Abstract
Background
Posttraumatic stress disorder (PTSD) and major depression are reliably associated with reductions in brain volume in markedly similar areas. To our knowledge, no volumetric studies have directly contrasted these conditions. We investigated which, if any, grey matter reductions would be uniquely associated with each disorder. We also investigated more subtle independent effects: specifically, correlations between brain volume and self-report measures of psychopathology.
Methods
We obtained structural magnetic resonance imaging scans from participants with PTSD, major depression and healthy controls exposed to trauma. Participants completed standardized self-report measures of anxiety and depression. We used voxel-based morphometry, applying the DARTEL algorithm within SPM5 to identify associated volumetric changes.
Results
We enrolled 24 patients with PTSD, 29 with major depression and 29 controls in our study. The clinical groups had regions of markedly smaller volume compared with the control group, particularly in prefrontal areas, but did not differ from each other. Greater self-reported anxiety was inversely related to volume in several areas, particularly the inferior temporal cortex, among patients with PTSD, but was associated with some volume increases in patients with major depression. Greater self-reported depression showed similar but weaker effects, being inversely related to brain volume in patients with PTSD but positively related to volume in the cuneus and precuneus of those with major depression.
Limitations
To maintain the representativeness of the sample, patients with PTSD were not excluded if they had typical comorbid conditions, such as depression. Patients were not all medication-free, but we controlled for group differences in antidepressant use in the analyses.
Conclusion
We identified commonalities in areas of brain volume in patients with PTSD and those with major depression, suggesting that existing findings concerning reductions in prefrontal areas in particular may not be specific to PTSD but rather related to features of the disorder that are shared with other conditions, such as depression. More subtle differences between patients with PTSD and those with major depression were represented by distinct structural correlates of self-reported anxiety and depression.
Introduction
Structural brain abnormalities in individuals with posttraumatic stress disorder (PTSD) and major depression show a marked overlap, Thus, meta-analyses of structural magnetic resonance imaging (MRI) studies on individuals with both PTSD and unipolar depression have consistently identified reductions in hippocampal size relative to healthy controls.1–3 This overlap is hard to interpret since PTSD and depression have a number of symptoms in common and are frequently comorbid.4 For example, in one study, reduced hippocampal size was found only in depressed individuals if they also reported early childhood trauma, but two-thirds of this group had comorbid PTSD.5 For these reasons, it is not known at present to what extent structural changes found in patients with PTSD are accounted for by comorbid depression. The present study used voxel-based morphometry (VBM) to test directly to what extent there are commonalities in brain volume reductions and whether there is evidence for structural changes that are unique to one or the other disorder.
In addition to reduced hippocampal size, PTSD has been associated with reductions in the dorsal anterior cingulate cortex6–9 and insular cortex.6,10,11 Differences in the rostral anterior cingulate cortex (ACC) have also been reported,10,12 although one study suggested that apparent differences in the latter were more a reflection of shape than volume.11 In addition to a smaller ACC, a meta-analysis1 found evidence for a reduction in the left amygdala of adult patients with PTSD and in the frontal/prefrontal cortex (but not the hippocampus) of pediatric patients with PTSD. As such, it has been suggested that PTSD is associated with abnormalities in multiple frontal-limbic structures. Studies of unipolar and psychotic depression have similarly found reduced volume in several regions of the ACC,13,14 and more specifically in the subgenual ACC.15–20 Other areas of reduced grey matter concentration or reduced volume include the amygdala,18,20–22 or-bitofrontal cortex,13,23,24 thalamus,24,25 striatum25,26 and several other prefrontal areas.14
Understanding these similar sets of results in both diagnoses is made difficult by the fact that there is typically a high level of comorbidity between PTSD and other anxiety disorders, particularly with depression. Any attempt to identify “pure” cases of PTSD would therefore result in a small, highly unrepresentative sample.27 Another complication is that because of a substantial degree of overlap in the diagnostic criteria, many of these comorbidities may be more apparent than real. Attempts to control for comorbidity risk removing effects that are actually those of interest. Our strategy, based on our previous work,28 was therefore to compare patients with PTSD regardless of comorbid depression with a similar group who met criteria for unipolar depression in the absence of PTSD. Our intention was to have 2 groups typical of those seen in clinical practice, matched as closely as possible on all relevant variables, e.g., severity of depression, use of antidepressants and early trauma, and differing only in the presence or absence of PTSD. A third, psychiatrically healthy, group provided a control for the effects of trauma exposure.
We hypothesized that there would be decreased grey matter volume in fronto-limbic structures and specifically the hippocampus and ACC in both clinical groups compared with the control group. Previous research has suggested the existence of more subtle structural effects relevant to psychopathology, independent of overall differences in volume, such as a correlation between self-reported anxiety and hippocampal volumes in depressed patients and healthy controls.29 Both self-reported anxiety and depression are likely to be elevated, relative to controls, in patients with PTSD and major depressive disorder. We sought to investigate whether any group differences in brain structure were accounted for by current levels of anxiety and depression and whether, independent of overall difference in volume, correlations between brain structure and self-reported anxiety and depression differed in patients with depression and those with PTSD. To improve the sensitivity of VBM, we used the DARTEL algorithm, an improved registration method applied to one of the preprocessing steps, which has recently been developed within SPM. A study with acutely depressed patients confirmed that VBM-DARTEL was more sensitive than standard VBM in detecting hippocampal abnormalities.30
Methods
Participants
T1-weighted MRI scans from participants were collected as part of 2 functional MRI studies. We recruited participants who were right-handed; were under age 50 years; were not currently abusing substances; and had no history of head injury, neurologic disorders or other major medical conditions. The PTSD group met diagnostic criteria for current PTSD when assessed with the Structured Clinical Interview for DSM-IV (SCID).31 The major depression group met diagnostic criteria for current major depressive disorder but not for PTSD when assessed with the SCID. The trauma control group did not meet diagnostic criteria for any psychiatric disorder when assessed with the SCID, and were not currently taking any psychotropic medication. All participants gave written informed consent, and all procedures were approved by the National Hospital for Neurology and Neurosurgery and Institute of Neurology Joint Research Ethics Committee, London, UK.
