Abstract
Background
Despite the increasing cure rates for children with acute lymphoblastic leukemia (ALL), patients who relapse continue to have poor prognosis. The Children’s Oncology Group (COG) conducted a limited institution Phase II trial of Campath-1H, a monoclonal antibody that targets CD52 on leukemic cells, in children with relapsed or refractory ALL.
Methods
From October 2005 through December 2006, 13 eligible patients were enrolled on the COG phase II study of Campath-1H (ADVL0222). Campath-1H was initially administered as an intravenous infusion over 2 hours, 5 times per week for 1 week, then 3 times per week for 3 additional weeks. Patients with stable disease or better on day 29 could continue on to combination therapy with Campath-1H, methotrexate, and 6-mercaptopurine for two additional cycles.
Results
One of 13 patients enrolled had a complete response to Campath-1H and four had stable disease. Dose limiting toxicity occurred in 2 out of 9 fully evaluable patients (Grade IV pain and Grade III allergic reaction/hypersensitivity). No patients received combination therapy. Serum Campath-1H concentrations appeared to be somewhat lower in children with ALL compared with adult patients with chronic lymphocytic leukemia.
Conclusion
Although a single complete response was observed, activity of single agent Campath-1H appears limited. Our study does not support future single agent evaluation of Campath-1H in children with relapsed ALL.
Keywords: Campath-1H, Acute Lymphoblastic Leukemia, Relapse
INTRODUCTION
Despite the success of treatment of childhood acute lymphoblastic leukemia (ALL) with cure rates exceeding 80%, the treatment outcomes in patients who relapse continues to be poor [1,2]. Of the approximately 25% of patients who relapse [3], only 40% in second or third relapse achieve a complete response and less than 10% are cured of their disease [4].
Campath-1H is a humanized monoclonal antibody that targets the CD52 antigen. CD52 is a 12 amino acid glycoprotein, membrane bound via a glycosylphosphatidylinositol lipid anchor, with a molecular weight of 21-28kDa [5]. CD52 is expressed on more than 95% of all normal B and T lymphocytes at various stages of differentiation, with the exception of plasma cells [6]. CD52 is highly expressed on lymphocytes, comprising up to an estimated 5% of the cell membrane surface area [7]. It is also present on cells of myeloid lineage, including monocytes, macrophage, eosinophils, and natural killer cells; however it is not present on granulocytes, erythrocytes, platelets, and hematopoietic stem cells [5]. CD52 is highly expressed in a variety of malignant cells, including T-cell prolymphocytic leukemia, CLL, hairy cell leukemia, NHL, and ALL [5,7,8].
Campath-1H targets CD52 on malignant cells and is hypothesized to initiate cell death via complement-dependent cytolysis, antibody-mediated cellular cytotoxicity, and apoptosis [8]. Thus, Campath-1H has been able to effectively remove malignant lymphocytes from the blood, bone marrow, and spleen without stem cell toxicity [9]. However, Campath-1H has not been as effective in eliminating tumor cells from lymph nodes and extra nodal masses [9]. Campath-1H has activity in patients with relapsed and refractory CLL, T-cell prolymphocytic leukemia, cutaneous T-cell lymphomas, and NHL [7,9-13]. Recently, Campath-1H has also shown utility in the treatment of autoimmune disease such as multiple sclerosis [14] and as immunosuppressive therapy for organ transplantation, stem cell transplantation, and graft vs. host disease [6].
The potential efficacy of Campath-1H for children with relapsed ALL has not been studied. We therefore conducted a Phase II trial of Campath-1H in children with ALL in second or greater relapse or primary induction failure after two different regimens. In this study, we planned to not only examine the effect of Campath-1H as monotherapy, but also when combined with methotrexate (MTX) and 6-mercaptopurine (MP).
