming results are presented for there was no statistically significant difference between swimming and swarming unless otherwise noted. The results aresummarized in Table 1 anddiscussed in the text when necessary.
Table 1. Motility of S. oneidensis flagellar mutantsa.
| Strain | Plasmidb | Gene on Plasmidc | RC of Swarmingd | RC of Swimminge |
| WT | 1 | 1 | ||
| WT | pHG101 | 1.01±0.04 | 0.98±0.03 | |
| WT | pHG102 | 0.99±0.04 | 1.03±0.04 | |
| ΔflgK | pHG101 | 0.04±0.02 | 0.07±0.03 | |
| ΔflgK | pHG101 | flgK | 1.05±0.04 | 1.04±0.04 |
| ΔflaB | pHG101 | 0.34±0.06 | 0.40±0.03 | |
| ΔflaB | pHG101 | flaB | 1.11±0.05 | 1.46±0.05 |
| ΔflaA | 0.97±0.06 | 1.08±0.05 | ||
| ΔflaAΔflaB | pHG101 | 0.03±0.01 | 0.05±0.02 | |
| ΔflaAΔflaB | pHG101 | flaB | 1.00±0.04 | 1.07±0.06 |
| ΔfliA | pHG102 | 0.05±0.03 | 0.03±0.01 | |
| ΔfliA | pHG102 | fliA | 0.95±0.04 | 0.99±0.03 |
| ΔfliD | pHG101 | 0.03±0.01 | 0.04±0.01 | |
| ΔfliD | pHG101 | fliD | 0.98±0.06 | 1.07±0.06 |
| ΔSO3234 | pHG102 | 0.64±0.07 | 0.57±0.07 | |
| ΔSO3234 | pHG102 | SO3234 | 0.95±0.08 | 1.01±0.06 |
| ΔpseB | pHG101 | 0.06±0.02 | 0.05±0.02 | |
| ΔpseB | pHG101 | pseB | 1.03±0.06 | 0.99±0.06 |
The motility – the distance from the culture spot to the edge of the haze of motility.
The vector in the strain to provide the same genetic background.
The designated vector borne gene in the strain for complementation.
RC of swarming, the swarming motility of each strain is normalized to that of the wild type strain on the same agar plate.
RC of swimming, the swimming motility of each strain is normalized to that of the wild type strain on the same agar plate.
S. oneidensis contains an intact and functional FlgK
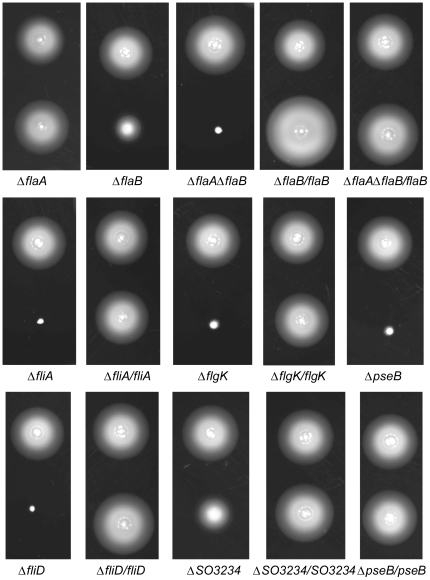
According to the genome sequence, two ORFs encoding proteins of 386 and 141 a.a. reside at the flgK locus. flgK encodes a hook-filament junction protein, which is essential for flagellar assembly and motility [11]. Given the synteny and the indispensible role of FlgK, it is unlikely that this crucial protein could be missing. To verify the sequence, an S. oneidensis culture with flagellated cells (confirmed by a microscope) was used as the chromosomal DNA source for gene amplification. Three independent sequencing results unambiguously indicated that SO3240 in the original annotation missed out A and C immediately after nt1134 and nt1505, which introduced frame-shift mutations. Addition of these two nucleotides allows translation of the full length ORF, resulting in a protein of 641 a.a., which displayed sequence similarities (full length) more than 80% and 50% to FlgK in other sequenced Shewanella and Vibrio species, respectively. Based on this result, we named SO3240 as flgK. To further validate the intactness of SO3240, we constructed an in-frame deletion strain. As expected, the mutant was non-motile, but the wild-type phenotype was restored when pHG101 carrying the gene was introduced into, confirming that FlgK is functional in S. oneidensis (Table 1) (Fig. 2).
Figure 2. Motility of S. oneidensis wild type and isogenic mutation strains.
In all panels, the mutant strain (lower) was compared to its parentalwild type (upper) in the swimming assay. Each mutant containing its complementation vector is presented as the mutant/the gene. For example, the ΔflaB/flaB strain refers to the ΔflaB mutant containing complementation vector pBBR-FLAB.
FlaB is the major flagellinsubunit for motility
In Shewanellae, genes encoding the flagellar filament subunits display the largest diversity asmentioned above, which warrants an in-depth analysis. Two flagellin genes are found in the S. oneidensis genome:flaA(SO3238)and flaB(SO3237). FlaA and FlaB share a sequence identity of 89% although they are rather short, only 272-3 a.a. in length compared to 376 a.a for V. cholera, 498 a.a. for E. coli, and 572 a.a. for Campylobacter jejuni [44]. Structural analyses on the 495 a.a. filament protein of S. typhimurium revealthat the protein consists of seven domains: D0-D1-D2-D3-D2-D1-D0, of which D2-D3-D2are not required for formation offull-length flagella for motility [37], [45]–[47]. We therefore speculate that the S. oneidensis subunits may retain only necessary domains. To test this hypothesis, alignments of the S. oneidensis FlaA and FlaB amino acid sequences with S. typhimurium LT2 FliC were performed. Indeed, the D2-D3-D2 domains were not found in either S. oneidensis flagellar filament subunit (Table 2).
Table 2. Alignment of S. typhimurium FliC with S. oneidensis FlaA and FlaB.
| Domain | Residues within domaina | %identity with FliCb | ||
| FliC | FlaA/FlaB | FlaA | FlaB | |
| D0 (N) | 1–45 | 1–45 | 66 | 68 (91)c |
| D1 (N) | 46–180 | 46–168 | 43 | 46 (86) |
| D2 (N) | 181–190 | – | – | – |
| D3 | 191–284 | – | – | – |
| D2 (C) | 285–407 | – | – | – |
| D1 (C) | 408–455 | 186–233 | 53 | 51(93) |
| D0 (C) | 456–495 | 234–272 | 57 | 58 (94) |
The domain boundaries within the S. oneidensis FlaA and FlaB proteins were assigned based on alignment with S. typhimurium FliC.
Per cent identity was determined using CLUSTALW with a gap penalty of 10.0 and a gap length penalty of 0.2.
Numbers in bracket represent %identity between S. oneidensis FlaA and FlaB.
In V. cholerae, mutation of flaA completely abolished motility whereas the other four flagellin genes were dispensable [48]. C. jejuniflaA was essential for motility but a flaB deletion strain produced full-length functional flagellar filaments [49]–[50]. Moreover, in V. parahaemolyticus, loss of one of six flagellar filament genes had little or no effect on motility or flagellar structure [11]. Consequently, in S. oneidensis it is possible that FlaA or FlaB may not be essential for formation of full-length flagella despite the high degree of conservation in all of the domains of these two proteins.