Measures
The Beck Anxiety Inventory (BAI)32 is a widely used self-report measure of the severity of physiologic and cognitive components of anxiety. It contains 21 items that are scored on a 4-point scale (possible range 0–63). We instructed participants to rate their moods during the week preceding the study.
The Beck Depression Inventory-II (BDI)33 is a widely-used 21-item self-report measure of depression severity. It contains 21 items that are scored on a 4-point scale (possible range 0–63). We instructed participants to rate their moods during the week preceding the study.
The Post-traumatic Diagnostic Scale (PDS)34 is a widely-used 17-item measure for use after a specific traumatic event. It assesses the frequency with which each symptom of PTSD has been experienced over the past month. Items are scored on a 4-point scale (possible range 0–51).
MRI acquisition
T1-weighted images were acquired on a Siemens 1.5-T Sonata whole-body scanner (Siemens Medical Systems), using a whole-body coil for radiofrequency transmission and a head coil for signal reception. Whole-brain structural scans were acquired using a modified driven equilibrium Fourier transform sequence35 with optimized parameters as described in the literature.36 For each volunteer, we acquired 176 sagittal partitions with an image matrix of 256 × 224 (read × phase). Two-fold oversampling was performed in read direction (head–foot direction) to prevent aliasing. The isotropic spatial resolution was 1 mm. Relevant imaging parameters were as follows: repetition time 12.24 ms, echo time 3.56 ms, inversion time 530 ms, bandwidth 106 Hz/Px, flip angle 23°. The total duration was 12 minutes. Spin tagging in the neck was performed to avoid flow artifacts in the vicinity of blood vessels. The flip angle of the tagging pulse was set at 110° to account for B1 losses in the neck. Special radiofrequency excitation pulses were used to compensate for B1 inhomogeneities of the transmit coil in superior–inferior37 and anterior–posterior38 directions. We reconstructed images by performing a standard 3-dimensional Fourier transformation, followed by modulus calculation. No data filtering was applied either in k-space or in the image domain.
MRI processing
T1-weighted image registration was achieved using the diffeomorphic registration algorithm implemented in the DARTEL toolbox39 for SPM5 (Wellcome Trust Centre for Neuroimaging), which has been found to optimize the sensitivity of such analyses.30,40 First, the anatomic images were manually reoriented so that the mm coordinate of the anterior commissure matched the origin (0, 0, 0), and the orientation approximated Montreal Neurological Institute (MNI) space. Next, T1-weighted images were classified into grey matter, white matter and cerebrospinal fluid (CSF) using the segmentation routine implemented in SPM5 applying standard settings. The resulting parameter files were imported into the DARTEL procedure to produce rigidly aligned grey and white matter tissue classes resliced to 1.5 × 1.5 × 1.5–mm voxel size. After the affine transformation, we used the rigidly aligned tissue class images to estimate the nonlinear deformations to best align all images. During this estimation stage, DARTEL iterates between building a template and registering tissue class images using the template. We used the resulting flow fields, which specify the parameterized deformations, to warp white and grey matter images for each participant. The spatially normalized images were rescaled by the Jacobian determinants of the deformations, using 64 time points for solving the partial differential equations. Seventh-degree B-spline interpolation was used when writing the normalized images. To obtain meaningful coordinates of volume alterations, the final DARTEL template was normalized to MNI space and the resulting deformations applied to the grey matter images of each participant using a Matlab script downloaded from the SPM email list (https://www.jiscmail.ac.uk/cgi-bin/wa.exe?A2=ind0807&L=SPM&P=R34150%20032749). Finally, the images were smoothed using an 8 × 8 × 8–mm Gaussian kernel. The input features for the subsequent analysis were the smoothed, modulated and normalized grey matter images.
Statistical analysis
Grey matter group differences were modelled using a factorial design with the factor group having 3 levels (PTSD, major depression, control). We followed up a significant main effect of group with comparisons between individual groups, inclusively masked with the results of the original group effect (at uncorrected mask p = 0.05) to avoid type-I errors. Measurements were assumed to be independent between levels, but variance was assumed to be unequal. Error covariance components were estimated by restricted maximum likelihood (REML) by SPM5 and used for inference and to adjust the degrees of freedom. We used absolute threshold masking at 0.05 to specify the voxels within the image volume that were to be assessed. As nonstationarity is problematic in cluster-level inferences in VBM data41,42 and requires adjustment of cluster sizes according to local smoothness of data,41,43 we adjusted our cluster-level results according to this method, as implemented in the VBM5 toolbox developed by C. Gaser (http://dbm.neuro.uni-jena.de/vbm/download/). Additionally, we created hippocampus and ACC region-of-interest (ROI) masks using the PickAtlas toolbox44 and applied them as small volume correction. For each region, 2 masks were created, 1 based on the Wake Forest University atlas and 1 based on the Anatomical Automatic Labeling (AAL) atlas.45
To examine the explanatory power of the continuous measures of anxiety and depression, the above analyses were repeated, including the BAI and BDI as covariates, centred to the overall mean. Finally, to investigate differential effects of BAI and BDI on PTSD and depression, we converted the BAI and BDI scores to z scores for each group separately. We excluded the control group from this analysis as there were floor effects on the BAI and BDI and hence little explanatory variance for either measure. The z scores (with scores for 1 group reversed [i.e., times –1]) were then used to specify regressors testing the interaction of group (PTSD, major depression) with anxiety and depression, respectively, with effects thresholded at p < 0.001. We also controlled for the effects of group differences in antidepressant use in the analyses. To restrict type-I errors, significant interactions were saved as threshold images and used to inclusively mask explanatory analyses of the effects of the BAI and BDI within each group separately.