METHODS
Eligibility
Patients enrolled on Children’s Oncology Group (COG) study ADVL0222 were 30 years of age or less at time of original diagnosis. Written informed consent/assent was obtained by parents/legal guardians and patients utilizing institutionally approved documents. Disease state at time of entry was ALL in second or greater bone marrow relapse or ALL that failed at least 2 regimens for remission induction; CD52 was expressed on at least 25% of malignant cells at relapse and patients with Ph+ ALL must have relapsed or progressed following treatment with imatinib mesylate. Patients had performance levels of Karnofsky ≥ 50% for age > 10years and Lansky ≥ 50 for age ≤ 10 years and had a life expectancy of ≥ 8 weeks. Patients must have fully recovered from any acute toxic effects from previous chemotherapy, immunotherapy or radiotherapy prior to study entry. Patients who relapsed while receiving standard ALL maintenance chemotherapy did not have to wait for study entry. Eligible patients had not received biological therapy for 8 weeks or radiation within 2 weeks of study entry. Post stem cell transplant (SCT) patients were eligible if ≥ 4 months post SCT and had no evidence of graft versus host disease or any prior Campath-1H therapy. All patients had acceptable organ function, a negative pregnancy test for post-menarche girls and did not receive any growth factor therapy within one week of study entry.
Study Design
Initial therapy consisted of single-agent Campath-1H. As acute adverse reactions, including fever, chills, hypotension, nausea and vomiting occur most frequently during the initial infusions of antibody, therapy began with a low dose that was then escalated within each individual patient. Campath-1H was administered as an intravenous infusion over 2 hours, 5 times per week for 1 week, then 3 times per week for 3 additional weeks (Table I). Treatment with preventive intrathecal chemotherapy (age adjusted MTX administered on day 1 of each cycle) or therapeutic intrathecal triple therapy (age adjusted MTX, cytarabine and hydrocortisone administered weekly until clearing of CSF blasts) was permitted. Patients with stable disease or better on day 29 could continue to receive combination therapy consisting of Campath-1H, intravenous and oral MTX, oral 6MP and intrathecal MTX or intrathecal triples.
TABLE I.
Course 1, Monotherapy Campath-1H
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|---|
| Week 1 | 0.06 mg/kg (max 3mg) plus ITT# |
0.2 mg/kg (max 9mg) |
0.3 mg/kg (max 15 mg) |
0.6 mg/kg (max 30 mg) |
0.6 mg/kg (max 30 mg) |
- | - |
| Week 2 | 0.6 mg/kg (max 30mg) plus ITT# |
- | 0.6 mg/kg (max 30mg) |
- | 0.6 mg/kg (max 30 mg) |
- | - |
| Week 3 | 0.6 mg/kg (max 30 mg) plus ITMTX* or ITT# |
- | 0.6 mg/kg (max 30 mg) |
- | 0.6 mg/kg (max 30 mg) |
- | - |
| Week 4 | 0.6 mg/kg (max 30 mg) plus ITT# |
- | 0.6 mg/kg (max 30 mg) |
- | 0.6 mg/kg (max 30 mg) |
- | - |
Response Criteria
All patients who received at least one dose of Campath-1H were considered evaluable for response assessment. Complete remission (CR) was defined as attainment of an M1 bone marrow (< 5% blasts) with no evidence of circulating blasts; partial remission (PR) as complete disappearance of circulating blasts and achievement of M2 marrow status (≥ 5% and < 25% blast cells); partial remission cytolytic (PRCL) as complete disappearance of circulating blasts and achievement of at least 50% reduction from baseline in bone marrow blast count; stable disease (SD) when the patient fails to qualify for either a CR, PR, PRCL or progressive disease; progressive disease (PD) as an increase of at least 25% in the absolute number of circulating absolute blast count, or development of new extramedullary disease.
Toxicity
Patients who received less than 85% of the intended first course doses and who did not develop dose limiting toxicity (DLT) were not considered fully evaluable for toxicity. Adverse events were graded according to the NCI Common Toxicity Criteria (version 3.0). Infusion related DLT was defined as any grade 3 or grade 4 toxicity associated with drug administration with the exception of grade 3 rigors/chills, grade 3 fever, grade 3 pruritis/itching without urticaria, grade 3 headache, grade 3 arthralgia, grade 3 myalgia and grade 3 bronchospasm/dyspnea (transient and in absence of other allergic reactions) not lasting for more than 24 hours.
Grade 3 and 4 non-hematological toxicities attributable to the investigational drug were also considered DLTs with the exception of grade 3 nausea and vomiting, grade 3 transaminase elevation ≤ 7 days in duration and grade 3 infection or fever. Hematologic DLT was defined as bone marrow aplasia lasting for more than 6 weeks; specifically, failure to recover with a peripheral ANC> 500/μL as documented by bone marrow aplasia without malignant infiltration, in bone marrow aspiration and/or biopsy.
