Abstract
Pitch perception was studied in a subject with a 10-mm cochlear implant (CI) in one ear and a 24-mm CI in the other ear. Both processors were programmed to allocate information from the same frequency range of 188-7938 Hz, despite the large differences in putative insertion depth and stimulated cochlear locations between the CIs. After 2 and 3 years of experience, pitch-matched electrode pairs between CIs were aligned closer to the processor-provided frequencies than to cochlear position. This finding suggests that pitch perception may have adapted to reduce perceived spectral discrepancies between bilateral CI inputs, despite 2-3 octave differences in tonotopic mapping.
INTRODUCTION
Cochlear implants (CIs) aim to utilize the tonotopic representation of sound frequency in the cochlea. Accordingly, electrode location, as well as stimulation rate, has been shown to influence the electrical pitch percept (e.g. Tong et al., 1982; Shannon, 1983; Townshend et al., 1987; Dorman et al., 1994; McKay et al., 2000). However, for a given stimulation rate and when compared with acoustic tones in the non-implanted ear, several studies found the electrical pitch percept to be 1-3 octaves lower than predicted from the Greenwood map for each electrode location (e.g. Dorman et al., 1994; Blamey et al., 1996; Boex et al., 2006). At least one study confirmed cochlear locations using imaging measurements and hypothesized that a one-octave discrepancy could be accounted for by stimulation of the spiral ganglion instead of basilar membrane (Boex et al., 2006). Other studies conducted immediately after implantation found pitch estimates to be close to basilar membrane predictions (Eddington et al., 1978; McDermott et al. 2009, Carlyon et al. 2009). Recently, a longitudinal study by Reiss et al (2007) showed that electric pitch can shift by as much as 2 octaves after several months of CI experience. The direction and the time scale of the shifts were variable; most, but not all, subjects had electric pitches that converged to match the CI frequency allocations. These findings suggest that long-term CI experience may also influence electric pitch perception relative to an acoustic reference.
Does long-term CI experience also influence electric pitch perception relationships between bilateral CIs? Here we address this question by presenting pitch perception data from a patient with bilateral CIs who had electrode arrays inserted to very different insertion depths and cochlear places of stimulation.
MATERIALS AND METHODS
Subject history
A 57-year old patient with a steeply-sloping high-frequency hearing loss was implanted with a 10-mm Hybrid CI in the left ear in 2002 (Gantz and Turner, 2003). Two years post-implantation, this patient lost residual hearing in both ears due to an autoimmune disorder, and was immediately implanted with a standard long-electrode CI (Cochlear Research Platform 8 or RP8) in the right ear in 2004.
The Hybrid CI processor was initially programmed to allocate a frequency range of 688-7938 Hz to the 6 channels on the Hybrid device, but after the residual hearing was lost, the patient preferred and switched to a full range of 188-7938 Hz in 2006. The long-electrode CI processor was also programmed to allocate 188-7938 Hz to 20 channels on the RP8 (the two basal-most electrodes were deactivated due to poor quality percepts). At the time of initial pitch testing in 2008, the patient had 2 years of experience with 188-7938 Hz frequency allocations in both devices (Table II).
Table II.
Frequency allocations for each electrode in the Hybrid and RP8 (long-electrode) devices. The first column gives the device type, the second column the electrode number, and the third and fourth columns the lower and upper boundaries of the frequencies allocated in the cochlear implant processor maps, respectively.
| Device | Electrode | Lower bound (Hz) | Upper bound (Hz) |
|---|---|---|---|
| RP8 | 22 | 188 | 313 |
| 21 | 313 | 438 | |
| 20 | 438 | 563 | |
| 19 | 563 | 688 | |
| 18 | 688 | 813 | |
| 17 | 813 | 938 | |
| 16 | 938 | 1063 | |
| 15 | 1063 | 1188 | |
| 14 | 1188 | 1438 | |
| 13 | 1438 | 1688 | |
| 12 | 1688 | 1938 | |
| 11 | 1938 | 2313 | |
| 10 | 2313 | 2688 | |
| 9 | 2688 | 3188 | |
| 8 | 3188 | 3688 | |
| 7 | 3688 | 4313 | |
| 6 | 4313 | 5063 | |
| 5 | 5063 | 5938 | |
| 4 | 5938 | 6938 | |
| 3 | 6938 | 7938 | |
| Hybrid | 6 | 188 | 563 |
| 5 | 563 | 1063 | |
| 4 | 1063 | 1813 | |
| 3 | 1813 | 2938 | |
| 2 | 2938 | 4813 | |
| 1 | 4813 | 7938 |
Electrode locations were not confirmed with imaging measurements. The policy at the University of Iowa Cochlear Implant Program is that unless there is indication of malfunction or other problems with an implant, patients are not subjected to imaging radiation. The surgeon's reports indicated normal (i.e. full) insertions for both arrays and all electrode impedances were in the normal range, Thus, no scans were obtained and the electrode insertion depths given in this study are based on the maximum possible array insertions.