Results
Participants
The PTSD group consisted of 24 individuals (9 men) with a mean age of 35.9 (range 25–47) years. They had experienced a range of traumas, including exposure to traumatic death (n = 11), robbery/assault (n = 5), accidents (n = 1), abuse in childhood (n = 6) and other (n = 1). Ten were currently taking antidepressant medication. The major depression group consisted of 29 individuals (8 men) with a mean age of 33.4 (range 24–47) years. They reported exposure to traumatic death (n = 4), robbery/assault (n = 5) and abuse in childhood (n = 3). Twenty-two were currently taking antidepressant medication. The control group consisted of 29 individuals (13 men) with a mean age of 32.45 (range 21–49) years. They reported exposure to traumatic death (n = 13), robbery/assault (n = 7), accidents (n = 7) and other (n = 2).
Demographic and symptom measures
The groups did not differ in sex composition, χ22 = 1.87 (p = 0.39), or age, F2,79 = 1.77 (p = 0.18). There were no group differences in estimated lifetime units of alcohol consumed, F2,77 = 2.92 (p = 0.06), but the major depression group was significantly more likely than the PTSD group to be taking anti-depressant medication, χ12 = 6.40, p = 0.011. We conducted an initial group (PTSD, major depression) × medication (on medication, off medication) analysis test for effects of antidepressant treatment on grey matter volume. No cluster-level significant main effect or interaction involving medication was found. In comparisons of grey matter volume between the PTSD and major depression groups, use of medication was modelled as a covariate of no interest owing to the relatively small groups (medication-free depression patients, n = 7). The PTSD group scored significantly higher than the control group on the PDS (mean 35.58 [standard deviation; SD 9.90] v. mean 8.48 [SD 5.92], respectively; t51 = 12.33, p < 0.001). Scores in the PTSD group were slightly in excess of those expected according to the authors of the PDS.34 There were group differences on the BAI (PTSD mean 30.39 [SD 14.44]; major depression mean 18.76 [SD 9.45]; control mean 6.14 [SD 5.91]; F2,78 = 36.79, p < 0.001). Post-hoc least significant difference tests indicated that all 3 groups differed significantly from each other. There were also group differences on the BDI (PTSD mean 31.12 [SD 11.49]; major depression mean 30.00 [SD 8.34]; control mean 5.21 [SD 6.55]; F2,79 = 76.76, p < 0.001). Post-hoc least significant difference tests indicated that the controls scored significantly lower than the 2 clinical groups, who did not differ from each other. The patients with PTSD were at least mildly depressed, scoring in the range of 14–61 on the BDI. Thus, the 2 clinical groups were matched on relevant variables, including severity of depression, and differed primarily on the variable of interest, PTSD symptoms.
VBM group differences: combined clinical groups show reduced volume compared with the control group
The 1-way analysis of variance showed a significant overall effect of group. Tests did not indicate any significant differences in brain volume between the major depression and PTSD groups, and subsequent analysis therefore focused on the contrast between the combined clinical groups and the control group. As shown in Table 1 and Figure 1, the clinical groups show clusters of decreased brain volume compared with controls in the middle portion of the cingulate cortex; in the medial prefrontal gyrus extending from the medial portions of the superior frontal gyrus at the precentral gyrus to the orbitofrontal cortex and ACC; and in an area within the right dorsolateral prefrontal cortex (DLPFC) spanning the middle frontal gyrus and superior frontal gyrus. Several other areas are significant at the cluster level but fail to survive family-wise error (FWE) correction. Applying small volume corrections reveals an additional cluster of volumetric reduction in the ACC.
Table 1.
Regions of significant brain volume reduction in the combined PTSD and major depression groups compared with controls*
| Foci of activation | Brodmann area | MNI coordinate
|
z score | Cluster size | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Middle cingulate cortex | 6 | 2 | −7 | 41 | 4.23 | 780 |
| Superior frontal gyrus/medial frontal gyrus/anterior cingulate gyrus | 8, 9, 10, 32 | 3 | 54 | 30 | 4.12 | 605 |
| Right middle frontal gyrus/superior frontal gyrus | 8, 9, 10 | 34 | 25 | 52 | 4.07 | 1260 |
| Right angular gyrus† | 39 | 55 | −55 | 37 | 3.92 | 304 |
| Right superior frontal gyrus† | 6 | 6 | −7 | 77 | 3.88 | 209 |
| Right postcentral gyrus† | 1 | 50 | −32 | 58 | 3.87 | 362 |
| Left superior parietal lobule/inferior parietal lobule† | 7 | −21 | −70 | 50 | 3.86 | 371 |
| Left precentral gyrus† | 6 | −19 | −21 | 63 | 3.85 | 366 |
| Left ventral inferior frontal gyrus/orbitofrontal cortex† | 47 | −35 | 24 | −26 | 3.76 | 319 |
| Right supramarginal gyrus† | 40 | 45 | −38 | 32 | 3.68 | 183 |
| Left parietal operculum† | 40/3b | −66 | −3 | 6 | 3.63 | 178 |
| Left anterior cingulate cortex‡ | 32 | −6 | 46 | 7 | 3.53 | 184 |
MNI = Montreal Neurological Institute; PTSD = posttraumatic stress disorder.