Pharmacokinetics
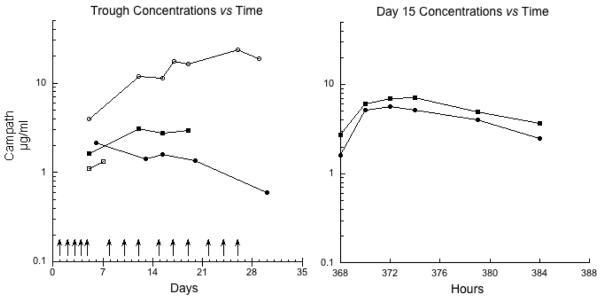
For subjects consenting to the pharmacokinetic portion of the study, blood samples were collected during the first treatment course. Extensive pharmacokinetic sampling occurred with the day 15 dose of antibody, along with acquisition of frequent trough concentration specimens, as detailed in Figure 1. Serum Camapth-1H concentrations were measured by BioAnaLab Limited (Oxford, UK) using a previously described, validated assay with a lower limit of quantification 0.5 μg/mL [15].
Figure 1.
Trough serum Campath-1H concentrations from 4 patients are shown in the left-panel, and the concentration time curves following the day 15 dose are shown in the right panel. Arrows denote days of antibody administration.
Serum samples were analyzed for the presence of anti-Campath-1H antibodies on day 1 and 29 of the first course. Antibodies were measured by BioAnaLab Limited (Oxford, UK) using a validated ELISA assay with a serum range of 444 to 8546 U/mL.
Statistical Analysis
A two-stage design was employed. Stage I required enrolling 10 patients. If none of the patients were to respond to single agent Campath-1H (CR, PR, PRCL), the agent would be defined as ineffective and the trial would be terminated. In case response was observed in ≥1 patient/s the trial proceeded to stage II. Stage II required enrolling an additional 15 patients of which if ≤4 patients responded, the agent will be declared ineffective and the trial will be terminated. On the other hand, if ≥ 5 patients responded, the agent will be defined as an effective agent and the trial will be terminated. This two stage design has a probability of 9.4% of erroneously concluding that the agent is effective when the true rate is 10 %, and probability of 0.90 of concluding agent as effective when the true response rate is 30%.
RESULTS
Patient Characteristics
Between October 2005 and December 2006, thirteen (8 male) patients, median (range) age 8 (3-20) years, were enrolled on study (Table II). Ten patients had B-precursor ALL and three had T cell disease. The median number (range) of prior chemotherapy regimens received was three (1-5). Six patients had prior bone marrow transplant. The median (range) of percent bone marrow CD52 positive cells at baseline in all eligible patients was 95% (56 -100%).
TABLE II.
Patient characteristics for eligible patients (n=13)
| Characteristic | Number (%) |
|---|---|
| Age (years) | |
| Median | 8 |
| Range | 3-20 |
| Sex | |
| Male | 8 (62) |
| Female | 5 (38) |
| Race | |
| White | 11 (85) |
| Black or African American | 1 (8) |
| Other | 1 (8) |
| Ethnicity | |
| Non-Hispanic | 10 (77) |
| Hispanic | 2 (15) |
| Puerto Rican | 1 (8) |
| Diagnosis | |
| B-precursor | 10 (77) |
| T Cell | 3 (23) |
| Prior Therapy | |
| Chemotherapy Regimens | |
| Median | 3 |
| Range | 1-5 |
| Number of Patients with Prior Radiation | 1 |
| Therapy | |
| Number of Patients with Prior Bone | 6 |
| Marrow Transplant |
Response
At the completion of course 1, only one patient had a complete response resulting in an objective response rate of 8% (95% confidence interval 0.2-36%), while 4 patients had stable disease. The CR occurred in a 6½ year old child previously treated for standard risk B-precursor ALL. Following initial relapse, the patient underwent two attempts at re-induction, initially with cytarabine/mitoxantrone and then with ifosfamide/etoposide. Upon study entry, the patient had a WBC of 0.2 ×103/microliter with 78% leukemic blasts on bone marrow aspirate. Upon achieving a CR, the patient was removed from protocol therapy with the intent to proceed to a stem cell transplant; the transplant was not pursued due to CMV reactivation. No patient proceeded onto the portion of the trial with combination therapy.
