Abstract
Background
Topiramate has been recognized as a drug that can induce memory and cognitive impairment. Using the one-trial inhibitory avoidance task, we sought to verify the effect of topiramate on consolidation and extinction of aversive memory. Our hypothesis was that topiramate inhibits the consolidation and enhances the extinction of this fear memory.
Methods
In experiment 1, which occured immediately or 3 hours after training, topiramate was administered to rats, and consolidation of memory was verified 18 days after the conditioning session. In experiment 2, which occured 18–22 days after the training session, rats were submitted to the extinction protocol. Rats received topiramate 14 days before or during the extinction protocol.
Results
Topiramate blocked fear memory retention (p < 0.01) and enhanced fear memory extinction (p < 0.001) only when administered during the extinction protocol.
Limitations
This experimental design did not allow us to determine whether topiramate also blocked the reconsolidation of fear memory.
Conclusion
Topiramate diminishes fear memory consolidation and promotes extinction of inhibitory avoidance memory.
Introduction
Topiramate is an antiepileptic drug with a complex molecular action, which includes stimulation of the γ-aminobutyric acid (GABA)ergic system, inhibition of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/ kainate glutamatergic receptors, activity-dependent attenuation of voltage-dependent sodium and calcium currents1 and inhibition of carbonic anhydrase.2 Topiramate has been recognized as a drug that can induce memory and cognitive impairment in doses effective for the treatment of epilepsy.3
Topiramate has been proposed for the treatment of some psychiatric disorders, such as alcohol abuse,4 bulimia and binge-eating disorder5 and, more recently, borderline personality disorder (BPD)6 and posttraumatic stress disorder (PTSD).7 Borderline personality disorder can be included in the new proposed category of overconsolidational spectrum disorders8 since it is associated with child abuse and neglect.9 Facilitation of memory extinction is one of the possible mechanisms by which topiramate is effective in the treatment of BPD and PTSD.10
To verify whether topiramate is effective in the acquisition and extinction of fear memory conditioning, we used one-trial inhibitory avoidance, a task in which an unconditioned stimulus (e.g., a shock given to the paws) is paired with a conditioned stimulus (e.g., the place where the shock is administered). In subsequent sessions, the presentation of only the conditioned stimulus extinguishes the previous learning.11
Methods
All animal procedures complied with institutional and national guidelines for animal care and were approved by the Ethics Committee for Animal Research of the Pontificia Universidade Católica do Rio Grande do Sul.
We used 83 adult male Wistar rats (270–340 g) from the Fundação Estadual de Produção de Pesquisa em Saúde (Porto Alegre). They were caged in groups of 5 with free access to food and water and maintained on a 12-hour light/ dark cycle (light on at 7:00 am) at a mean temperature of 23°C (standard deviation 1°C).
Behavioural procedures
Conditioning session
In the conditioning session, each animal was submitted to one-trial step-down inhibitory avoidance.11 The apparatus used was a transparent acrylic box measuring 50 × 25 × 25 cm and whose floor consisted of parallel 1.0-mm diameter stainless steel wires spaced 1.0 cm apart. A Formica-covered wooden platform measuring 7.0 × 5.0 × 25.0 cm was located on the left side of the floor. The animal was placed on the platform, and immediately after stepping down and placing its 4 paws on the grid, it received a 1.0-mA 2-second scrambled electric footshock.
Experiment 1: effect of topiramate on conditioned fear memory consolidation
Animals were divided in 3 groups. Group 1, the control group (n = 16), received a single dose of apple juice (Sulavan) diluted in 50% water by gavage immediately after the fear conditioning session. Group 2 (n = 13) received a single 10-mg/kg dose of topiramate (Topamax; Janssen-Cilag) by gavage immediately after the conditioning session. Group 3 (n = 13) received a single 10-mg/kg dose of topiramate by gavage 3 hours after the conditioning session.
Eighteen days after the conditioning session, all animals were submitted to a retention test session, during which the animals were again placed on the training box platform, and we measured step-down latency up to a maximum of 600 seconds.