Significant at p < 0.05, family wise error–corrected, whole-brain cluster level–corrected.
Significant at the cluster level, but does not survive whole-brain correction.
Small volume corrections significant at p < 0.05.
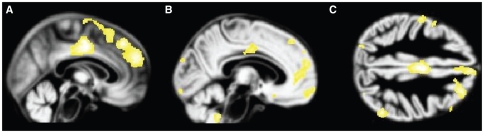
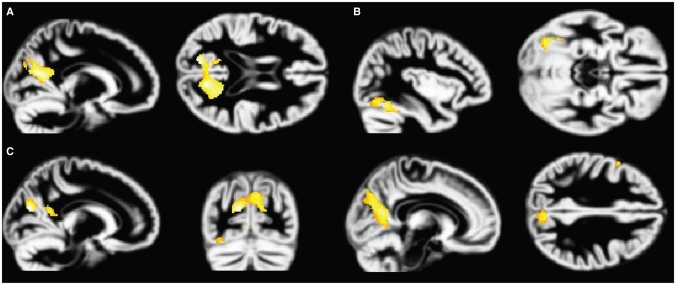
Fig. 1.
Volume reduction in the combined posttraumatic stress disorder and major depression groups compared with controls. Reduced grey matter volume in the combined clinical groups compared with the control group is displayed on the normalized DARTEL grey matter template over all 3 groups. Volume reductions in the (A) middle cingulate gyrus and medial prefrontal cortex, (B) anterior cingulate gyrus and orbitofrontal cortex and (C) dorsolateral prefrontal cortex are observed, thresholded at p = 0.001.
The BAI and BDI scores explain away volumetric reductions in combined clinical groups compared with the control group
To identify explanations for the observed volume reduction, we investigated morphologic differences between the combined clinical groups and the control group, controlling for individual scores on the BAI and BDI. These contrasts yielded no voxel-level significant results that survived FWE correction for the whole-brain volume. Additionally, no differences were found using small volume corrections for the ACC and hippocampus. Inclusion of continuous psychopathology scores as a covariate thus explained away group differences.
Effect of group × BAI interaction
To investigate differences between the groups in correlations between brain structure and self-reported anxiety, independent of overall differences in brain volume, we tested the group (PTSD, major depression) × BAI interaction. This revealed a cluster, including anterior areas of the left inferior temporal gyrus and middle temporal gyrus spanning the temporal pole; a left-sided cluster, including the fusiform gyrus, lingual gyrus and cerebellar vermis; a right-sided cluster across the anterior inferior temporal gyrus and temporal pole; and a posterior cluster in the inferior temporal gyrus spreading to the middle temporal gyrus (Appendix 1, available at www.cma.ca/jpn). Several other areas were significant at the cluster level but failed to survive FWE correction.
Further analysis of this interaction by assessing volumetric changes within each group individually, inclusively masked by the overall interaction effect, revealed that for the PTSD group, BAI scores were inversely associated with grey matter volumes in the fusiform gyrus, middle frontal gyrus, inferior temporal gyrus, transverse gyrus, cerebellum, middle temporal gyrus, putamen and frontal operculum (Table 2 and Fig. 2). In contrast, the major depression group showed a positive association between BAI scores and grey matter volumes in the middle temporal gyrus and superior frontal gyrus (Table 3 and Fig. 3).
Table 2.
Regions of significant brain volume reduction in the PTSD group associated with increasing Beck Anxiety Inventory32 scores*
| Foci of activation | Brodmann area | MNI coordinate
|
z score | Cluster size | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Fusiform gyrus | 18 | −26 | −71 | −10 | 4.95 | 662 |
| Left middle frontal gyrus | 9 | −35 | 43 | 33 | 4.52 | 275 |
| Left inferior temporal gyrus | 20 | −47 | −27 | −28 | 4.14 | 949 |
| Right transverse temporal gyrus | 42 | 58 | −14 | 8 | 4.11 | 119 |
| Left inferior temporal gyrus | 20 | −36 | −2 | −45 | 4.02 | 143 |
| Right anterior cerebellar lobe | 27 | −57 | −28 | 3.89 | 67 | |
| Right putamen | 26 | 11 | 10 | 3.72 | 86 | |
| Right middle temporal gyrus | 21 | 61 | −30 | −12 | 3.69 | 634 |
| Right inferior temporal gyrus/temporal pole | 38 | 45 | 7 | −39 | 3.65 | 147 |
| Left frontal operculum | 44 | −39 | 8 | 19 | 3.46 | 69 |
| Left cerebellar tonsil | −21 | −33 | −38 | 3.44 | 117 | |
| Right medial temporal pole | 38 | 31 | 6 | −36 | 3.29 | 54 |
| Right inferior temporal gyrus | 20 | 48 | −16 | −26 | 3.27 | 82 |
MNI = Montreal Neurological Institute; PTSD = posttraumatic stress disorder.
Significant at p < 0.05, family wise error–corrected, whole-brain cluster level–corrected.