Toxicity
Four of the 13 patients were not considered fully evaluable for toxicity because they received less than 85% of the intended dose and did not develop DLT: two patients had early disease progression, one patient received incorrect dosing, and one patient was found to have Philadelphia chromosome positive leukemia and thus removed from study to be treated with imatinib (Table III). Of the nine patients fully evaluable for toxicity, two experienced DLT, one each with Grade IV pain and Grade III allergic reaction/hypersensitivity. The most common non-dose limiting grade II or greater events included grade II infusion related events, allergic reaction/hypersensitivity, hypotension, fever, rigors/chills, rash/desquamation, cough, edema and hypoxia (Table IV).
TABLE III.
Patient Outcome
| Subject Number |
Age (years) |
Diagnosis | # days of therapy | DLT | Response | Note |
|---|---|---|---|---|---|---|
| 1 | 9.6 | T cell | 6 | IE* | PD | |
| 2 | 9.4 | B-precursor | 28 | No | SD | |
| 3 | 5.2 | B-precursor | 4 | IE* | - | Found to have Ph+ ALL |
| 4 | 6.8 | B-precursor | 28 | No | CR | |
| 5 | 20.8 | T cell | 45 | No | SD | |
| 6 | 15.2 | B-precursor | 42 | No | SD | |
| 7 | 11.0 | B-precursor | 29 | No | SD | |
| 8 | 7.2 | B-precursor | 25 | No | PD | |
| 9 | 8.0 | B-precursor | 1 | Yes | - | Grade 4 infusion related toxicity lasting > 24 hrs |
| 10 | 3.2 | T cell | 1 | Yes | - | Grade 3 infusion related toxicity. Family withdrew |
| 11 | 7.2 | B-precursor | 4 | IE* | - | Incorrect doses administered |
| 12 | 4.4 | B-precursor | 25 | No | PD | |
| 13 | 9.2 | B-precursor | 18 | IE* | PD |
Patient not fully evaluable for toxicity if they received less than 85 % of the intended dose; IE, inevaluable; CR, complete remission; SD, stable disease; PD, progressive disease.
TABLE IV.
Course 1 Non-Hematologic toxicities related to protocol therapy observed in 9 evaluable patients.
| Toxicity Type | Course 1 (total, 9 courses) | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Allergic reaction/hypersensitivity (including drug fever) | 1 | 2 | ||
| Hypotension | 1 | |||
| Fever (in the absence of neutropenia, where neutropenia is defined as ANC <1.0 × 10e9/L) |
3 | |||
| Rigors/chills | 1 | 4 | ||
| Rash/desquamation | 2 | |||
| Diarrhea | 1 | |||
| Febrile neutropenia (fever of unknown origin without clinically or microbiologically documented infection)(ANC <1.0 × 10e9/L, fever >=38.5 d |
1 | |||
| Hypoalbuminemia | 2 | |||
| ALT, SGPT | 1 | |||
| AST, SGOT | 1 | |||
| Hypocalcemia | 1 | |||
| Hypercalcemia | 1 | |||
| Hyperglycemia | 1 | |||
| Hypomagnesemia | 1 | |||
| Hypokalemia | 2 | |||
| Hypernatremia | 1 | |||
| Hyponatremia | 1 | |||
| Pain Buttock | 1 | |||
| Pain headache | 2 | |||
| PAIN NOS | 1 | |||
| Cough | 1 | |||
| Edema, larynx | 1 | |||
| Hypoxia | 1 | |||
Campath-1H Pharmacokinetics and Immunogenicity
Samples for pharmacokinetics were obtained in four subjects (Figure 1). The median (range) peak Campath-1H concentration post initial dose was 6.5 μg/ml (1.3-23.5). A total of 11 samples were examined for anti-Campath-1H antibodies. All samples were below the limit of quantification for anti-Campath-1H antibodies (< 444 U/ml).
DISCUSSION
In children with relapsed ALL, Campath-1H has limited activity, with 1/13 children achieving a CR. If the two patients who received an inadequate dose are excluded, the estimated response rate is response rate is 9% (1/11) with a 95% CI of 0.2-41.3%. Despite the initial intent to proceed to stage 2 of this trial, poor accrual led to termination of the trial. This study highlights some of the challenges of developing novel therapeutic agents for children with relapsed ALL, as the rapidity of disease progression, coupled with the desire of clinicians to use multi-agent therapy or to proceed to repeated transplants, limit enrollment to single agent novel therapy trials, even of short duration.