To determine the role of these two genes, we constructed S. oneidensis strains containing an in-frame deletion in flaA, flaB, or flaAflaB. Motility assaysdemonstrated that compared to the wild type strain,the mutation in flaAhad little or no effect on swimming motility but ΔflaB retained only about 40% of its swimming/swarming capabilitybased on the radius of the swimming rings (Table 1, Fig. 2). The double mutantΔflaAΔflaB, however, lost motility completely. To confirm the observation, ΔflaB wastransformed with the pHG101 containing flgB. Surprisingly, the strain with plasmid-borne flgB showed a stronger capacity for swimming than its parental wild type, which is likely due to multiple copies of the gene in the complemented strain. However, in the case of swarming, the motility increased much less significantly. This suggests that over-expressed flaBmay elevate motility of individual cells as swarming motility is a form of movement in multicellular groups rather than as individuals [51]. To test whether over-expressed flaB alone is enough to promote motility, ΔflaAΔflaB was complemented with flaB. A swimming assay showed that the plasmid borne flaB was only able to elevate motility to a level equal to that of the wild type. Examination of the ΔflaB cells by electron microscopy revealed that the flaB mutant produced heavily truncated flagella (data not shown). In contrast,ΔflaA displayed filament morphology similar to the wild type. All of these results indicate that both flagellin subunits are constituents of the filaments with FlaB dominating.
flaB but not flaA is under the control of σ28
Our observation that one of the flagellins is predominantin the S. oneidensis flagellum is supported by other examples. In V. cholerae, transcription of the essential flaA was directed by σ54 whereas the other four non-essential flagellin genes were controlled by σ28 [48]. In contrast, in C. jejuni the essential flagellar filament gene flaA was under the control of σ28whereas the σ54-controlled flaB played a minor role [49]–[50]. Given the high degree of sequence identity between S. oneidensis FlaA and FlaB, the contrasting phenotypes of these two mutants are likely to bedue to differences in gene expression levels.
To elucidate the underlying mechanism determining the essentiality of FlaB in S. oneidensis, we first examined the promoter regions of flaB and flaA. The σ28–dependent promoters in the S. oneidensis genome have been predicted using pattern matching (PM) and iterative position specific score matrix (PSSM)-based approaches [52]. PM and PSSM identified σ28 binding sites in upstream regions of 9 and 12 genes, respectively, and only 6 were in common supporting the use of these programs. We then constructed the σ28 binding weight matrix using the experimentally verified σ28 binding sequences from E. coli, S. typhimurium and V. cholerae to screen for the σ28 binding sites in the S. oneidensis genome [13], [40], [43], [53]. In total, 169 putative σ28 binding sites were identified using RSAT with the default setting [54] as given in the supplemental material (Table S1). The σ28 recognition sites were found in the promoter regions of flagellar genes including motY, flaB, flgM, and pomA, all of which were among the top 15 most confident. Furthermore, these four sites are the only sites that have been identified by all three approaches (in our method, the top 15 were chosen for comparison). These results strongly suggest that motY, flaB, flgM, and pomA are under the control of σ28 in S. oneidensis in agreement with similar results in V. cholerae [13], [31].
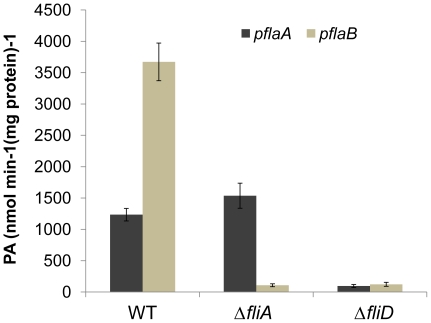
To experimentally validate that flaB is under the direct control of σ28, we fused each of the flaA and flaB promoters to the full-length lac operon in a newly developed reporter system [43]. For the promoter activity assay, a fliA null mutant was constructed to provide a genetic background lacking σ28. As expected, the mutant produced a truncated flagellum and lost motility completely, matching the phenotype of fliA defective mutants from various bacteria (Table 1, Fig. 2& 3). The reporter plasmids were introduced into the wild type and aΔfliAstrain and the activity of the flaA and flaB promoters was measured. Although both flaA and flaB were actively transcribed in the wild type background, the promoter activity of flaB was approximately three times higher than that of flaA (Fig. 4). In contrast, in the fliA defective background, flaB was barely transcribed whereas expression of flaA was not significantly altered. These results verify that flaB but not flaA is under the control of σ28.
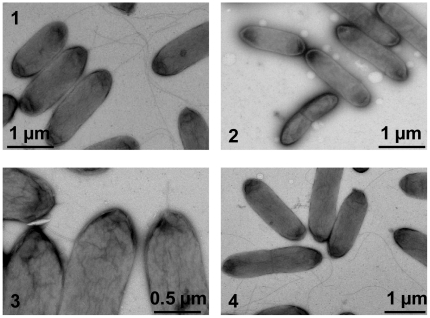
Figure 3. Transmission electron micrographs of S. oneidensis wild type and flagellar mutantstrains.
Cells were stained with 1% phophotungstic acid and applied to TEM. 1. The wild type. 2.ΔflgK, aflagellated. 3. ΔfliD, truncated filaments. 4. ΔSO3234, flagella indistinguishable from that of the wild type.
Figure 4. Promoter activities of flaA and flaB in S. oneidensis strains.
Whole-cell lysates were prepared from S. oneidensis cultures in mid-exponential growth phase and assayed as described in Experimental Procedures. Promoter activities were determined by measuring β-galactosidase levels using flaA-lacZ and flaB-lacZ reporter constructs in the wild type, ΔfliA, and ΔfliD strains. Quantitation of the promoter activities was normalized to the total protein in each strain.
Both FlaA and FlaB are under the feedback control
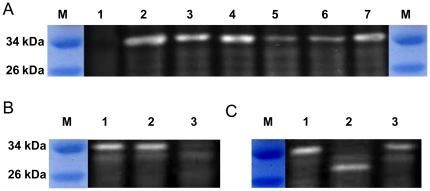
The relative transcription levels of flaA and flaB agree well with the contents of their products inthe flagella filament. However, in recent studies it was shown that the relative amounts of flagellins is subject to multiple levels of regulation and modification, including post-transcriptional, posttranslational controls as well as secretion [17], [55]–[57]. To test whether mechanisms at other levels account for the contrasting phenotypes of the flaA and flaB mutants, we raised antibodies against a peptide fragment shared by FlaA and FlaB and used them to detect flagellin subunits in the ΔflaA, ΔflaB, ΔfliA strains and in their parental wild type strains. Western blotting revealed a single band in the wild type but not in the ΔflaAΔflaB double mutation strain. At the same position, a band was visible in both ΔflaA and ΔflaB, indicating that the two flagellins migrated identically on sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) (Fig. 5). This single band was at a position approximately 4∼5 kDa higher than expected for the deduced molecular masses of each of the flagellins (∼29 kDa), suggesting the possibility of posttranslational modification, mostly likely glycosylation. The band in ΔflaA was much stronger than that in ΔflaB, supporting other evidence that FlaB is produced in substantially greater amount than FlaA. Additionally, results showed that ΔfliA possessed the smallest number of flagellins proteins, all presumably FlaA. The immunoblotting assay also confirmed the successful complementation of mutations in fliA and flaB. It is noteworthy that the plasmid borne flaB promoted over-production of FlaB flagellin in comparison to the amount of this protein in the wild type.
Figure 5. Western blot analysis of FlaA and FlaB.
Whole-cell lysates were prepared from S. oneidensis cultures in mid-exponential growth phase, loaded equally, separated on 12% SDS-PAGE, and electrophoretically transferred to polyvinylidene difluoride (PVDF). S. oneidensis flagellins were probed with polyclonal antibodiesthat recognize both FlaA and FlaB and detected by chemiluminescence. In all panels, M represents the protein marker. The samples in each lane are the (A) 1, ΔflaAΔflaB strain, 2, ΔflaB/flaB strain, 3, ΔfliA/fliA strain, 4, wild type strain, 5, ΔfliA strain, 6, ΔflaB strain, 7, ΔflaA strain; (B) 1, wild type strain, 2, ΔfliD/fliD strain, 3, ΔfliD strain; (C) 1, wild type strain, 2, ΔpseB strain, 3, ΔpseB/pseB strain.