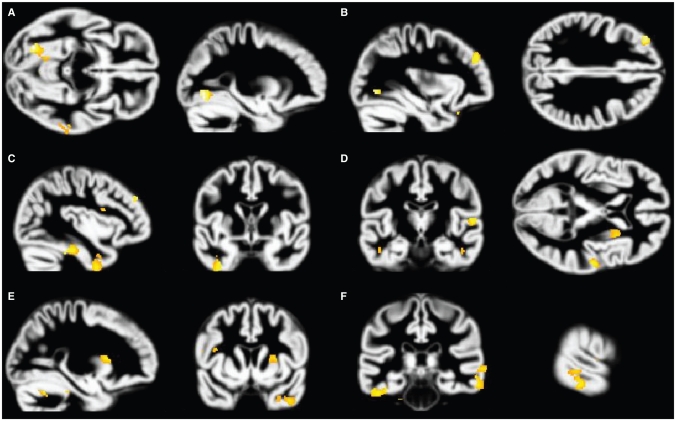
Fig. 2.
Volume reductions in the posttraumatic stress disorder (PTSD) group associated with increasing Beck Anxiety Inventory (BAI)32 scores. Increased BAI scores are associated with grey matter volume decreases in the PTSD group. Analyses are inclusively masked with the BAI × group (PTSD, major depression) interaction effect, and results are displayed on the normalized DARTEL grey matter template over all 3 groups. Volume reduction in the (A) fusiform gyrus, (B) middle frontal gyrus, (C) inferior temporal gyrus, (D) transverse gyrus, (D right and E) putamen and (F) middle temporal gyrus are presented, thresholded at p = 0.001.
Table 3.
Regions of significant brain volume increases in the major depression group associated with increasing Beck Anxiety Inventory32 scores*
| Foci of activation | Brodmann area | MNI coordinate
|
z score | Cluster size | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Left middle temporal gyrus | 20 | −49 | −17 | −23 | 3.90 | 106 |
| Right superior frontal gyrus | 6 | 23 | 11 | 55 | 3.83 | 93 |
MNI = Montreal Neurological Institute.
Significant at p < 0.05, family wise error–corrected, whole-brain cluster level–corrected.
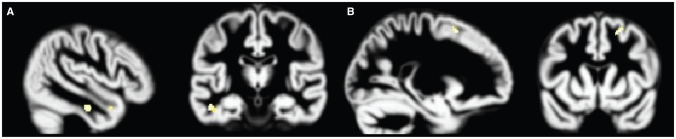
Fig. 3.
Volume increases in the major depression group associated with increasing Beck Anxiety Inventory (BAI)32 scores. Increased BAI scores are associated with grey matter volume increases in the major depression group. Analyses are inclusively masked with the BAI × group (posttraumatic stress disorder, major depression) interaction effect, and results are displayed on the normalized DARTEL grey matter template over all 3 groups. Volume increases in the (A) middle temporal gyrus and (B) superior frontal gyrus are presented, thresholded at p = 0.001.
Effect of group × BDI interaction
To investigate similar differences between the groups in correlations between brain structure and self-reported depression, we tested the group (PTSD, major depression) × BDI interaction on grey matter volume. This revealed a cluster, including the right lingual gyrus, bilateral cuneus and right precuneus; and a cluster within the left fusiform gyrus (Appendix 1). Several other areas were significant at the cluster level but failed to survive FWE correction.
Following the masking approach used to examine the earlier interaction, BDI scores in the PTSD group were inversely associated with grey matter volumes in the superior frontal gyrus, fusiform gyrus, lingual gyrus and cuneus (Table 4 and Fig. 4). In contrast, BDI scores in the major depression group were positively associated with grey matter volumes in the cuneus and precuneus, fusiform gyrus and middle frontal gyrus (Table 5 and Fig. 5).
Table 4.
Regions of significant brain volume reduction in the PTSD group with increasing Beck Depression Inventory33 scores*
| Foci of activation | Brodmann area | MNI coordinate
|
z score | Cluster size | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right superior frontal gyrus | 9 | 15 | 60 | 35 | 3.95 | 101 |
| Right fusiform gyrus | 37 | 32 | −57 | −9 | 3.45 | 82 |
| Right lingual gyrus | 18 | 5 | −65 | 4 | 3.39 | 139 |
| Left cuneus | 19 | 3 | −89 | 26 | 3.34 | 24 |
MNI = Montreal Neurological Institute; PTSD = posttraumatic stress disorder.
Significant at p < 0.05, family wise error–corrected, whole-brain cluster level–corrected.
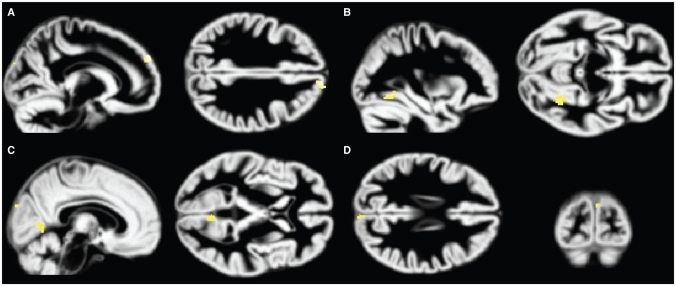
Fig. 4.
Volume reductions in the posttraumatic stress disorder (PTSD) group associated with increasing Beck Depression Inventory-II (BDI)33 scores. Increased BDI scores are associated with grey matter volume decreases in the PTSD group. Analyses are inclusively masked with the BDI × group (PTSD, major depression) interaction effect, and results are displayed on the normalized DARTEL grey matter template over all 3 groups. Volume decreases in the (A) superior frontal gyrus, (B) fusiform gyrus, (C) lingual gyrus and (D) cuneus are presented, thresholded at p = 0.001.