Pitch matching procedure and analysis
Two computers with Custom Sound software (Cochlear) were used to stimulate the two devices/ears independently. Each pitch comparison trial consisted of first stimulating an electrode in one device/ear, then stimulating an electrode in the other device/ear, with varying order. Stimulation of each electrode consisted of three 1200 pps pulse trains of 500 msec duration with 500 msec interstimulus intervals. The 1200 pps stimulation rate was chosen to be high enough to saturate stimulation rate effects on pitch perception (Zeng, 2002). After an electrode pair was stimulated, the subject was asked to verbally report the second electrode as higher (H), lower (L), or similar (S) in pitch to the first electrode.
Four runs of pitch comparisons were conducted for each Hybrid electrode. Hybrid electrodes 2, 4, and 6 were tested, for a total of twelve runs. Generally, in each run, one Hybrid electrode was compared with odd-numbered RP8 electrodes from 3-21. The exception was the fourth run in which even-numbered electrodes from 4-22 were tested; results were combined with odd-numbered electrodes from runs 1-3 for adjacent electrodes 3/4, 5/6, and so on. The run sequences were varied to reduce order effects, with run 1 ascending from basal to apical electrodes, runs 2-3 in pseudorandom sequence (Latin-square, with run 2 in 6-electrode steps, i.e. E5, E11, E17, E3, E9, ... and run 3 in 8-electrode steps), and run 4 descending. The presence of strong order effects on responses in the ascending and descending runs (Table I) was noted after the completion of data collection. Therefore, the data from even-numbered electrodes and odd-numbered electrodes were combined to minimize these order effects. Repeat testing was conducted one year later with the same run sequences to verify the repeatability of the results with exclusively odd-numbered electrodes.
Table I.
Results of pitch comparisons between various electrode pairs compared between Hybrid and RP8 (long-electrode) devices. Results for the initial and repeat measurement are shown at top and bottom, respectively. The Hybrid electrode number is varied along the major columns and the RP8 electrode number is varied along the rows. Within each major column are two minor columns; the first column shows the ordered responses for all four runs, where L (lower), S (similar), or H (higher) indicate the relation of the Hybrid pitch to the RP8 electrode pitch of that row. The second minor column is the result of the formula F as defined in Eqn. 1 in the Methods. The bold text and shaded regions indicate the pitch-matched electrode pairs within the range defined by F ≤ 1. . Asterisks indicate electrode pairs with F > 1 that were included in the pitch matched range because they were flanked by electrode pairs with F ≤ 1.
| Hybrid electrode 6 | Hybrid electrode 4 | Hybrid electrode 2 | ||||
|---|---|---|---|---|---|---|
| RP8 electrode | Runs 1-4 | F | Runs 1-4 | F | Runs 1-4 | F |
| 3/4 | LLLL | 4 | LLLL | 4 | LSLL | 3 |
| 5/6 | LLLL | 4 | SLLL | 3 | LSLL | 3 |
| 7/8 | LLLL | 4 | SLLL | 3 | SSSL | 1 |
| 9/10 | LLLL | 4 | LSLL | 3 | HHSL | 1 |
| 11/12 | LLSL | 3 | SSSL | 1 | HHHL | 2* |
| 13/14 | SLLL | 3 | HHLL | 0 | HHSL | 1 |
| 15/16 | SSSL | 1 | HHHL | 2* | HHHL | 2 |
| 17/18 | HSSS | 1 | SHLL | 1 | HHHL | 2 |
| 19/20 | HSLL | 1 | HLLL | 2 | HSHS | 2 |
| 21/22 | HHSS | 2 | HHHH | 4 | HHHH | 4 |
| RP8 electrode – repeat measurement | ||||||
| 3 | LLLL | 4 | LLLL | 4 | LLLL | 4 |
| 5 | LLLL | 4 | LLLL | 4 | LLLL | 4 |
| 7 | LLLL | 4 | LLLL | 4 | HLLL | 2 |
| 9 | LLLL | 4 | LLLL | 4 | HHSL | 1 |
| 11 | SLLL | 3 | HLLL | 2 | HHHL | 2* |
| 13 | SLLL | 3 | HSLL | 1 | HSLL | 1 |
| 15 | SSSL | 1 | HHLL | 0 | HHHL | 2 |
| 17 | HSHL | 1 | HHHS | 3* | HHHL | 2 |