As a considerable number of FlaA and FlaB proteins were present, a fliD mutant was constructed and assayed by immunoblotting to demonstrate whether these flagellin subunits are subjected to the feedback control. Results showed that the fliD mutant was completely non-motile on swarming or swimming plates and the plasmid borne fliD fully rescued the defect (Table 1, Fig. 2). Consistently, TEM studies demonstrated that the mutant cells possessed a truncated flagellum (Fig. 3), a characteristics of non-cap mutants in other bacteria [58]. Additionally, the mutation reduced the amount of flagellin drastically(lane 3 in Fig. 5B). The fliD gene on pHG101 complemented the fliD mutation resulting in similar levels of flagellin. To further investigate whether the feedback control occurs at the transcriptional level, we performed the β-Galactosidase assay to monitor the activity of the flaA and flaB promoters in the fliD mutant. Consistent with the Western blotting results, both promotersshowed substantially reduced activities, confirming other evidence that feedback control inhibits transcription of both genes (Fig. 4). These results indicate that both flaA and flaB are under feedback control and this control exertedprimarily at the transcription level.
FliS interacts with FlaA and FlaB
A recent study supported a novel mechanism for flagellar gene expression is dictated by the rate of protein secretion [59]. As a consequence, intracellular levels of flagellins may have inhibitory effects on their own production. The flagellin subunits are synthesized in the cytosol and transported as monomers to the nascent end of the flagellum [1]. Before transport, chaperon FliS functions to prevent flagellin polymerization in the cytosol by binding to the C-terminal D0 domain of the flagellin protein [60]–[61]. We then asked whether FliS recognizes FlaA and FlaB differently, causing one of them to be favorably secreted.
To test this, we generated plasmid constructs capable of detecting protein-protein interactions between FliS and FlaA as well as FliS and FlaB using the BacterioMatch II Two-Hybrid System. Plasmids containing ‘bait’ (FlaA or FlaB) and ‘target’ (FliS) were co-transformed into BacterioMatch II Validation Reporter Competent Cells for interaction detection. Positive protein interactions allow growth of the reporter strain on 3-AT (see Methods). As shown in Table 3, cells containing either FlaA/FliS or FlaB/FliS were able to form colonies on 3-AT in less than 24 h, similar to cells hosting the positive control plasmid pair. These positive interactions were confirmed by growth of these colonies on plates containing both 3-AT and streptomycin (12.5 µg/ml). In contrast, cells with the negative control plasmid pair failed to produce any colonies in 40 h. These results indicate that FliS is able to interact with both FlaA and FlaB. Meanwhile, we performed the same assay on FlaG and FliS. As expected, no interactions were detected between these two proteins, providing additional evidence that FlaG is not a flagellin subunit.
Table 3. Bacterial two-hybrid assay of FliS-FlaA, FliS-FlaB, and SO3234-FliD.
| Bait | Target | Colonies on nonselective platesa | Colonies on Selective platesb | Confirmationc | Result Interpretation |
| pBT | pTRG | 201 | 0 | – | no bait + no target – no interaction |
| pBT | pTRG-Gal11P | 177 | 0 | – | no bait + target Gal11P – no interaction |
| pBT-LGF2 | pTRG-Gal11P | 188 | 159 | 159 | bait LGF2 + target Gal11P – strong interaction |
| pBT | pTRG-FliS | 231 | 0 | – | no bait + target PliS – no interaction |
| pBT-FlaA | pTRG | 143 | 0 | – | bait FlaA + no target – no interaction |
| pBT-FlaA | pTRG-FliS | 150 | 47 | 47 | Bait FlaA + target FliS – strong interaction |
| pBT-FlaB | pTRG | 128 | 0 | – | bait FlaB + no target – no interaction |
| pBT-FlaB | pTRG-FliS | 192 | 104 | 104 | Bait FlaB+ target FliS – strong interaction |
| pBT-FlaG | pTRG | 222 | 0 | – | Bait FlaG+ no target – no interaction |
| pBT-FlaG | pTRG-FliS | 266 | 0 | – | Bait FlaG+ target FliS – no interaction |
| pBT | pTRG-SO3234 | 163 | 0 | – | no bait + target SO3234 – no interaction |
| pBT-FliD | pTRG | 145 | 0 | – | bait FliD + no target – no interaction |
| pBT-FliD | pTRG-SO3234 | 138 | 0 | – | Bait FliD+ target SO3234 – no interaction |
M9 agar +25 µg/ml chloramphenicol +12.5 µg/ml tetracycline.
a+5 mM 3-AT.
b+12.5 µg/ml streptomycin.
Both FlaA and FlaB are glycosylated
The apparent low migration rates of FlaA and FlaB on SDS-PAGE can be explained by posttranslational modification. The most abundant flagellin modification is glycosylation by the O-glycan pathways [17], [62]. Extensive studies on C. jejuni and Helicobacter pylori have shown that the predominant O-glycans on flagellins are derivatives of pseudaminic acid (Pse) or legionaminic acid (Leg) [63]. We reasoned that S. oneidensis uses the same strategy to modify flagellins thus promoting flagella assembly. The genome screening for flagellin glycosylation proteins identified SO3271 and SO3270 as promising candidates showing a sequence identity of greater than 30% to known components of the Pse and Leg pathways. SO3271and SO3270, predicted to be co-transcribed, share approximately 66% and 36% identity with PseB (C. jejuni CJ1293 and H. pylori HP0840) and PseC (C. jejuni CJ1294 and H. pylori HP0366), respectively. PseB (NAD(P)-dependent dehydratase/epimerase) and PseC (PLP-dependent aminotransferase), catalyze the first two steps in the pathway, converting UDP-α-D-GlcNac to UDP-2-acetamido-2,6-dideoxyb-l-arabino-hexos-4-ulose, then to UDP-4-amino-4,6-dideoxy-b-L-AltNAc [64].
To test the role of PseB in flagellin modification, we created an SO3271 (pseB) in-frame deletion strain. Like ΔpseB of H. pylori, the mutant was non-motile on swimming or swarming plates (Table 1, Fig. 2), indicating the essential role of this protein in motility [65]. Complementation with SO3271 on pHG101restored motility comparable to the parental wild type (Fig. 2, Table 1). To confirm that the motility phenotype resulted from flagellin modification, extracts from the mutant were subjected to Western blotting analysis (Fig. 5C). Flagellins from theΔpseB strain migrated much faster than the comparable proteins from the wild type. The position exactly matched the calculated molecular mass for both FlaA and FlaB, illustrating that flagellins in ΔpseBare in un-modified form. Additionally, glycosylated flagellins were not detected in the mutant strain in the analysis, suggesting that glycosylation by the PseBpathwayfunctions for both FlaA and FlaB. On the basis of similarities in sequence and functionality between SO3271 and PseB, we designated SO3271 of S. oneidensis as pseB.
SO3234 is importantfor motility but may not be the chaperon of FliD
In E. coli and S. typhimurium, operon fliDST encodes the cap FliD, the flagellin chaperon FliS, and the FliD chaperon FliT [1]. Since FliT is a regulator that inhibits the flagellar master regulatory proteins FlhDC by direct binding [66], FliD can function as an anti-regulator by sequestering FliT from FlhDC [1]. Unlike the fliD defect mutantsthat possess truncated flagella and lose motility fully, the fliT mutants produce flagellar structures indistinguishable from those produced by their parental wild type [67]. Consequently, the mutants are as motile as the wild type [68]. In S. oneidensis, the genes downstream of fliD are SO3234 and fliS. Intriguingly, although this type of organization is also observed in a variety of bacteria including V. cholera, little has been done to elucidate the role of SO3234 and its analogs in flagellar assembly. The question arose whether SO3234 (106 a.a.)is functionally equivalent to FliT.