Table 5.
Regions of significant brain volume increases in the major depression group with increasing Beck Depression Inventory33 scores*
| Foci of activation | Brodmann area | MNI coordinate
|
z score | Cluster size | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Left cuneus/right precuneus | 18, 19 | −13 | −77 | 20 | 5.23 | 3123 |
| Left fusiform gyrus | 37 | −37 | −57 | −20 | 4.68 | 472 |
| Left precuneus | 19 | −15 | −49 | 15 | 4.17 | 84 |
| Left cuneus | 19 | −7 | −78 | 40 | 3.25 | 15 |
MNI = Montreal Neurological Institute.
Significant at p < 0.05, family wise error–corrected, whole-brain cluster level–corrected.
Fig. 5.
Volume increases in the major depression group associated with increasing Beck Depression Inventory-II (BDI)33 scores. Increased BDI scores are associated with grey matter volume increases in the depressed group. Analyses are inclusively masked with the BDI × group (posttraumatic stress disorder, major depression) interaction effect and results are displayed on the normalized DARTEL grey matter template over all 3 groups. Volume increases in the (A) cuneus/precuneus, (B) fusiform gyrus and (C) cuneus and precuneus depicting locations in both regions are presented, thresholded at p = 0.001.
Discussion
To the best of our knowledge, this is the first study to have directly compared brain volumes in matched samples of patients with PTSD and major depression who had similar levels of depression and differed only based on the presence of PTSD. Consistent with the marked overlap in areas of brain volume reduction identified in previous studies of each disorder separately, we were not able to find evidence for unique structural changes associated with PTSD. Volume reductions were found in common predominantly in frontal areas, such as the dorsal ACC, consistent with prior studies of PTSD6–9 and depression.13 Reductions in the rostral ACC also mirrored previous findings in studies of PTSD10,12 and depression.15,16,18–20 Other areas in which we detected reduced volume in common included the medial orbitofrontal cortex, as previously implicated in PTSD46 and depression.13,23,24 Reduced DLPFC volume has previously been found in young adults exposed to harsh physical punishment,47 and patients with PTSD show abnormal recruitment of DLPFC activity during a working-memory updating task.48 The DLPFC is also implicated in the pathophysiology of depression.14
Of note is the absence of evidence for hippocampal reduction in the clinical samples. Findings concerning hippocampal volume have been heterogeneous, with many studies of individuals with PTSD failing to report abnormalities in this area.49–53 There may be as yet undetected associations such that specific trauma histories (e.g., prolonged or childhood trauma) are more likely to be associated with changes in these areas. Hippocampal reduction may also be a preexisting vulnerability factor rather than a general consequence of PTSD.54
As previously noted,12 the convergent structural neuro-imaging findings in patients with PTSD and those with depression raise issues about whether models of the neurocircuitry of PTSD55 reflect common vulnerabilities to different types of psychopathology. Similar models have also been put forward to account for mood alterations in patients with depression.14,17 Our findings could be taken to suggest that many of the neurobiologic observations in patients with PTSD relate to common mechanisms of emotional control rather than being specific to PTSD or, alternatively, that they are due to comorbid depression.
Despite the overall lack of differences in brain volume, PTSD and depression could be distinguished by more subtle effects involving the association between scores on the continuous measures of anxiety, depression and brain volume. This adds to a previous study that found differences in functional responses to script-driven imagery in patients with PTSD with and without comorbid major depression.56 In our data, in patients with PTSD the BAI scores were inversely associated with volumes in a number of areas, primarily involving the inferior temporal lobes, but also including frontal areas, the frontal operculum and other temporal areas. Neuro-anatomical experiments in monkeys have shown that the orbital and medial prefrontal cortex is associated with an extended cortical circuit (“the orbital prefrontal network”), which includes visual association areas in the inferior temporal cortex and somatic–sensory association areas in the insula and frontal operculum.14 The functions of this network, which is likely to be implicated in PTSD, are thought to include sensory integration and affective coding.
Integration of visual stimuli is performed by the inferior temporal structures that form part of the ventral visual stream, allowing visual events to be placed with a general temporal and spatial context. Unlike the dorsal visual stream, which supports egocentric representations of visual experience that retain a close link with the original perceptual input, visual processing in the ventral stream and related structures allows allocentric representations of scenes to be manipulated and combined in novel ways.57,58 It has recently been proposed59,60 that flashbacks in patients with PTSD arise from egocentric trauma representations within the dorsal stream that have received inadequate contextualization within the ventral visual stream. The fact that flashbacks are a symptom associated with PTSD and not depression61 may be related to the differential patterns of correlation identified in this study.
In patients with PTSD the Beck Depression Inventory scores were inversely associated with volumes in similar regions, as well as in the superior frontal gyrus. This area has been implicated in the selection of action sets62 and response inhibition.63 More strikingly, however, in the major depression group BDI scores were positively associated with volumes in the cuneus and precuneus, areas involved in visual processing and imagery. It is well-established that intrusive imagery involving past life stressors is extremely common in patients with depression,61 and more frequent intrusions are regularly associated with higher depression scores on the BDI.64,65
Limitations
Among the limitations of our study was that the sample consisted of individuals with PTSD arising from a variety of sources and was too small to permit the analysis of subgroups. Although this suggests the results are more likely to be generalizable, it leaves open the possibility that different results would be obtained in more homogeneous samples. A limitation of our analysis of the group effect of antidepressant use was the relatively small groups (medication-free patients with depression, n = 7). Much of the available literature is based on samples of combat veterans or sexual abuse survivors, and this may account for the fact that we did not detect volume changes in some previously identified areas, such as the hippocampus. Given the evidence that prior trauma, particularly in childhood, is a risk factor for PTSD,66,67 and that cumulative childhood trauma predicts greater symptom complexity,68 future studies should enrol separate samples of patients with PTSD following single and multiple traumatic exposure and separately evaluate the effects of childhood trauma.