Overall, children with ALL appeared to tolerate Campath-1H reasonably well. As shown in Table IV, two of the 9 patients who were fully evaluable for toxicity had DLT (Grade IV pain and Grade III allergic reaction/hypersensitivity). This experience appears similar to adult trials in other hematologic malignancies [11,12,16,17].
Studying the pharmacokinetics of Campath-1H is inherently challenging, as the antibody is known to have non-linear, time-dependent pharmacokinetics [18]. With its multiple-dosing schedules and dependence of WBC count for clearance, Campath-1H’s half-life increases over consecutive doses and clearance decreases as the tumor burden is decreased. [18]. Our limited data suggest that drug exposure in children with ALL may be somewhat lower than that observed in adults with CLL (Figure 1). Mould et al. found that adult patients with CLL who had Campath-1H trough concentrations >13.2 μg/mL had a 50% chance of achieving either CR or PR [18], while Montillo et al. found that all patients with an AUC0-12 >5 μg h−1 ml−1 following Campath-1H administered as consolidation therapy achieved a CR [19]. In a study of 30 CLL patients, Hale et al. found that higher blood concentrations correlated with better clinical response, with peak plasma concentrations ranging from 2.8 μg/ml to 26.4 μg/mL (mean 10.7 μg/ml) and trough ranging from less than 0.5 μg/mL to 18.3 μg/ml (mean 5.4 μg/mL) [20]. If confirmed, the lower exposures in children may potentially be the result of more rapid clearance.
Although we were unable to determine the efficacy of the combination of Campath-1H with chemotherapy, limited data from adult trials support such an approach. Kennedy et al. showed the utility of Campath-1H in combination therapy in which 5 out of 6 patients with B-cell CLL, who were previously refractory to both Campath-1H and fludarabine alone, responded to combination therapy [12]. More recently, Elter et al. [17] studied the efficacy of combination therapy with Campath-1H and fludarabine in a larger cohort of relapsed CLL patients, with an overall response rate of 83%. Of the 36 patients with B-CLL who were recruited, 22 had previously been treated with fludarabine either as a single agent or in combination with other cytotoxic agents or rituximab; nine of these 22 patients (41%) were refractory to fludarabine treatment; four of the 36 patients were treated with alemtuzumab alone (agents received greater than 6 mo prior to study entry). Of the nine (41%) of 22 patients that had experienced treatment failure with fludarabine, six of these patients responded to fludarabine and Campath-1H. Three of four patients with Campath 1-H pretreatment showed a partial response and one achieved stable disease.
As a single agent, Campath-1H appears to have limited efficacy for the treatment of children with relapsed ALL. Novel trial designs will need to be pursued for the development of new agents in children with ALL, as accrual and evaluability for treatment tolerability remain ongoing challenges. One such approach was demonstrated with our recently completed phase 1 trial of the anti-CD22 antibody epratuzumab [21]. As a single agent, Campath-1H appears to have limited efficacy for the treatment of children with relapsed ALL. Novel trial designs will need to be pursued for the development of new agents in children with ALL, as accrual and evaluability for treatment tolerability remain ongoing challenges.
ACKNOWLEDGMENT
Jeffrey Pride Fund for Leukemia Research and the Jeffrey Pride Foundation Grants U-01 CA09452; U-10 CA98413 and U-10 CA98543 of the Children’s Oncology Group from the National Cancer Institute, N.I.H. We thank Bayer Schering Pharma for providing Campath-1H for this clinical trial.
Footnotes
Conflict of Interest The authors have no present or perceived conflicts of interest to disclose.