Our mutational analysis has validated that the annotated fliD indeed encodes the cap of the flagellar filament so we constructedan S. oneidensis strain devoid of SO3234. The mutant ΔSO3234 displayed a decrease in motility to approximately 56% relative to its parental strain (Table 1, Fig. 2), in contrast to the findings that fliT mutants produced functional flagella in general [38], [67]–[69]. When pHG101 containing fliD-SO3234fused to the arcApromoter was inserted, both mutant strains had their motility fully restored (Fig. 2, Table 1). Surprisingly, the mutants appeared to produce full-length flagella (Fig. 3), indicating that the reduced motility may not be due to impaired filament formation.
To further dissect the role of SO3234, the bacterial two-hybrid system was employed to examine the direct interaction of SO3234 and FliD. The SO3234 gene was cloned into the bait plasmid while fliD was used as the target, and these were co-transformed into the E. coli reporter strain. In contrast to the positive control, no colonies were found on plates containing 3-AT after 24 hours of incubation (Table 3). Additional incubation for 16 hours which allows the growth of cells containing weak interactors did not help. These results rule out the possibility that FliD interacts with SO3234 in vivo, thus confirming that SO3234 is unlikely to be the chaperone for FliD.
Discussion
Flagellar synthesis in S. oneidensishas been presumed to be similar to that in V. cholera, the research paradigm for polarly flagellated bacteria, despite apparent permutations in the flagellar gene contents and organization betweenthe two genomes [13], [33], [34], [70]. As a result, only a couple of studies have been done in deciphering the S. oneidensismotility system in contrast to extensive investigation on its anaerobic respiration and metal reduction for more than two decades [21], [26], [36], [39]. In this study, we have performed a relatively comprehensive genetic analysis of the polar flagellumin S. oneidensis, generating three contributions to the current understanding of polar flagellar synthesis. First, we provide insights about the two flagellin subunits in respect to their functionality and regulation. Second, we demonstrated that both FlaA and FlaB are glycosylated by anovel pathway utilizing PseB. Third, we present data that SO3234 may not be the chaperon for FliT, arguing that the polar flagella may not require chaperons for their assembly.
Most of polar flagellated bacteria possess multiple flagellins which are similar to one another [11], [49], [71]–[73]. These microbes can be readily classified into two groups based on whether functionally predominant flagellins are present. V. parahaemolyticus, Caulobacter crescentus, and Aeromonas hydrophilaare representatives of the group without dominant filament subunits [11], [71], [73]. For bacteria in the other group, the removal of the predominant flagellin subunits causes a loss in motility [11], [49], [74]–[75]. Meanwhile, the minor flagellins are so insignificant that their mRNAs (C. jejuni) may not be above a detectable level [49], [74]. Consistently, major flagellins outnumber the minor ones hundreds or thousands of times. This phenomenon has largely been accredited to the fact that flagellin genes are under the control of different promoters, recognized by specialized regulatory proteins and/or sigma factors. In V. cholera, the major flagellin gene is σ54–dependent [11] while C. jejuni and C. coli employs σ28 to direct expression of the predominant flagellin [49], [74]. In S. oneidensis, disruption of either flagellin gene fails to lead to a complete non-motile phenotype although σ28–dependent FlaB is evidently more important than FlaA in motility. More intriguingly, transcription of both genesis considerable as is the amounts of FlaA and FlaB. Collectively, compared to the other studied polarly flagellated bacteria S. oneidensis seems to assemble flagella with ‘weaker’ major and ‘stronger’ minor flagellins, thus representing a novel model.
Flagella production is a metabolically costly endeavor for bacterial cells and therefore is tightly regulated, particularly at the transcriptional level. To prevent production of unnecessary flagellar subunits, additionalfeedback loops are exploited to ensure that during flagellar assembly every stage is signaled prior to synthesis of the components for the next stage [76]. In a recent study, it was reported that secretion efficiency of flagellins plays an important role [44]. The secretion signal modulating efficiency is located in the amino-terminal sequence, more precisely the N-terminal D0 domain of flagellin [77]. For example, protein-specific conserved residues are identified between C. jejuni FlaA and FlaB in the D0 domain by sequence alignment of both proteins from multiple strains [44]. These protein-specific residues dictate the secretion efficiency. In S. oneidensis, however, this may not be the case. Amino acid sequence alignment of the D0 domains of FlaA and FlaB from 23 sequenced Shewanella strains reveals a similar degree of conservation in N- and C-terminal D0 domains. More importantly, no protein-specific residues are found within either domain, suggesting that secretion of FlaA and FlaB may be similar if it is dependent on this terminal sequence. In addition, the chaperon FliS may not make a difference because the D0 domain binding regionsof these two flagellinsshare the highest identity [60]–[61]. However, there are some features residing in these two flagellins that distinguish them because the greater motility of the S. oneidensis strain with the over-expressed FlaB is only found in the presence of FlaA. This certainly merits further investigation.
Although great efforts have been made to increase our understanding of the polar flagellar system in recent years,the function of FliT is still elusive. In bacteria with lateral flagella such as Salmonella, chaperon FliT ofFliD inhibits the transcriptional activator FlhD4C2, suggesting that FliT is a regulatory component conserved throughoutthese flagellar systems [66], [78]–[79]. Interestingly, despite multiple roles of FliT the mutation in this gene did not affect either flagellar structure or motility [67] but export of FliD was significantly reduced in the mutant [68].
Unlike most bacteria with multiple separating segments for flagellar genes, S. oneidensis allocates only one major region, making it an ideal organism for identification of unknown flagellar genes. Among a few unknown flagellar genes, SO3234 appears most likely to be the counterpart of FliT because it shares conserved synteny with fliT in peritrichously flagellated bacteria and is also similar in size. We found that although the SO3234 null mutant showed reduced motility, it produced a flagellum indistinguishable from that in its parental strain. This observation is resonant with the finding by Capdevila et al. [38], indicating that the SO3234mutant is phenotypically different from fliT mutants. Furthermore, SO3234 is unable to interact with FliD, eliminating the possibility of chaperoning FliD. Given that most of the polarly flagellated bacteria also lack the second interaction partner of FliT, FlhC, we therefore assume that FliT is unlikely to be present in this group of microorganism. However, whether FliT is only conserved among enteric bacteria as suggested by Imada et al. [79] demands further investigation because FlhDC has been identified in a polarly flagellated bacterium, Burkholderia glumae [25].
Strikingly, both flagellins of S. oneidensis lack the D2–D3 domains, retaining only one glycosylation siteas identified in the flagellins of C. jejuni 81–176 (Fig. 6). Assuming that the basic structure of S. oneidensis flagellin is similar to that of Salmonella [20], it is unlikely that the majority of glycosylation sites are located in the D0–D1 domains because they are not exposed. If this holds, there are at most 7 residues suitable for glycosylation. Consequently, it is almost certain that Pse (MW = 316) is not the pathway glycosylating flagellins because it needs at least 15 sites to account for the observed mass increase of 4∼5 kDa. However, S. oneidensis apparently employs PseB to initiate glycosylation. This is not surprising because the bacterium has a complete set of enzymes and transporters for GlcNac metabolism to generate abundant substrates [80]. Therefore, flagellar glycosylation in S. oneidensis may be carried out by anovel pathway by components which may evolve independently as promiscuous enzymes that work in multiple pathways. Additional work is in progress to identify other genes in the pathway and to dissect the role of glycosylation in the flagellar assembly in S. oneidensis.
Figure 6. Potential glycosylation sites in FlaA and FlaB of S. oneidensis.
S. oneidensis flagellins (SoFlaA and SoFlaB) are aligned with FlaA and FlaB of C. jejuni 81–176 (CjFlaA and CjFlaB). The domain information is based on the domains of Salmonella enterica serovar Typhimurium flagellin. Positions of the glycosylation sites of CjFlaA are in purple (S206, found in S. oneidensis) and in yellow (T481, missing in S. oneidenisis, only one is shown). Serine and threonineresidues in S. oneidensis flagellins are in green and blue, respectively.