| 19 | HHSL | 1 | SLHL | 1 | HHSS | 2 |
| 21 | HHHH | 4 | HHHH | 4 | HHHH | 4 |
The subject's responses were highly variable due to the order effects, with some comparisons yielding both H and L but no S responses (Table I), so that a pitch-match could not always be determined simply by looking for electrodes with S responses. However, as with a psychometric function: 1) equal numbers of opposite responses can be considered an indicator of similar pitch for an electrode pair; 2) exclusively H or L responses definitively indicate that the pair is not pitch matched. Thus, the pitch-matched range was determined by quantifying the consistency of the response, using the following formula:
| (1) |
where NL and NH are the number of instances of L and H, respectively, for that electrode pair. A large value of F indicates consistent L or H responses, and a small F indicates either S responses or inconsistent L and H responses. Electrode pairs with F ≤ 1 were considered to be matched in pitch; this corresponds to a consistently H or L response 25% of the time or less. In addition, the pitch-matched range was constrained to be continuous; if an electrode pair had a value of F > 1, but was flanked by electrode pairs with F ≤ 1, the pair was included in the matched range.
To ensure that the patient could perform the pitch comparison task, the patient was also tested on the ability to pitch rank electrodes 1-6 within the Hybrid device and electrodes 5, 7, 9, 11, 13, 15, 17, 19, and 21 within the long-electrode device. All possible pair combinations were tested at least twice and the patient correctly ranked all pairs tested, i.e. the rankings matched the sequential order of the electrode locations.
RESULTS
The pitch comparison responses and the calculated F values are shown in Table I for each electrode pair. Bold text and darker shaded regions indicate pitch-matched ranges. In the initial results at top, Hybrid electrode 6 was matched in pitch to the range of RP8 electrodes 15-20, Hybrid electrode 4 to RP8 electrodes 11-18, and Hybrid electrode 2 to RP8 electrodes 7-14. Similar results were found on repeat testing of exclusively odd-numbered electrodes one year later, shown at bottom. The main difference was that the ranges for Hybrid electrodes 4 and 2 were shifted downward on the apical side. The repeat results for run 4 with odd-numbered electrodes were unchanged from the first run with even-numbered electrodes.
For both sets of results, the large contribution of run sequence to response variability is apparent when comparing the first and the last runs for each electrode pair. This contrasts to the observed repeatability of responses for repeat runs with the same sequence.
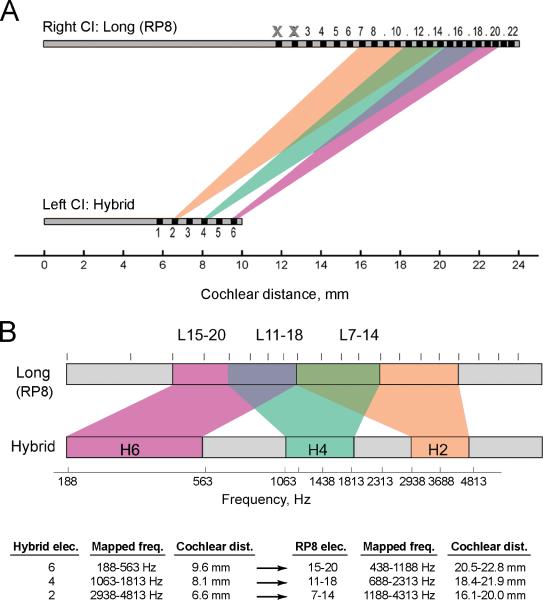
The pitch-matched electrode pairs for the initial results are shown graphically in Fig. 1. Figure 1A shows the electrode pair matches between the two devices when plotted as a function of cochlear position. Note the much deeper maximum potential insertion of the RP8 at 24 mm, compared to the Hybrid at 10 mm. Clearly, there is a large mismatch in cochlear position between the pitch-matched electrode pairs; for example, the deepest electrode in the Hybrid (elec. 6 at 9.6 mm) is matched to the eight deepest electrodes (elec. 15-20 at 20.5-22.8 mm) in the RP8. These correspond to theoretical cochlear frequencies of ~5000 Hz for the Hybrid electrode 6 versus ~590-890 Hz for RP8 electrodes 15-20, based on basilar membrane cochlear frequency maps; spiral ganglion frequency estimates differ <10% from basilar membrane estimates in this frequency range (Greenwood, 1990; Stakhovskaya et al., 2007). The other Hybrid electrodes are similarly matched to RP8 electrodes at deeper cochlear locations.