Experiment 2: effect of topiramate on conditioned fear memory extinction
Animals were divided in 3 groups. Group 1, the control group (n = 16), received apple juice (Sulavan) diluted in 50% water by gavage twice a day at 9 am and 5 pm for 14 days beginning 36 hours after the conditioning session (day 3). After a 24-hour interval (on day 18 after the conditioning session), they were submitted to the extinction protocol in which each animal was placed on the platform once a day during 5 consecutive days (days 18–22 after the conditioning session) and again after a 1-day interval (day 24 after the conditioning session). During the first 5 extinction procedure days, the animals received apple juice as described earlier. After each extinction session, the animal was placed in its residence cage. Group 2 (n = 13) underwent the same procedures as the control group, but received 10 mg/kg of topiramate administered by gavage twice daily (9 am and 5 pm) instead of the apple juice during the first gavage period, beginning 36 hours after the conditioning session and continuing for 14 days (days 3–16 after the conditioning session). Group 3 (n = 13) also underwent the same experimental procedures as the control group, but with the administration of 10 mg/kg of topiramate twice daily (9 am and 5 pm) instead of the apple juice during the second gavage period (i.e., during the extinction sessions from days 18–22 after the conditioning session).
Extinction session
In the extinction session, each animal was placed on the platform, and we measured step-down latency up to a maximum of 600 seconds. No electric shock was administered, and the animal was allowed to stay on the metal base for 30 seconds to permit learning.
Statistical analyses
Since the variable being analyzed (step-down latency) does not follow a normal distribution and we limited the observation to 600 seconds, data are expressed as medians and inter-quartile ranges. We analyzed the data using the Friedman test for repeated measures or the Kruskal–Wallis analysis of variance, followed by the Mann–Whitney nonparametric test.
Results
Experiment 1
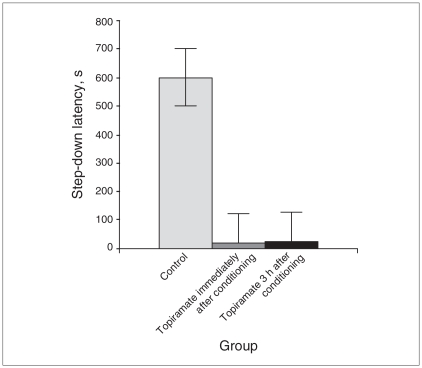
The effect of topiramate in inhibitory avoidance memory consolidation is shown in Figure 1. When given immediately or 3 hours postconditioning, topiramate blocked memory retention, as evaluated 14 days after conditioning: D2 = 10.32, p = 0.006.
Fig. 1.
Consolidation of inhibitory avoidance memory is inhibited by a single dose of topiramate (10 mg/kg) administrated immediately (n = 13) or 3 hours after conditioning (n = 13) in comparison with a control group (n = 16). Animals were tested 18 days after training. We measured step-down latency in seconds and presented the results as medians and interquartile ranges.
Experiment 2
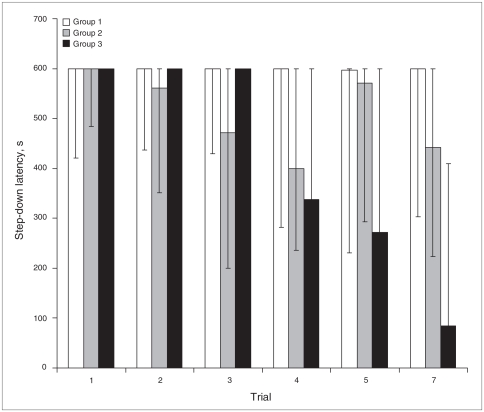
The effect of topiramate in inhibitory avoidance memory extinction is shown in Figure 2. Topiramate was effective in promoting memory extinction when administrated during the extinction procedure (group 3; F13,5 = 21.44, p < 0.001), but it was ineffective if administered for 2 weeks before extinction (group 2; F14,5 = 10.34, p = 0.07). The reduction of step-down latency decreased between each section of exposure but was statistically significant only in the last trial of extinction (i.e., on day 24 after the conditioning session): D2 = 9.60, p = 0.008.
Fig. 2.
Extinction of inhibitory avoidance memory was enhanced in the last trial of extinction (2 d after the last drug administration) in group 3 (n = 13; 10 mg/kg of topiramate twice daily at 9 am and 5 pm administered during the first 5 d of extinction procedure, p < 0.001) but not in group 2 (n = 13, 10 mg/kg of topiramate twice daily at 9 am and 5 pm administered for 2 wk before extinction, p = 0.07). We measured step-down latency in seconds and presented the results as medians and interquartile ranges (P25 – P75).