Methods
Bacterial strains, plasmids, and culture conditions
A list of all bacterial strains and plasmids used in this studyis given in Table 4 [81]. For genetic manipulation, E. coli and S. oneidensis strains weregrown under aerobic conditions in Luria-Bertani (LB) medium at 37 and 30°C,respectively. When needed, the growth medium was supplementedwith antibiotics at the following concentrations: ampicillin at50 µg/ml, kanamycin at50 µg/ml,and gentamycin at 15 µg/ml.
Table 4. Strains and plasmids used in this study.
| Strain or plasmid | Description | Reference or source |
| E. coli strain | ||
| WM3064 | Donor strain for conjugation; ΔdapA | [81] |
| S. oneidensis strains | ||
| MR-1 | Wild-type | ATCC 700550 |
| HG3210 | fliA deletion mutant derived from MR-1; ΔfliA | This study |
| HG3234 | SO3234 deletion mutant derived from MR-1; ΔSO3234 | This study |
| HG3235 | fliD deletion mutant derived from MR-1; ΔfliD | This study |
| HG3237 | flaB deletion mutant derived from MR-1; ΔflaB | This study |
| HG3238 | flaA deletion mutant derived from MR-1; ΔflaA | This study |
| HG3240 | flgK deletion mutant derived from MR-1; ΔflgK | This study |
| HG3237-8 | flaB and flaA double deletion mutant derived from MR-1; ΔflaBΔflaA | This study |
| HG3210-3238 | fliA and flaA double deletion mutant derived from MR-1; ΔfliAΔflaA | This study |
| Plasmids | ||
| pDS3.0 | Apr, Gmr, derivative from suicide vector pCVD442 | [82] |
| pDS-FLIA | pDS3.0 containing the PCR fragment for deleting fliA | This study |
| pDS-3234 | pDS3.0 containing the PCR fragment for deleting 3234 | This study |
| pDS-FLID | pDS3.0 containing the PCR fragment for deleting fliD | This study |
| pDS-FLAA | pDS3.0 containing the PCR fragment for deleting flaA | This study |
| pDS-FLAB | pDS3.0 containing the PCR fragment for deleting flaB | This study |
| pDS-FLGK | pDS3.0 containing the PCR fragment for deleting flgK | This study |
| pTP327 | Broad host lacZ reporter vector | [43] |
| pTP327-FLAAp | pTP327 containing the S. oneidensisflaA promoter | This study |
| pTP327-FLABp | pTP327 containing the S. oneidensisflaB promoter | This study |
| pBBR1MCS-2 | Broad host Kmr vector used for complementation | [83] |
| pHG101 | Promoterless broad host Kmr vector used for complementation | This study |
| pHG102 | pHG101 containing the arcA promoter | This study |
Construction and complementation of in-frame deletion mutants
In this study, in-frame deletion strains were constructed using the Fusion PCR method [82]. Primers used for generating PCR products for mutagenesis are available upon request. In brief, two fragments flanking the targeted gene were amplifiedindependently first and joined together by the second round of PCR. The resulting fusion fragment for each individual gene was introduced into plasmidpDS3.0. The resulting mutagenesis vector was transformed into E. coli WM3064, and then transferred into S. oneidensis by conjugation. Integration of the mutagenesis constructinto the chromosome was selected by gentamycin resistanceand confirmed by PCR. Verified transconjugants were grown in LB broth in the absence of NaCl and plated on LB supplemented with 10% of sucrose. Gentamycin-sensitive and sucrose-resistant colonies were screened by PCR for deletion of the targeted gene. The deletion mutation was then verified by sequencing of the mutatedregion.
To facilitate complementation experiments, two plasmids were constructed in this study. The first plasmid, pHG101, was formed by replacing lacZα and its promoter region on pBBR1MCS with the amplified MCS of the same plasmid [83]. The second plasmid, pHG102, was derived from pGH101 by placing the S. oneidensis arcA promoter in the front of the MCS. For complementation of genes next to their promoter, a fragment containing the targeted gene and its native promoter was generated by PCR and cloned into pHG101. For other genes, the targeted gene was amplified and inserted into MCS of pHG102 under the control of the arcA promoter. Introduction of each verified complementation vector into the corresponding mutant wasdone by mating with WM3064 containing the vector, andconfirmed by plasmid extraction and restriction enzyme mapping.
Physiological characterization
Growth of deletion strains in LB was measured by recording cell densities of cultures at 600 nm under aerobic conditions in triplicate with strain MR-1 as the control. To determine the swarm and swim motility of mutants, one microliter of an overnight culture was spotted in the middleof a swarm LB plate (0.5% agar) or a swim LB plate (0.25%agar) and allowed to dry for 1 h at room temperature. All plateswere incubated at 30°C for 10 h or as noted otherwise. Forphase-contrast microscopic analysis, swarmor swim cells werescraped from the leading edges of each swarm and then visualized inNB or saline on a glass slide.
Electron microscopy visualization
For transmission electron microscopy (TEM), cells grown overnighton 1% tryptone agar plates were suspended in sterile distilledwater, spread onto carbon-Formvar copper grids, and then negativelystained with 1% phosphotungstic acid (pH 7.4). Preparations were viewedunder a CM12 Philips TEM.
β-Galactosidase activity assay
A lacZ reporter system for S. oneidensis has been developed [43]. To construct the flaA-lacZ and flaB-lacZ reporters, the flaA and flaB promoter DNA fragments were generated by PCR (primers available upon request), cloned into pTP327, and verified by sequencing. The reporter plasmids were moved into S. oneidensisΔfliA or the MR-1 strain by conjugation. Cells in the log phase (30°C, OD600 = 0.4) were harvested by centrifugation, washed with PBS(phosphate buffered saline), and treated with lysis buffer (0.25 M Tris/HCl, (pH 7.5), 0.5% Trion-X100). The resulting soluble protein was collected after centrifugation and used for enzyme assaysemploying the High Sensitivity β-Galactosidase Assay Kit (Stratagene) according to manufacturer's instructions. β-galactosidaseactivity was determined by monitoring color development at 575 nm every minute for 30 min by using a Synergy 2 Multi-Detection Microplate Reader. The protein concentration of the cell lysates was determined using a Bradford assay with BSA as a standard (Bio-Rad).
Bacterial two-hybrid assay
The BacterioMatch II Two-Hybrid system (Stratagene) wasused to investigate protein-protein interaction in vivo in E. coli cells according to manufacturer's instructions. Briefly, plasmid constructs were created by cloning the target and bait proteins in the pTRG and pBT vectors and verified by sequencing. The resultant plasmids were used to co-transform BacterioMatch II Validation Reporter Competent Cells on M9 salt agar plates containing 25 µg/ml chloramphenicoland 12.5 µg/ml tetracycline with or without 3-amino-1,2,4-triazole (3-AT). pBT-LGF2, pTRG-Gal11P,and empty pBT and pTRG constructs were used as positive andnegative controls. The plates were incubated at 37°C for 24 h and then moved to room temperature for an additional 16 h (the colonies indicating a positiveinteraction usually appeared between 18 and 24 h). The positiveinteractions were confirmed by streaking colonies on platescontaining both 3-AT and streptomycin (12.5 µg/ml).