Figure 1.
Pitch matched electrode pairs for the Long-electrode (RP8) and Hybrid devices as a function of cochlear position (A) and frequency allocation (B). In each plot, the RP8 is shown at top and the Hybrid at bottom. Each shaded region indicates the range of RP8 electrodes that were pitch-matched to a specific Hybrid electrode; orange shows the RP8 electrodes matched with Hybrid electrode 2, green shows the RP8 electrodes matched with Hybrid electrode 4, and violet shows the RP8 electrodes matched with Hybrid electrode 6. A. Pitch-matched electrode pairs plotted versus electrode cochlear position. Clearly, pitch-matched electrode pairs between devices show a large mismatch in cochlear position. B. Pitch-matched electrode pairs plotted versus frequencies allocated by the speech processor. Note that the x-axis is reversed relative to insertion depth and cochlear location to show ascending frequency. The overlap in the frequency axis shows that pitch-matched electrode pairs between devices are aligned by their speech processor frequency allocations. The table below summarizes the frequency allocations and cochlear distances for matched Hybrid and RP8 electrodes.
Pitch perception was studied in a subject with a 10-mm cochlear implant (CI) in one ear and a 24-mm CI in the other ear. Both processors were programmed to allocate information from the same frequency range of 188-7938 Hz, despite the large differences in putative insertion depth and stimulated cochlear locations between the CIs. After 2 and 3 years of experience, pitch-matched electrode pairs between CIs were aligned closer to the processor-provided frequencies than to cochlear position. This finding suggests that pitch perception may have adapted to reduce perceived spectral discrepancies between bilateral CI inputs, despite 2-3 octave differences in tonotopic mapping.
In contrast, Figure 1B shows that when plotted as a function of the frequencies allocated to the electrodes, the pitch-matched electrodes overlap more between devices. The allocated frequencies show considerable overlap for the RP8 electrodes matched to Hybrid electrodes 4 and 2. The RP8 electrodes matched to Hybrid electrode 6 overlap in frequency allocation, but not completely for the lowest frequencies between 188-438 Hz, which may be due in part to order effects biasing the response for electrodes 21/22.
DISCUSSION
This case study of a bilateral cochlear implant patient is highly unusual in that the two devices were implanted at very different insertion depths, but provided information from exactly the same frequency range. Thus, for any single sound frequency from the environment, there was a large discrepancy in cochlear place of stimulation between the two ears. The results suggest that pitch-matched electrode pairs between ears were aligned more by similarity of frequency allocation than by similarity of cochlear position. In other words, spectral mismatches between ears introduced by the CI programming may have caused perceptual adaptation to reduce the perceived discrepancy. Changes in electric pitch relative to acoustic hearing may also be driven by spectral mismatches between electric and acoustic inputs introduced by the CI programming (Reiss et al., 2007; 2008).
It is possible that the same pattern of pitch-matched electrode pairs existed before experience with these frequency allocations; for instance, the long-electrode array could have been bent or kinked so that it did not reach full insertion. However, this is unlikely because clinicians reported uneventful insertion and initial device programming (e.g. no shorts were found), the patient was able to correctly pitch-rank all electrodes tested, and the patient was an exceptional CI user with scores of 90% - 100% for CNC word recognition with the long-electrode CI alone (mean scores in 2008: 35% with Hybrid, 97% with RP8, 94% bilateral).
Note that the results should be interpreted with caution because of the relatively small data set and the order effects on the pitch-matching data. The data provide preliminary evidence that CI users may undergo adaptation to match frequency inputs through the two ears. The observed order effects on the data also illustrate the difficulty in obtaining accurate pitch-match judgments from CI users, likely due to weak or variable pitch strength and differences in sound quality between comparison stimuli.