Discussion
These experiments were an attempt to verify whether topiramate is capable of modulating consolidation and/or extinction of fear memory. On the other hand, owing to the highly complex action mechanisms of this drug,1 which involve numerous neurochemical targets, it might be difficult to determine the role of topiramate in memory processing.
Topiramate inhibited consolidation of one-trial inhibitory avoidance and enhanced its extinction. Among the multiple neurochemical actions of this drug, the antagonist effect on AMPA/kainite glutamate receptors, attenuation of voltage-dependent calcium currents and inhibition of carbonic anhydrase could partially explain our results.
The inhibition of consolidation of one-trial inhibitory avoidance suggests that topiramate could be useful for the prevention of PTSD. The effect may be owing to the drug’s antagonistic effect on AMPA receptors since one-trial memory consolidation appears to be accompanied and followed by AMPA receptor–dependent processes11,12 in the hippocampus, amygdala and other brain regions. Topiramate has a smaller effect on AMPA receptors than kainite receptors, although even at low concentrations this antagonistic effect on AMPA receptors can be demonstrated.1 The attenuation of voltage-dependent calcium currents promoted by topiramate could also play a role in the inhibition of memory consolidation.11
Similarly, the inhibition of carbonic anhydrase may play a role, as it prevents this enzyme from switching the effect of GABA from an inhibitory to an excitatory one.2 This enzyme catalyzes the reversible reaction between CO2 hydration and HCO3 dehydration. The activation of the enzyme promotes a rapid rise in the levels of HCO3 in memory-related neural circuits.2,13,14 The increased HCO3 efflux through the GABAA channels facilitates depolarization of the cell, thus switching the effect of GABA activation from an inhibitory to an excitatory one.13 Carbonic anhydrase inhibition maintains the inhibitory action of GABA, impeding the flexible role of this receptor.2 Several mechanisms, such as intracellular release of Ca+, activate carbonic anhydrase. Topiramate inhibits the activation of isoenzymes I–IV of carbonic anhydrase.15 This may be an important mechanism involved in the impairment of the consolidation of fear conditioning, since the central administration of acetazolamide (a sulfonamide compound, as is topiramate) impairs spatial learning,16 which is a kind of memory biochemically and anatomically related to one-step avoidance fear memory.11
In the second experiment, we observed enhanced memory extinction in the group that received topiramate during repeated exposure to the same fear-inducing situation. This finding is in accordance with what was expected. Memory extinction is a special kind of learning17 that is context-dependent.18 To allow extinction, the animal must be repeatedly placed in the same context where the fear was conditioned but without the aversive stimulus. Thus, it can learn that this context, that was previously aversive, fails to produce aversion. Since topiramate may have impaired memory retrieval,3 we withdrew the medication after 5 extinction sessions and retested the animals 2 days later. As they stepped down quickly, we can conclude that the conditioned fear memory was extinguished and that topiramate somehow facilitated this extinction.
To extinguish memory, the rat must step down at some time during the extinction procedure. If it fails to do so, the rat will not learn that the electric shock has been withdrawn. So, in the case of an intense fear memory, which can even produce freezing, the rat must be less fearful to face the challenge of trying to step down from the platform. Topiramate can decrease anxiety through its action on GABA receptors,19,20 and this effect may contribute to allowing animals to step down.
Topiramate may improve fear memory extinction in 2 different ways: by decreasing fear owing to its sedative and antianxiety effects and allowing the animal to step down from the platform and learn, or by increasing learning itself. Enhancement of learning is not an effect expected by clinicians familiar with the use of topiramate, since this drug may provoke cognitive side effects that include memory complaints.3 However, the neurobiologic effect produced by topiramate involves brain structures, circuits and receptors that are implicated in memory extinction.