Immunoblotting assay
Rabbit polyclonal antibodies against a fragment of FlaB (CRDLTIQSENGANST) were prepared in accordance with standard protocols provided by the manufacturer (Genscript) and used for immunoblotting analysis. Bacterial cells in log phase (30°C, OD600 = 0.4) were used. For these experiments, cell samples were washed once with TE buffer (10 mM Tris [pH 8.0], 1 mM EDTA), and resuspended to an optical density at 600 nm (OD600) of 1.0 inlysis buffer (50 mM Tris [pH 8.0], 1 mM EDTA, 100 mM NaCl). The total protein concentration of the cell lysateswas then determined by the bicinchoninic acid assay (PierceChemical). Samples were loaded onto SDS-12% polyacrylamide gels and either stained with Coomassie brilliant blue or electrophoretically transferred to polyvinylidene difluoride (PVDF) according to the manufacturer's instructions (Bio-Rad). The gels were blotted for 1 h at 50 V using a Criterion blotter(Bio-Rad). The blotting membrane was probed with anti-FlaB antibody, followed by a 1∶10,000 dilution of goat anti-mouseimmunoglobulin G-alkaline phosphatase conjugate, and thealkaline phosphatase was detected using a chemiluminescence Western blotting kit (Roche Diagnostics) inaccordance with the manufacturer's instructions. Images were visualized with the UVP Imaging System.
Identification of flagellar genes under the control of σ28
The operons under the control of σ28 in E. coli, Salmonella typhimurium and V. cholerae were from references [13], [53]. Common σ28 binding motif identification, weight matrix construction, and genome screening were performed as described previously [43].
Supporting Information
Organization of flagellin genes in Shewanellae . Among sequenced Shewanella strains,12 including S. oneidensis possess two genes encoding flagellins of 265–275 a.a.. The second largest group consists of 5 strains including Shewanella sp. MR-4, MR-7, S. benthica, S. violacea, and S. frigidimarina, whosegenes encode flagellin subunits of 465–482 a.a.. S. baltica OS185 and OS195 possess four genes encoding flagellins, which appear to have resulted from transposition events. The other two strains S. pealeana and S. piezotolerans contain genes encoding flagellins of 393–394 a.a. and 434–463 a.a., respectively.
(PDF)
σ28-dependent genes in Shewanella oneidensis.
(PDF)
Acknowledgments
We thank Arthur Aronson (Purdue U. USA) for taking the time to carefully read and edit this paper, and Yuzhong Zhang (Shangdong U., China) for valuable assistance with the bacterial two-hybrid assay.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by Major State Basic Research Development Program (973 Progam: 2010CB833803) and by the Fundamental Research Funds for the Central Universities to HG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chevance FFV, Hughes KT. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Micro. 2008;6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moens S, Vanderleyden J. Functions of Bacterial Flagella. Crit Rev Microbiol. 1996;22:67–100. doi: 10.3109/10408419609106456. [DOI] [PubMed] [Google Scholar]
- 3.Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella motility chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 4.Young GM, Schmiel DH, Miller VL. A new pathway for the secretion of virulence factors by bacteria: The flagellar export apparatus functions as a protein-secretion system. Proc Natl Acad Sci USA. 1999;96:6456–6461. doi: 10.1073/pnas.96.11.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Childers SE, Ciufo S, Lovley DR. Geobacter metallireducens accesses insoluble Fe(iii) oxide by chemotaxis. Nature. 2002;416:767–769. doi: 10.1038/416767a. [DOI] [PubMed] [Google Scholar]
- 6.Caccavo F, Das A. Adhesion of dissimilatory Fe(III)-reducing bacteria to Fe(III) minerals. Geomicrobiol J. 2002;19:161–177. [Google Scholar]
- 7.Stewart BJ, McCarter LL. Lateral flagellar gene system of Vibrio parahaemolyticus. J Bacteriol. 2003;185:4508–4518. doi: 10.1128/JB.185.15.4508-4518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merino S, Shaw JG, Tomás JM. Bacterial lateral flagella: an inducible flagella system. FEMS Microbiol Lett. 2006;263:127–135. doi: 10.1111/j.1574-6968.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 9.Bardy SL, Ng SYM, Jarrell KF. Prokaryotic motility structures. Microbiology. 2003;149:295–304. doi: 10.1099/mic.0.25948-0. [DOI] [PubMed] [Google Scholar]
- 10.Macnab RM. How bacteria assemble flagella. Annu Rev Microbiol. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- 11.McCarter LL. Polar Flagellar Motility of the Vibrionaceae. Microbiol Mol Biol Rev. 2001;65:445–462. doi: 10.1128/MMBR.65.3.445-462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soutourina OA, Bertin PN. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol Rev. 2003;27:505–523. doi: 10.1016/S0168-6445(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 13.Prouty MG, Correa NE, Klose KE. The novel σ54- and σ28-dependent flagellar gene transcription hierarchy of Vibrio cholera. Mol Microbiol. 2001;39:1595–1609. doi: 10.1046/j.1365-2958.2001.02348.x. [DOI] [PubMed] [Google Scholar]
- 14.McCarter LL. Regulation of flagella. Curr Opin Microbiol. 2006;9:180–186. doi: 10.1016/j.mib.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Schoenhofen IC, McNally DJ, Brisson JR, Logan SM. Elucidation of the CMP-pseudaminic acid pathway in Helicobacter pylori: synthesis from UDP-N-acetylglucosamine by a single enzymatic reaction. Glycobiology. 2006;16:8–14. doi: 10.1093/glycob/cwl010. [DOI] [PubMed] [Google Scholar]
- 16.Schoenhofen IC, Vinogradov E, Whitfield DM, Brisson JR, Logan SM. The CMP-legionaminic acid pathway in Campylobacter: Biosynthesis involving novel GDP-linked precursors. Glycobiology. 2009;19:715–725. doi: 10.1093/glycob/cwp039. [DOI] [PubMed] [Google Scholar]
- 17.Nothaft H, Szymanski CM. Protein glycosylation in bacteria: sweeter than ever. Nat Rev Micro. 2010;8:765–778. doi: 10.1038/nrmicro2383. [DOI] [PubMed] [Google Scholar]
- 18.Thibault P, Logan SM, Kelly JF, Brisson JR, Ewing CP, et al. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J BiolChem. 2001;276:34862–34870. doi: 10.1074/jbc.M104529200. [DOI] [PubMed] [Google Scholar]
- 19.Ewing CP, Andreishcheva E, Guerry P. Functional characterization of flagellin glycosylation in Campylobacter jejuni 81–176. J Bacteriol. 2009;191:7086–7093. doi: 10.1128/JB.00378-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samatey FA, Imada K, Nagashima S, Vonderviszt F, Kumasaka T, et al. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature. 2001;410:331–337. doi: 10.1038/35066504. [DOI] [PubMed] [Google Scholar]
- 21.Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, et al. Towards environmental systems biology of Shewanella. Nat Rev Micro. 2008;6:592–603. doi: 10.1038/nrmicro1947. [DOI] [PubMed] [Google Scholar]
- 22.Heidelberg JF, Paulsen IT, Nelson KE, Gaidos EJ, Nelson WC, et al. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat Biotech. 2002;20:1118–1123. doi: 10.1038/nbt749. [DOI] [PubMed] [Google Scholar]
- 23.Pallen MJ, Matzke NJ. From the origin of species to the origin of bacterial flagella. Nat Rev Micro. 2006;4:784–790. doi: 10.1038/nrmicro1493. [DOI] [PubMed] [Google Scholar]
- 24.Liu R, Ochman H. Stepwise formation of the bacterial flagellar system. Proc Natl Acad Sci USA. 2007;104:7116–7121. doi: 10.1073/pnas.0700266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Kang Y, Choi O, Jeong Y, Jeong J-E, et al. Regulation of polar flagellum genes is mediated by quorum sensing and FlhDC in Burkholderia glumae. Mol Microbiol. 2007;64:165–179. doi: 10.1111/j.1365-2958.2007.05646.x. [DOI] [PubMed] [Google Scholar]
- 26.Paulick A, Koerdt A, Lassak J, Huntley S, Wilms I, et al. Two different stator systems drive a single polar flagellum in Shewanella oneidensis MR-1. Mol Microbiol. 2009;71:836–850. doi: 10.1111/j.1365-2958.2008.06570.x. [DOI] [PubMed] [Google Scholar]
- 27.Nealson KH, Scott J. Ecophysiology of the genus Shewanella. The Prokaryotes. 2006. Edited by D in M Springer-Verlag.