Conclusion
We were unable to identify differences between regions of reduced brain volumes specifically associated with PTSD and depression. Overall, areas of volume reduction are very similar, suggesting some shared deficits in mechanisms for regulating emotion. Nevertheless, each disorder reveals a distinct pattern in the relation of brain volume to continuous measures of anxiety and depression, with anxiety inversely associated with inferior temporal volumes in patients with PTSD and with depression positively associated with volumes in the cuneus and precuneus of patients with major depression. The findings underscore the fact that PTSD and depression appear to be different kinds of disorders despite high levels of comorbidity and emphasize the need for more investigations that study both conditions simultaneously and attempt to isolate their unique pathophysiologic mechanisms.
Footnotes
Competing interests: None declared for Ms. Kroes or Drs. Rugg and Whalley. Dr. Brewin declared having received grant and travel support from the Wellcome Trust.
Contributors: Drs. Rugg, Whalley and Brewin designed the study. Dr. Whalley acquired the data, which Ms. Kroes and Dr. Brewin analyzed. Ms. Kroes and Dr. Brewin wrote the article, which Drs. Rugg and Whalley reviewed. All authors approved publication of the article.
References
- 1.Karl A, Schaefer M, Malta LS, et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–31. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- 3.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 4.Campbell DG, Felker B, Liu C, et al. Prevalence of depression-PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. J Gen Intern Med. 2007;22:711–8. doi: 10.1007/s11606-006-0101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vythilingam M, Heim C, Newport J, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–80. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Xia W, Li L, et al. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Res. 2006;146:65–72. doi: 10.1016/j.pscychresns.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord. 2006;90:171–4. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodward SH, Kaloupek DG, Streeter CC, et al. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59:582–7. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 9.Yamasue H, Kasai K, Iwanami A, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100:9039–43. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasai K, Yamasue H, Gilbertson MW, et al. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry. 2008;63:550–6. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbo V, Clément M-H, Armony JL, et al. Size versus shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biol Psychiatry. 2005;58:119–24. doi: 10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Rauch SL, Shin LM, Segal E, et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–6. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- 13.Diwadkar V, Lacerda A, Keshavan M, et al. Voxel-based and region-of-interest morphometry convergently reveal regional gray matter abnormalities in unipolar depression. Proc Intl Soc Mag Reson Med. 2003;11:182. [Google Scholar]
- 14.Drevets WC, Price J, Furey M. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botteron KN, Raichle ME, Drevets WC, et al. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–4. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- 16.Coryell W, Nopoulos P, Drevets W, et al. Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. Am J Psychiatry. 2005;162:1706–12. doi: 10.1176/appi.ajp.162.9.1706. [DOI] [PubMed] [Google Scholar]
- 17.Drevets WC, Price JL, Simpson JR, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 18.Hastings RS, Parsey RV, Oquendo MA, et al. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–9. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- 19.Hirayasu Y, Shenton ME, Salisbury DF, et al. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156:1091–3. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y, Wang F, Xie G, et al. Reduced ventral anterior cingulate and amygdala volumes in medication-naïve females with major depressive disorder: a voxel-based morphometric magnetic resonance imaging study. Psychiatry Res. 2007;156:83–6. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–9. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–8. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 23.Bremner JD, Vythilingam M, Vermetten E, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–9. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 24.Vasic N, Walter H, Höse A, et al. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J Affect Disord. 2008;109:107–16. doi: 10.1016/j.jad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Kim MJ, Hamilton JP, Ian HG. Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Res. 2008;164:114–22. doi: 10.1016/j.pscychresns.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parashos I, Tupler L, Blitchington T, et al. Magnetic-resonance morphometry in patients with major depression. Psychiatry Res. 1998;84:7–15. doi: 10.1016/s0925-4927(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 27.Lanius RA, Brewin CR, Bremner JD, et al. Does neuroimaging research examining the pathophysiology of posttraumatic stress disorder require medication-free patients? J Psychiatry Neurosci. 2010;35:80–9. doi: 10.1503/jpn.090047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whalley MG, Rugg MD, Smith APR, et al. Incidental retrieval of emotional contexts in post-traumatic stress disorder and depression: an fMRI study. Brain Cogn. 2009;69:98–107. doi: 10.1016/j.bandc.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rusch BD, Abercrombie HC, Oakes TR, et al. Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biol Psychiatry. 2001;50:960–4. doi: 10.1016/s0006-3223(01)01248-3. [DOI] [PubMed] [Google Scholar]
- 30.Bergouignan L, Chupin M, Czechowska Y, et al. Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage. 2009;45:29–37. doi: 10.1016/j.neuroimage.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 31.First MB, Williams JB, Spitzer RL. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version. Washington (DC): American Psychiatric Press; 1997. [Google Scholar]
- 32.Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 33.Beck AT, Steer RA, Brown GK, editors. Manual for the Beck Depression Inventory-II. San Antonio (TX): Psychological Corporation; 1996. [Google Scholar]