The term “order effects” here refers to the influence of the previous pitch comparison pair on the judgment of the following comparison pair. Based on the results of this study and previous studies with acoustic-to-electric pitch matching, it is recommended that order effects in CI pitch-matching studies be minimized with pseudo-random sequences that sample every first-order interaction, or at least a subset of such sequences together with counterbalanced mirror sequences. Adaptive procedures and unidirectional pitch comparison sequences (e.g. high to low only) are unlikely to yield repeatable pitch judgments for difficult pitch comparisons because of the strong dependence of the results on the sequence.
The order effects, when not properly minimized, may also account for why other studies did not find acoustic-to-electric pitch matches to align with frequency allocations (Nardo et al., 2007, 2008). Another explanation for the differing results may simply be the variability in adaptation seen across subjects in acoustic-to-electric pitch matching studies (Reiss et al., 2008).
The data suggest that similar to combined electric and acoustic stimulation, the brain can also minimize spectral discrepancies between bilateral CIs. If pitch adaptation is possible for stimulation of a Hybrid CI and a standard CI at very disparate cochlear locations, then pitch adaptation is certainly possible for stimulation of two standard CIs at less disparate cochlear locations. Previous studies with the Hybrid CI suggest that this adaptation may take months, but the time scale may also depend on the amount of discrepancy and the individual's ability to adapt. An analogous study is the re-alignment of the auditory-visual maps in the adult barn owl after 10 weeks of experience with visual prisms, which shift the visual space map relative to the auditory space map; a correlation was found between the amount of adaptation and subsequent localization performance (Bergan et al., 2005). However, other recent studies showed interaural frequency map changes in auditory cortex after just 6 weeks of stimulation with a single frequency tone (Pienkowski and Eggermont, 2009) or experience with asymmetric hearing loss (Cheung et al., 2009).
The results raise an interesting possibility that two spectrally “abnormal” inputs can be realigned relative to each other rather than relative to a “normal” input such as acoustic hearing; this suggests a model of perceptual adaptation based on matching temporally correlated inputs, rather than a model based on matching a preexisting template of pitch perception. The perceptual adaptation model utilizes the neural mechanism of spike-timing dependent plasticity (STDP), the strengthening of weak synaptic inputs that fire simultaneously with other inputs and the converse, the weakening of synaptic inputs that do not fire with other inputs. Thus, correlated temporal fluctuations in the amplitude envelopes of spike activity between inputs, at the time scale of neural integration, can induce plasticity. Note that such a model would not require the final pitch associated with any electrode to be tonotopically correct, just matched in pitch to other inputs that are stimulated simultaneously together with this electrode in everyday listening conditions, such as another electrode that receives the same frequency range of information. This model also does not require the pitch percept to be a pure tone; the pitch may be noise-like or even multidimensional in quality (Collins and Throckmorton, 2000). Finally, the comparison of electric to electric inputs, rather than electric to acoustic inputs, eliminates any possible confounds due to limited residual hearing frequency range, potential shifts in peripheral frequency coding in the hearing-impaired ear, and large differences in sound quality between electric and acoustic hearing, which may account for some of the variability seen in previous studies comparing electric to acoustic pitch. Thus, this case study also provides a clearer picture of the relationship of the pitch alignment of multiple electrodes to the CI programming than seen previously for unilateral CI subjects.
ACKNOWLEDGMENTS
The authors would like to thank Marjorie Leek and four anonymous reviewers for helpful comments on the manuscript.