- 28.Thormann KM, Duttler S, Saville RM, Hyodo M, Shukla S, et al. Control of formation and cellular detachment from Shewanella oneidensis MR-1 biofilms by cyclic di-GMP. J Bacteriol. 2006;188:2681–2691. doi: 10.1128/JB.188.7.2681-2691.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang Y, Gao H, Chen J, Dong Y, Wu L, et al. Pellicle formation in Shewanella oneidensis. BMC Microbiol. 2010;10:291. doi: 10.1186/1471-2180-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Romine MF, Ward MJ. Identification and analysis of a highly conserved chemotaxis gene cluster in Shewanella species. FEMS Microbiol Lett. 2007;273:180–186. doi: 10.1111/j.1574-6968.2007.00810.x. [DOI] [PubMed] [Google Scholar]
- 31.Syed M, Karpinets TV, Leuze M, Kora G, Romine MF, et al. Shewregdb: Database and visualization environment for experimental and predicted regulatory information in Shewanella oneidensis MR-1. Bioinformation. 2009;4:169–172. doi: 10.6026/97320630004169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karpinets TV, Romine MF, Schmoyer DD, Kora GH, Syed MH, et al. Shewanella knowledgebase: integration of the experimental data and computational predictions suggests a biological role for transcription of intergenic regions. 2010. Databasedoi: 101093/database/baq012. [DOI] [PMC free article] [PubMed]
- 33.Martinez RM, Jude BA, Kirn TJ, Skorupski K, Taylor RK. Role of FlgT in anchoring the flagellum of Vibrio cholera. J Bacteriol. 2010;192:2085–2092. doi: 10.1128/JB.01562-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez RM, Dharmasena MN, Kirn TJ, Taylor RK. Characterization of two outer membrane proteins FlgO and FlgP that influence Vibrio cholerae motility. J Bacteriol. 2009;191:5669–5679. doi: 10.1128/JB.00632-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terashima H, Koike M, Kojima S, Homma M. The flagellar basal body-associated protein FlgT is essential for a novel ring structure in the sodium-driven Vibrio motor. J Bacteriol. 2010;192:5609–5615. doi: 10.1128/JB.00720-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouhenni RA, Vora GJ, Biffinger JC, Shirodkar S, Brockman K, et al. The role of Shewanella oneidensis MR-1 outer surface structures in extracellular electron transfer. Electroanalysis. 22:856–864. [Google Scholar]
- 37.Kuwajima G. Construction of a minimum-size functional flagellin of Escherichia coli. J Bacteriol. 1988;170:3305–3309. doi: 10.1128/jb.170.7.3305-3309.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capdevila S, Martinez-Granero FM, Sanchez-Contreras M, Rivilla R, Martin M. Analysis of Pseudomonas fluorescens F113 genes implicated in flagellar filament synthesis and their role in competitive root colonization. Microbiology. 2004;150:3889–3897. doi: 10.1099/mic.0.27362-0. [DOI] [PubMed] [Google Scholar]
- 39.Koerdt A, Paulick A, Mock M, Jost K, Thormann KM. MotX and MotY are required for flagellar rotation in Shewanella oneidensis MR-1. J Bacteriol. 2009;191:5085–5093. doi: 10.1128/JB.00206-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao H, Wang X, Yang Z, Palzkill T, Zhou J. Probing regulon of ArcA in Shewanella oneidensis MR-1 by integrated genomic analyses. BMC Genomics. 2008;9:42. doi: 10.1186/1471-2164-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao H, Yang ZK, Barua S, Reed SB, Romine MF, et al. Reduction of nitrate in Shewanella oneidensis depends on atypical NAP and NRF systems with NapB as a preferred electron transport protein from CymA to NapA. ISME J. 2009;3:966–976. doi: 10.1038/ismej.2009.40. [DOI] [PubMed] [Google Scholar]
- 42.Gao H, Barua S, Liang Y, Wu L, Dong Y, et al. Impacts of Shewanella oneidensis c-type cytochromes on aerobic and anaerobic respiration. Microbial Biotech. 2010;3:455–466. doi: 10.1111/j.1751-7915.2010.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao H, Wang X, Yang ZK, Chen J, Liang Y, et al. Physiological roles of Arca, Crp and Etra and their interactive control on aerobic and anaerobic respiration in Shewanella oneidensis. PLoS ONE. 2010;5:e15295. doi: 10.1371/journal.pone.0015295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neal-McKinney JM, Christensen JE, Konkel ME. Amino-terminal residues dictate the export efficiency of the Campylobacter jejuni filament proteins via the flagellum. Mol Microbiol. 2010;76:918–931. doi: 10.1111/j.1365-2958.2010.07144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshioka K, Aizawa S, Yamaguchi S. Flagellar filament structure and cell motility of Salmonella typhimurium mutants lacking part of the outer domain of flagellin. J Bacteriol. 1995;177:1090–1093. doi: 10.1128/jb.177.4.1090-1093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yonekura K, Maki-Yonekura S, Namba K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature. 2003;424:643–650. doi: 10.1038/nature01830. [DOI] [PubMed] [Google Scholar]
- 47.Samatey FA, Imada K, Nagashima S, Vonderviszt F, Kumasaka T, et al. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature. 2001;410:331–337. doi: 10.1038/35066504. [DOI] [PubMed] [Google Scholar]
- 48.Klose KE, Mekalanos JJ. Differential regulation of multiple flagellins in Vibrio cholera. J Bacteriol. 1998;180:303–316. doi: 10.1128/jb.180.2.303-316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guerry P, Alm RA, Power ME, Logan SM, Trust TJ. Role of two flagellin genes in Campylobacter motility. J Bacteriol. 1991;173:4757–4764. doi: 10.1128/jb.173.15.4757-4764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wassenaar TM, Bleumink-Pluym NM, van der Zeijst BA. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991;10:2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kearns DB. A field guide to bacterial swarming motility. Nat Rev Micro. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song W, Maiste PJ, Naiman DQ, Ward MJ. Sigma 28 promoter prediction in members of the Gammaproteobacteria. FEMS Microbiol Lett. 2007;271:222–229. doi: 10.1111/j.1574-6968.2007.00720.x. [DOI] [PubMed] [Google Scholar]
- 53.Kutsukake K, Ohya Y, Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol. 1990;172:741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turatsinze J-V, Thomas-Chollier M, Defrance M, van Helden J. Using RSAT to scan genome sequences for transcription factor binding sites and cis-regulatory modules. Nat Protocols. 2008;3:1578–1588. doi: 10.1038/nprot.2008.97. [DOI] [PubMed] [Google Scholar]
- 55.Anderson PE, Gober JW. FlbT the post-transcriptional regulator of flagellin synthesis in Caulobacter crescentus interacts with the 5′ untranslated region of flagellin mRNA. Mol Microbiol. 2000;38:41–52. doi: 10.1046/j.1365-2958.2000.02108.x. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto S, Kutsukake K. FljA-mediated posttranscriptional control of phase 1 flagellin expression in flagellar phase variation of Salmonella enterica serovar Typhimurium. J Bacteriol. 2006;188:958–967. doi: 10.1128/JB.188.3.958-967.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Douillard FP, Ryan KA, Caly DL, Hinds J, Witney AA, et al. Posttranscriptional regulation of flagellin synthesis in Helicobacter pylori by the RpoN chaperone HP0958. J Bacteriol. 2008;190:7975–7984. doi: 10.1128/JB.00879-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JS, Chang JH, Chung SI, Yum JS. Molecular cloning and characterization of the Helicobacter pylori fliD Gene an essential factor in flagellar structure and motility. J Bacteriol. 1999;181:6969–6976. doi: 10.1128/jb.181.22.6969-6976.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown JD, Saini S, Aldridge C, Herbert J, Rao CV, et al. The rate of protein secretion dictates the temporal dynamics of flagellar gene expression. Mol Microbiol. 2008;70:924–937. doi: 10.1111/j.1365-2958.2008.06455.x. [DOI] [PubMed] [Google Scholar]