- 34.Foa E, Cashman L, Jaycox L, et al. The validation of a self-report measure of PTSD: the Posttraumatic Diagnostic Scale. Psychol Assess. 1997;9:445–51. [Google Scholar]
- 35.Ugurbil K, Garwood M, Ellermann J, et al. Imaging at high magnetic fields: initial experiences at 4 T. Magn Reson Q. 1993;9:259–77. [PubMed] [Google Scholar]
- 36.Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage. 2004;21:757–67. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- 37.Deichmann R, Good CD, Josephs O, et al. Optimization of 3-D MP-RAGE sequences for structural brain imaging. Neuroimage. 2000;12:112–27. doi: 10.1006/nimg.2000.0601. [DOI] [PubMed] [Google Scholar]
- 38.Deichmann R, Good CD, Turner R. RF inhomogeneity compensation in structural brain imaging. Magn Reson Med. 2002;47:398–402. doi: 10.1002/mrm.10050. [DOI] [PubMed] [Google Scholar]
- 39.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Klein A, Andersson J, Ardekani B, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worsley KJ, Andermann M, Koulis T, et al. Detecting changes in nonisotropic images. Hum Brain Mapp. 1999;8:98–101. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<98::AID-HBM5>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moorhead TWJ, Job DE, Spencer MD, et al. Empirical comparison of maximal voxel and non-isotropic adjusted cluster extent results in a voxel-based morphometry study of comorbid learning disability with schizophrenia. Neuroimage. 2005;28:544–52. doi: 10.1016/j.neuroimage.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 43.Hayasaka S, Phan KL, Liberzon I, et al. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–87. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 44.Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 45.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automatic anatomical labeling of activations in SPM using a microscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 46.Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci. 2007;1121:546–61. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- 47.Tomoda A, Suzuki H, Rabi K, et al. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage. 2009;47:T66–71. doi: 10.1016/j.neuroimage.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moores KA, Clark CR, McFarlane AC, et al. Abnormal recruitment of working memory up-dating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Res. 2008;163:156–70. doi: 10.1016/j.pscychresns.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Bonne O, Brandes D, Gilboa A, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–51. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fennema-Notestine C, Stein MB, Kennedy CM, et al. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:1089–101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 51.Jatzko A, Rothenhöfer S, Schmitt A, et al. Hippocampal volume in chronic posttraumatic stress disorder (PTSD): MRI study using two different evaluation methods. J Affect Disord. 2006;94:121–6. doi: 10.1016/j.jad.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Pederson CL, Maurer SH, Kaminski PL, et al. Hippocampal volume and memory performance in a community-based sample of women with posttraumatic stress disorder secondary to child abuse. J Trauma Stress. 2004;17:37–40. doi: 10.1023/B:JOTS.0000014674.84517.46. [DOI] [PubMed] [Google Scholar]
- 53.Winter H, Irle E. Hippocampal volume in adult burn patients with and without posttraumatic stress disorder. Am J Psychiatry. 2004;161:2194–200. doi: 10.1176/appi.ajp.161.12.2194. [DOI] [PubMed] [Google Scholar]
- 54.Gilbertson MW, Williston SK, Paulus LA, et al. Configural cue performance in identical twins discordant for posttraumatic stress disorder: theoretical implications for the role of hippocampal function. Biol Psychiatry. 2007;62:513–20. doi: 10.1016/j.biopsych.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauch SL, Shin LM, Whalen PJ, et al. Neuroimaging and the neuroanatomy of PTSD. CNS Spectr. 1998;3(Suppl):30–41. [Google Scholar]
- 56.Lanius RA, Frewen PA, Girotti M, et al. Neural correlates of trauma script-imagery in post-traumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res. 2007;155:45–56. doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Burgess N, Becker S, King JA, et al. Memory for events and their spatial context: models and experiments. Philos Trans R Soc Lond B Biol Sci. 2001;356:1493–503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev. 2007;114:340–75. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bisby JA, King JA, Brewin CR, et al. Acute effects of alcohol on intrusive memory development and viewpoint dependence in spatial memory support a dual representation model. Biol Psychiatry. 2010;68:280–6. doi: 10.1016/j.biopsych.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 60.Brewin CR, Gregory JD, Lipton M, et al. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol Rev. 2010;117:210–32. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brewin CR, Lanius R, Novac A, et al. Reformulating PTSD for DSM-V: life after criterion A. J Trauma Stress. 2009;22:366–73. doi: 10.1002/jts.20443. [DOI] [PubMed] [Google Scholar]
- 62.Rushworth MFS, Walton ME, Kennerley SW, et al. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–7. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Leung H-C, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J Neurosci. 2007;27:9893–900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brewin CR, Reynolds M, Tata P. Autobiographical memory processes and the course of depression. J Abnorm Psychol. 1999;108:511–7. doi: 10.1037//0021-843x.108.3.511. [DOI] [PubMed] [Google Scholar]
- 65.Kuyken W, Brewin CR. Intrusive memories of childhood abuse during depressive episodes. Behav Res Ther. 1994;32:525–8. doi: 10.1016/0005-7967(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 66.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–66. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 67.Cougle JR, Resnick H, Kilpatrick D. Does prior exposure to interpersonal violence increase risk of PTSD following subsequent exposure? Behav Res Ther. 2009;47:1012–7. doi: 10.1016/j.brat.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cloitre M, Stolbach BC, Herman JL, et al. A developmental approach to complex PTSD: childhood and adult cumulative trauma as predictors of symptom complexity. J Trauma Stress. 2009;22:399–408. doi: 10.1002/jts.20444. [DOI] [PubMed] [Google Scholar]