This work was supported by NIDCD grants F32 DC009157, RO1DC000377 and 2P50 DC00242, and by GCRC/NCRR grant RR00059
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Blamey PJ, Dooley GJ, Parisi ES, Clark GM. Pitch comparisons of acoustically and electrically evoked auditory sensations. Hear Res. 1996;99(1–2):139–150. doi: 10.1016/s0378-5955(96)00095-0. [DOI] [PubMed] [Google Scholar]
- Bergan JF, Ro P, Ro D, Knudsen EI. Hunting increases adapting auditory map plasticity in adult barn owls. J Neurosci. 2005;25(42):9816–9820. doi: 10.1523/JNEUROSCI.2533-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boex C, Baud L, Cosendai G, Sigrist A, Kos MI, Pelizzone M. Acoustic to electric pitch comparisons in cochlear implant subjects with residual hearing. J Assoc Res Otolaryngol. 2006;7(2):110–124. doi: 10.1007/s10162-005-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyon RP, Macherey O, Frijans J, Axon P, Kalkmann R, Boyle P, Baguley D, Briggs J, Deeks J, Briaire J, Barreau X, Dauman R. Pitch matching between acoustic and electric stimulation by cochlear implant patients with normal hearing in the unimplanted ear.. Abstracts of the 2009 Assoc for Res in Otolaryngol Midwinter Meeting, #448..2009. [Google Scholar]
- Cheung SM, Bonham BH, Schreiner CE, Godey B, Copenhaver DA. Realignment of interaural cortical maps in asymmetric hearing loss. J Neurosci. 2009;29(21):7065–7078. doi: 10.1523/JNEUROSCI.6072-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Throckmorton CS. Investigating perceptual features of electrode stimulation via a multidimensional scaling paradigm. J Acoust Soc Am. 2000;108(5 Pt 1):2353–2365. doi: 10.1121/1.1314320. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Smith M, Smith L, Parkin JL. The pitch of electrically presented sinusoids. J. Acoust. Soc. Am. 1994;95:1677–1679. doi: 10.1121/1.408558. [DOI] [PubMed] [Google Scholar]
- Eddington DK, Dobelle WH, Brackmann DE, Mladejovsky MG, Parkin JL. Auditory prostheses research with multiple channel intracochlear stimulation in man. Ann Otol Rhinol Laryngol. 1978;87(6 Pt 2):1–39. [PubMed] [Google Scholar]
- Gantz BJ, Turner CW. Combining acoustic and electric hearing. Laryngoscope. 2003;113:1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Greenwood D. A cochlear frequency-position function for several species--29 years later. J. Acoust Soc Am. 1990;87(6):2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- McDermott H, Sucher C, Simpson A. Electro-Acoustic Stimulation. Audiol Neurootol. 2009;14(Suppl 1):2–7. doi: 10.1159/000206489. [DOI] [PubMed] [Google Scholar]
- McKay CM, McDermott HJ, Carlyon RP. Place and temporal cues in pitch perception: Are they truly independent? ARLO. 2000;1:25–30. [Google Scholar]
- Nardo WD, Cantore I, Cianfrone F, Melillo P, Fetoni AR, Paludetti G. Differences between electrode-assigned frequencies and cochlear implant recipient pitch perception. Acta Oto-Laryngol. 2007;127:370–377. doi: 10.1080/00016480601158765. [DOI] [PubMed] [Google Scholar]
- Nardo WD, Cantore I, Marchese MR, Cianfrone F, Scorpecci A, Glannantonio S, Paludetti G. Electric to acoustic pitch matching: a possible way to improve individual cochlear implant fitting. Eur Arch Otorhinolaryngol. 2008;265:1321–1328. doi: 10.1007/s00405-008-0655-3. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Eggermont J. Long-term, partially reversible reorganization of frequency tuning in mature cats can be induced by passive exposure to moderate-level sounds. Hear Res. 2009;257(1-2):24–40. doi: 10.1016/j.heares.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Reiss LA, Turner CW, Erenberg SR, Gantz BJ. Changes in pitch with a cochlear implant over time. J Assoc Res Otolaryngol. 2007;8(2):241–257. doi: 10.1007/s10162-007-0077-8. Epub 2007 Mar 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss LA, Gantz BJ, Turner CW. Cochlear implant speech processor frequency allocations may influence pitch perception. Otol Neurotol. 2008;29(2):160–167. doi: 10.1097/mao.0b013e31815aedf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV. Multichannel electrical stimulation of the auditory nerve in man. I. Basic psychophysics. Hear Res. 1983;11:157–189. doi: 10.1016/0378-5955(83)90077-1. [DOI] [PubMed] [Google Scholar]
- Stakhovskaya O, Sridhar D, Bonham BM, Leake PA. Frequency map for the human cochlear spiral ganglion: Implications for cochlear implants. J Assoc Res Otolaryngol. 2007;8(2):220–233. doi: 10.1007/s10162-007-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong YC, Clark GM, Blamey PJ, Busby PA, Dowell RC. Psychophysical studies for two multiple-channel cochlear implant patients. J Acoust Soc Am. 1982;71:153–160. doi: 10.1121/1.387342. [DOI] [PubMed] [Google Scholar]
- Townshend B, Cotter N, van Compernolle D, White RL. Pitch perception by cochlear implant subjects. J Acoust Soc Am. 1987;82:106–115. doi: 10.1121/1.395554. [DOI] [PubMed] [Google Scholar]
- Zeng FG. Temporal pitch in electric hearing. Hear Res. 2002;174:101–106. doi: 10.1016/s0378-5955(02)00644-5. [DOI] [PubMed] [Google Scholar]