- 60.Evdokimov AG, Phan J, Tropea JE, Routzahn KM, Peters HK, et al. Similar modes of polypeptide recognition by export chaperones in flagellar biosynthesis and type III secretion. Nat Struct Mol Biol. 2003;10:789–793. doi: 10.1038/nsb982. [DOI] [PubMed] [Google Scholar]
- 61.Ozin AJ, Claret L, Auvray F, Hughes C. The FliS chaperone selectively binds the disordered flagellin C-terminal D0 domain central to polymerization. FEMS Microbiol Lett. 2003;219:219–224. doi: 10.1016/S0378-1097(02)01208-9. [DOI] [PubMed] [Google Scholar]
- 62.Logan SM. Flagellar glycosylation - a new component of the motility repertoire? Microbiology. 2006;152:1249–1262. doi: 10.1099/mic.0.28735-0. [DOI] [PubMed] [Google Scholar]
- 63.Guerry P, Szymanski CM. Campylobacter sugars sticking out. Trends Microbiol. 2008;16:428–435. doi: 10.1016/j.tim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Schoenhofen IC, Lunin VV, Julien JP, Li Y, Ajamian E, et al. Structural and functional characterization of PseC, an aminotransferase involved in the biosynthesis of pseudaminic acid, an essential flagellar modification in Helicobacter pylori. J Biol Chem. 2006;281:8907–8916. doi: 10.1074/jbc.M512987200. [DOI] [PubMed] [Google Scholar]
- 65.Schoenhofen IC, McNally DJ, Vinogradov E, Whitfield D, Young NM, et al. Functional characterization of dehydratase/aminotransferase pairs from Helicobacter and Campylobacter. J Biol Chem. 2006;281:723–732. doi: 10.1074/jbc.M511021200. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto S, Kutsukake K. FliT acts as an anti-FlhD2C2 factor in the transcriptional control of the flagellar regulon in Salmonellaenterica serovar Typhimurium. J Bacteriol. 2006;188:6703–6708. doi: 10.1128/JB.00799-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yokoseki T, Kutsukake K, Ohnishi K, lino T. Functional analysis of the flagellar genes in the fliD operon of Salmonella typhimurium. Microbiology. 1995;141:1715–1722. doi: 10.1099/13500872-141-7-1715. [DOI] [PubMed] [Google Scholar]
- 68.Bennett JCQ, Thomas J, Fraser GM, Hughes C. Substrate complexes and domain organization of the Salmonella flagellar export chaperones FlgN and FliT. Mol Microbiol. 2001;39:781–791. doi: 10.1046/j.1365-2958.2001.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bresolin G, Trcek J, Scherer S, Fuchs TM. Presence of a functional flagellar cluster Flag-2 and low-temperature expression of flagellar genes in Yersinia enterocolitica W22703. Microbiology. 2008;154:196–206. doi: 10.1099/mic.0.2007/008458-0. [DOI] [PubMed] [Google Scholar]
- 70.Cameron DE, Urbach JM, Mekalanos JJ. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholera. Proc Natl Acad Sci USA. 2008;105:8736–8741. doi: 10.1073/pnas.0803281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canals R, Ramirez S, Vilches S, Horsburgh G, Shaw JG, et al. Polar flagellum biogenesis in Aeromonas hydrophila. J Bacteriol. 2006;188:542–555. doi: 10.1128/JB.188.2.542-555.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lambert C, Evans KJ, Till R, Hobley L, Capeness M, et al. Characterizing the flagellar filament and the role of motility in bacterial prey-penetration by Bdellovibrio bacteriovorus. Mol Microbiol. 2006;60:274–286. doi: 10.1111/j.1365-2958.2006.05081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minnich SA, Ohta N, Taylor N, Newton A. Role of the 25- 27- and 29-kilodalton flagellins in Caulobacter crescentus cell motility: method for construction of deletion and Tn5 insertion mutants by gene replacement. J Bacteriol. 1988;170:3953–3960. doi: 10.1128/jb.170.9.3953-3960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nuijten PJ, van Asten FJ, Gaastra W, van der Zeijst BA. Structural and functional analysis of two Campylobacter jejuni flagellin genes. J Biol Chem. 1990;265:17798–17804. [PubMed] [Google Scholar]
- 75.Kostrzynska M, Betts JD, Austin JW, Trust TJ. Identification characterization and spatial localization of two flagellin species in Helicobacter pylori flagella. J Bacteriol. 1991;173:937–946. doi: 10.1128/jb.173.3.937-946.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aldridge P, Hughes KT. Regulation of flagellar assembly. Curr Opin Microbiol. 2002;5:160–165. doi: 10.1016/s1369-5274(02)00302-8. [DOI] [PubMed] [Google Scholar]
- 77.Végh BM, Gál P, Dobó J, Závodszky P, Vonderviszt F. Localization of the flagellum-specific secretion signal in Salmonella flagellin. Biochem Bioph Res Co. 2006;345:93–98. doi: 10.1016/j.bbrc.2006.04.055. [DOI] [PubMed] [Google Scholar]
- 78.Wang S, Fleming RT, Westbrook EM, Matsumura P, McKay DB. Structure of the Escherichia coli FlhDC complex a prokaryotic heteromeric regulator of transcription. J Mol Biol. 2006;355:798–808. doi: 10.1016/j.jmb.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 79.Imada K, Minamino T, Kinoshita M, Furukawa Y, Namba K. Structural insight into the regulatory mechanisms of interactions of the flagellar type III chaperone FliT with its binding partners. Proc Natl Acad Sci USA. 2010;107:8812–8817. doi: 10.1073/pnas.1001866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang C, Rodionov DA, Li X, Laikova ON, Gelfand MS, et al. Comparative genomics and experimental characterization of N-acetylglucosamine utilization pathway of Shewanella oneidensis. J Biol Chem. 2006;281:29872–29885. doi: 10.1074/jbc.M605052200. [DOI] [PubMed] [Google Scholar]
- 81.Saltikov CW, Newman DK. Genetic identification of a respiratory arsenate reductase. Proc Natl Acad Sci USA. 2003;100:10983–10988. doi: 10.1073/pnas.1834303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao WM, Liu YQ, Giometti CS, Tollaksen SL, Khare T, et al. Knock-out of SO1377 gene which encodes the member of a conserved hypothetical bacterial protein family COG2268 results in alteration of iron metabolism increased spontaneous mutation and hydrogen peroxide sensitivity in Shewanella oneidensis MR-1. BMC Genomics. 2006;7:76. doi: 10.1186/1471-2164-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Organization of flagellin genes in Shewanellae . Among sequenced Shewanella strains,12 including S. oneidensis possess two genes encoding flagellins of 265–275 a.a.. The second largest group consists of 5 strains including Shewanella sp. MR-4, MR-7, S. benthica, S. violacea, and S. frigidimarina, whosegenes encode flagellin subunits of 465–482 a.a.. S. baltica OS185 and OS195 possess four genes encoding flagellins, which appear to have resulted from transposition events. The other two strains S. pealeana and S. piezotolerans contain genes encoding flagellins of 393–394 a.a. and 434–463 a.a., respectively.
(PDF)
σ28-dependent genes in Shewanella oneidensis.
(PDF)