REFERENCES
- 1.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371(9617):1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Abreu D, Bordoni A, Zucca E. Epidemiology of hematological malignancies. Ann Oncol. 2007;18(Suppl 1):i3–i8. doi: 10.1093/annonc/mdl443. [DOI] [PubMed] [Google Scholar]
- 3.Moppett J, Burke GA, Steward CG, et al. The clinical relevance of detection of minimal residual disease in childhood acute lymphoblastic leukaemia. J Clin Pathol. 2003;56(4):249–253. doi: 10.1136/jcp.56.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131(5):579–587. doi: 10.1111/j.1365-2141.2005.05773.x. [DOI] [PubMed] [Google Scholar]
- 5.Tibes R, Keating MJ, Ferrajoli A, et al. Activity of alemtuzumab in patients with CD52-positive acute leukemia. Cancer. 2006;106(12):2645–2651. doi: 10.1002/cncr.21901. [DOI] [PubMed] [Google Scholar]
- 6.Magliocca JF, Knechtle SJ. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl Int. 2006;19(9):705–714. doi: 10.1111/j.1432-2277.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 7.Alinari L, Lapalombella R, Andritsos L, et al. Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene. 2007;26(25):3644–3653. doi: 10.1038/sj.onc.1210380. [DOI] [PubMed] [Google Scholar]
- 8.Rodig SJ, Abramson JS, Pinkus GS, et al. Heterogeneous CD52 expression among hematologic neoplasms: implications for the use of alemtuzumab (CAMPATH-1H) Clin Cancer Res. 2006;12(23):7174–7179. doi: 10.1158/1078-0432.CCR-06-1275. [DOI] [PubMed] [Google Scholar]
- 9.Robak T. Monoclonal antibodies in the treatment of chronic lymphoid leukemias. Leuk Lymphoma. 2004;45(2):205–219. doi: 10.1080/1042819031000139666. [DOI] [PubMed] [Google Scholar]
- 10.Dearden CE, Matutes E, Cazin B, et al. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood. 2001;98(6):1721–1726. doi: 10.1182/blood.v98.6.1721. [DOI] [PubMed] [Google Scholar]
- 11.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99(10):3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy B, Rawstron A, Carter C, et al. Campath-1H and fludarabine in combination are highly active in refractory chronic lymphocytic leukemia. Blood. 2002;99(6):2245–2247. doi: 10.1182/blood.v99.6.2245. [DOI] [PubMed] [Google Scholar]
- 13.Rai KR, Freter CE, Mercier RJ, et al. Alemtuzumab in previously treated chronic lymphocytic leukemia patients who also had received fludarabine. J Clin Oncol. 2002;20(18):3891–3897. doi: 10.1200/JCO.2002.06.119. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen PS. The gap between effect of drugs and effectiveness of treatments. J Neurol Sci. 2007;259(1-2):128–132. doi: 10.1016/j.jns.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Rebello P, Hale G. Pharmacokinetics of CAMPATH-1H: assay development and validation. J Immunol Methods. 2002;260(1-2):285–302. doi: 10.1016/s0022-1759(01)00556-7. [DOI] [PubMed] [Google Scholar]
- 16.Keating MJ, O’Brien S, Kontoyiannis D, et al. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43(9):1755–1762. doi: 10.1080/1042819021000006547. [DOI] [PubMed] [Google Scholar]
- 17.Elter T, Borchmann P, Schulz H, et al. Fludarabine in combination with alemtuzumab is effective and feasible in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: results of a phase II trial. J Clin Oncol. 2005;23(28):7024–7031. doi: 10.1200/JCO.2005.01.9950. [DOI] [PubMed] [Google Scholar]
- 18.Mould DR, Baumann A, Kuhlmann J, et al. Population pharmacokinetics-pharmacodynamics of alemtuzumab (Campath) in patients with chronic lymphocytic leukaemia and its link to treatment response. Br J Clin Pharmacol. 2007;64(3):278–291. doi: 10.1111/j.1365-2125.2007.02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montillo M, Tedeschi A, Miqueleiz S, et al. Alemtuzumab as consolidation after a response to fludarabine is effective in purging residual disease in patients with chronic lymphocytic leukemia. J Clin Oncol. 2006;24(15):2337–2342. doi: 10.1200/JCO.2005.04.6037. [DOI] [PubMed] [Google Scholar]
- 20.Hale G, Rebello P, Brettman LR, et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood. 2004;104(4):948–955. doi: 10.1182/blood-2004-02-0593. [DOI] [PubMed] [Google Scholar]
- 21.Raetz EA, Cairo MS, Borowitz MJ, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children’s Oncology Group Pilot Study. J Clin Oncol. 2008;26(22):3756–3762. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]