Extinction involves mainly the ventral medial prefrontal cortex,17,21 amygdala22 and hippocampus.11,18 The amygdala has a central role in fear extinction, particularly the basolateral amygdala, as demonstrated in several different ways, especially with electrophysiologic studies.22 The GluR5 kainate receptors are highly expressed in the basolateral and medial nuclei of the amygdala and hippocampus,23 and topiramate has an antagonist effect on these receptors.1 In addition, GluR5 is presynaptically present in GABA neurons, modulating the release of GABA in a dose-dependent, bidirectional manner.24 A low concentration of glutamate, as well as basal activity, facilitates the release of GABA, but high levels of glutamate suppress GABA release from the interneuron to pyramidal cells in the basolateral amygdala.24 Topiramate’s inhibition of GluR5 may allow the release of GABA even with high glutamate activity. γ-Aminobutyric acid neuro-transmission seems to be involved in fear extinction: glutamatergic inputs from the medial prefrontal cortex synapse on amygdala GABA neurons, and in this way, may provide an important inhibitory effect to the amygdala.25 Furthermore, the infusion of the GABA agonist muscimol to the basolateral amygdala after the extinction session facilitated extinction.24
Prior to extinction, memories must be retrieved. This is a complex mechanism involving different areas of the brain, such as the hippocampus, entorhinal cortex, posterior parietal and cingulated cortices and basolateral amygdala.26 Among the multiple neurochemical mechanisms involved in retrieval, an AMPA activation–dependent process in the amygdala is required.27 The amygdala does not participate in the informational process itself (that is mainly carried out by the hippocampus) but plays a modulatory role in retrieval.28 In the case of fear memory conditioning, the amygdala adds aversive emotional significance to memories, enhancing some aspects of the experience but impairing others that are not relevant for the emotional meaning attributed.29 It has also been demonstrated that extinction promotes diminished GluR1 AMPA subunit expression in the basolateral amygdala.22 Topiramate has an antagonist effect on AMPA receptors,1 which may have diminished the emotional (fear) significance of the memory to be extinguished in our experiment, thus allowing the rat to step down from the platform to the wire grid and preventing these memories from being reconsolidated.
Limitations
A problem associated with the investigation of the effect of a drug on memory extinction is the need to verify whether there is a true effect on extinction or whether the effect is due to a state-dependent memory phenomenon (i.e., when the physiologic state of the organism promotes or inhibits memory retrieval). Our results seem to be owing to extinction since the rat stepped down quickly in the absence of the drug on the last day of the experiment. In the case of state dependency, the effect would probably be the opposite.
Finally, at first sight it may seem incongruent that a drug that promotes extinction, a process that requires new learning, could also inhibit consolidation of a fear memory. Here, we can speculate as to whether the mechanisms involved in fear memory consolidation also have a synergic effect on fear memory extinction. To be extinguished, these memory traces must be evoked, and as they provoke fear, these memories may be reconsolidated. Mechanisms that diminish fear consolidation may also diminish the probability of memory reconsolidation.
Conclusion
Our results offer 2 opportunities for clinical investigation: first, the use of topiramate as a drug for the prevention of PTSD and, second, for extinction of overconsolidated traumatic memories, such as those that possibly occur in BPD. This may allow 2 original approaches in psychopharmacology: prevention based on diminished memory consolidation and treatment based on enhanced memory extinction.
Footnotes
Competing interests: Dr. Prado-Lima declared having received travel support from Janssen-Cilag, Eli Lilly and Novartis, as well as consultancy and lecture fees from Wyeth. Dr. Kristensen declared having received travel assistance from Novartis. None declared for Drs. Perrenoud, Cammarota and Izquierdo.
Contributors: All authors helped design the study and approved the article for publication. Dr. Perrenoud acquired the data, which was analyzed by all other authors. Drs. Prado-Lima and Kristensen wrote the article, which Drs. Perrenoud, Cammarota and Izquierdo reviewed.
References
- 1.Gryder DS, Rogawski MA. Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J Neurosci. 2003;23:7069–74. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun MK, Alkon DL. Carbonic anhydrase gating of attention: memory therapy and enhancement. Trends Pharmacol Sci. 2002;23:83–9. doi: 10.1016/s0165-6147(02)01899-0. [DOI] [PubMed] [Google Scholar]
- 3.Lee HW, Jung DK, Suh CK, et al. Cognitive effects of low-dose topiramate monotherapy in epilepsy patient: a 1-year follow-up. Epilepsy Behav. 2006;8:736–41. doi: 10.1016/j.yebeh.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomized controlled trial. Lancet. 2003;361:1677–85. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- 5.McElroy SL, Hudson JI, Capece JA, et al. Topiramate for treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry. 2007;61:1039–48. doi: 10.1016/j.biopsych.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt do Prado-Lima PA, Bacaltchuck J. Topiramate in treatment-resistant depression and binge-eating disorder. Bipolar Disord. 2002;4:271–3. doi: 10.1034/j.1399-5618.2002.01182.x. [DOI] [PubMed] [Google Scholar]
- 7.Tucker P, Trautman RP, Wyatt DB, et al. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68:201–6. doi: 10.4088/jcp.v68n0204. [DOI] [PubMed] [Google Scholar]
- 8.Bracha HS. Human brain evolution and the “neuroevolutionary time-depth principle:” implications for the reclassification of fear-circuitry-related traits in DSM-V and for studying resilience to warzone-related posttraumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:827–53. doi: 10.1016/j.pnpbp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley R, Jenei J, Westen D. Etiology of borderline personality disorder: disentangling the contributions of intercorrelated antecedents. J Nerv Ment Dis. 2005;193:24–31. doi: 10.1097/01.nmd.0000149215.88020.7c. [DOI] [PubMed] [Google Scholar]
- 10.do Prado-Lima P, Kristensen CH, Bacaltchuck J. Can childhood trauma predict response to topiramate in borderline personality disorder? J Clin Pharm Ther. 2006;31:193–6. doi: 10.1111/j.1365-2710.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 11.Izquierdo I, Bevilaqua LRM, Rossato JI, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- 13.Collin C, Devane WA, Dahl D, et al. Long-term synaptic transformation of hippocampal CA1 gamma-aminobutyric acid synapses and the effect of anandamide. Proc Natl Acd Sci U S A. 1995;92:10167–71. doi: 10.1073/pnas.92.22.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun MK, Alkon DL. Pharmacological enhancement of synaptic efficacy, spatial learning, and memory through carbonic anhydrase activation in rats. J Pharmacol Exp Ther. 2001;297:961–7. [PubMed] [Google Scholar]
- 15.Leniger T, Thöne J, Wiemann M. Topiramte modulates pH of hyppocampal CA3 neurons by combined effects of carbonic anhydrase and Cl−/HCO3− exchange. Br J Pharmacol. 2004;142:831–42. doi: 10.1038/sj.bjp.0705850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun MK, Zhao WK, Nelson TJ, et al. Theta rhythm of hyppocampal CA1 neuron activity: gating by GABAergic synaptic depolarization. J Neurophysiol. 2001;85:269–79. doi: 10.1152/jn.2001.85.1.269. [DOI] [PubMed] [Google Scholar]
- 17.Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–36. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Bouton ME, Westbrook RF, Corcoran KA, et al. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–60. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Kuzniecky R, Ho S, Pan J, et al. Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology. 2002;58:368–72. doi: 10.1212/wnl.58.3.368. [DOI] [PubMed] [Google Scholar]
- 20.White HS, Brown SD, Woodhead JH, et al. Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia. 2000;41 (Suppl 1):S17–20. [PubMed] [Google Scholar]
- 21.Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Barad M, Gean PW, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry. 2006;60:322–8. doi: 10.1016/j.biopsych.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Aroniadou-Anderjaska V, Qashu F, Braga MFM. Mechanisms regulating GABAergic inhibitory transmission in the basolateral amygdala: implications for epilepsy and anxiety disorders. Amino Acids. 2007;32:305–15. doi: 10.1007/s00726-006-0415-x. [DOI] [PubMed] [Google Scholar]
- 24.Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABAA agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;23:758–64. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- 25.Akirav I, Maroun M. The role of the medial prefrontal cortex–amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007;2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cammarota M, Bevilaqua LRM, Barros DM, et al. Retrieval and the extinction of memory. Cell Mol Neurobiol. 2005;25:465–74. doi: 10.1007/s10571-005-4009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barros DM, Izquierdo LA, Mello e Souza T, et al. Molecular signaling pathways in the cerebral cortex are required for retrieval of one-trial avoidance learning in rats. Behav Brain Res. 2000;114:183–92. doi: 10.1016/s0166-4328(00)00226-6. [DOI] [PubMed] [Google Scholar]
- 28.Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78:578–95. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- 29.Tsoory MM, Vouimba RM, Akirav I, et al. Amygdala modulation of memory-related processes in the hippocampus: potential relevance to PTSD. Prog Brain Res. 2008;167:35–51. doi: 10.1016/S0079-6123(07)67003-4. [DOI] [PubMed] [Google Scholar]