Summary
Axin1 is a critical negative regulator of the canonical Wnt-signaling pathway. It is a concentration-limiting factor in the β-catenin degradation complex. Axin1 null mutant mouse embryos died at embryonic day 9.5, precluding direct genetic analysis of the roles of Axin1 in many developmental and physiological processes using these mutant mice. In this study, we have generated mice carrying two directly repeated loxP sites flanking the exon 2 region of the Axin1 gene. We show that floxed-allele-carrying mice (Axin1fx/fx) mice appear normal and fertile. Upon crossing the Axin1fx/fx mice to the CMV-Cre transgenic mice, the loxP-flanked exon 2 region that encodes the N-terminus and the conserved regulation of G-protein signaling domain was efficiently deleted by Cre-mediated excision in vivo. Moreover, we show that mouse embryos homozygous for the Cre/loxP-mediated deletion of exon 2 of the Axin1 gene display embryonic lethality and developmental defects similar to those reported for Axin1−/− mice. Thus, this Axin1fx/fx mouse model will be valuable for systematic tissue-specific dissection of the roles of Axin1 in embryonic and postnatal development and diseases.
Keywords: Axin1, conditional inactivation, Cre-LoxP
INTRODUCTION
Axin1 is a multidomain scaffolding protein that interacts with multiple proteins and serves as a key negative regulator of canonical Wnt signaling by the β-catenin destruction complex (Clevers, 2006; MacDonald et al., 2009). Axin1 has been reported to be the rate limiting factor for the β-catenin destruction complex assembly (Lee et al., 2003; Salic et al., 2000). In addition, Axin1 is also involved in regulation of TGF-β, SAPK/JNK, and P53 signaling pathways (Liu et al., 2006; Rui et al., 2004; Zhang et al., 1999). Recent data demonstrated that Axin1 is a central coordinator of Myc degradation (Arnold et al., 2009).
Complete inactivation of Axin1 function leads to early embryonic lethality at E9.5 with forebrain truncation, neural tube defects, and embryonic axis duplications (Chia and Costantini, 2005; Chia et al., 2009; Perry et al., 1995; Zeng et al., 1997). This does not allow identification of potential function in later developmental processes in which Axin1 is also likely to play critical roles (e.g., bone development and remodeling). To investigate Axin1 function at later stages of embryogenesis and in postnatal mice, a conditional allele for Axin1 inactivation where Axin1 function is disrupted in tissue- and stage-specific manner would be valuable.
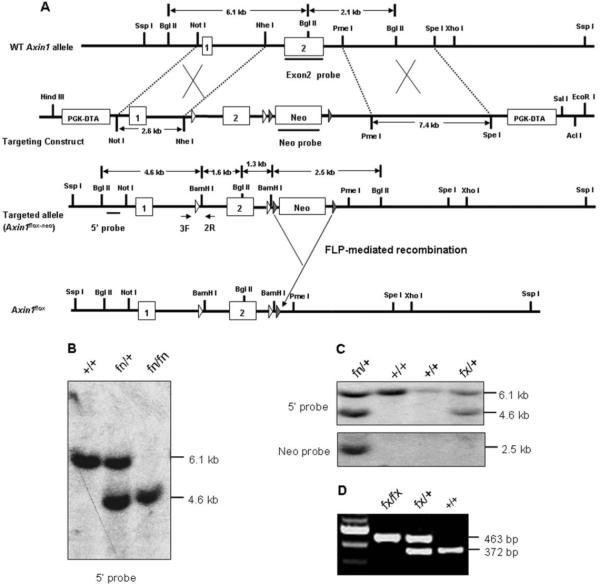
The Axin1 gene consists of 10 exons spanning ~56 kb (Zeng et al., 1997). In the Axin1 null allele (Perry et al., 1995), exon 2 and parts of the two flanking introns are deleted and exons 1 and 3 are separated by a ~600-kb transgene insertion (Zeng et al., 1997). We designed a targeting construct to disrupt exon 2, which encodes the first two AUG codons and highly conserved regulation of G-protein signaling domain (Zeng et al., 1997). Two loxP sites were inserted in the same orientation upstream and downstream of exon 2. A Neo-TK (thymidine kinase) cassette with two flanking Frt sites was inserted downstream of second loxP site. The AclI-linearized targeting vector was electroporated into SV129 embryonic stem (ES) cells. One hundred and twenty G418-resistant ES cell colonies were selected and then screened by Southern blot analysis for homologous recombination using 5′ external probe (Fig. 1A). Two colonies demonstrated the 6.1-kb wild-type and 4.6-kb targeted bands by Southern blotting with the 5′ probe (Fig. 1B). Two Axin1-targeted ES clones were injected into C57BL/6J blastocysts for the production of chimeric animals. Three male chimeras produced only one transmitted the floxed allele to the germline as demonstrated by PCR genotyping and Southern blot analysis. The F1 heterozygous (fn/+; fn denotes floxP-neo) mice were mated, and homozygous (fn/fn) progenies were identified by PCR and Southern blot analysis (Fig. 1B). Both Axin1fn/+ and Axin1fn/fn mice were viable, fertile, and did not display any obvious phenotypic abnormality, indicating that the insertion of loxP and Frt-Neo-Frt cassette does not alter Axin1 gene expression.
FIG. 1.
Generation of mice carrying Axin1fn (flox-neo) and Axin1fx/fx alleles. A: Targeting construct for inactivation of the Axin1 gene. The wild-type Axin1 locus, targeting construct, targeted allele, and flox allele are shown schematically. The boxes numbered 1–2 represent the exons 1 and 2. Open triangles represent the loxP sequence. The filled triangles represent the frt sites. The PKG-promoter-driven DT-A and Neo-TK cassette are indicated by boxes. The bars represent the probes for Southern blot analysis. The flox allele was generated by crossing targeted allele with FLPeR mice. A 2.6-kb NotI/NheI fragment represents 5′ homologous arm. A 7.4-kb PmeI/SpeI fragment represents the 3′ homology arm. The arrows indicate the priming position for primers 3F and 2R, which were used to detect both wild-type and floxed alleles. B: Southern blot analysis of BamHI-BglII genomic DNA prepared from tail biopsies of wild-type (+/+), heterozygous (fn/+, fn denotes floxP-neo), and homozygous (fn/fn) Axin1 flox-neo mice using 5′ external probe. Presence of wild-type and targeted alleles are indicated by 6.1-kb and 4.6-kb fragments, respectively. C: Southern blot analysis of genomic DNA using 5′ probe and Neo probe confirmed that Neo-TK cassette was removed by the Flp recombinase. The 2.5-kb band represents the Neo-TK cassette. D: A representative agarose gel image of PCR genotyping using primers 3F and 2R. A 372-bp band represents wild-type alleles, and the 463-bp band represents the floxed allele.
The Frt-flanked neo selection marker was deleted in vivo by breeding the Axin1fn/+ or Axin1fn/fn mice with the FLPeR mice, which constitutively express the FLPe recombinase in most tissue types, including cells of the developing germ line (Farley et al., 2000). The Axin1fx/+ (fx denotes floxP with neo cassette deleted) mice, which have two loxP sites flanking Exon 2 of the Axin1 gene and one Frt site in the Axin1 allele, were confirmed by PCR and Southern blot analysis using 5′ and neo probes (Fig. 1C). Axin1fx/+ mice were then inter-crossed to generate the homozygous Axin1fx/fx conditional mice (Fig. 1D). The Axin1fx/fx mice were viable and fertile and did not present any recognizable phenotype.
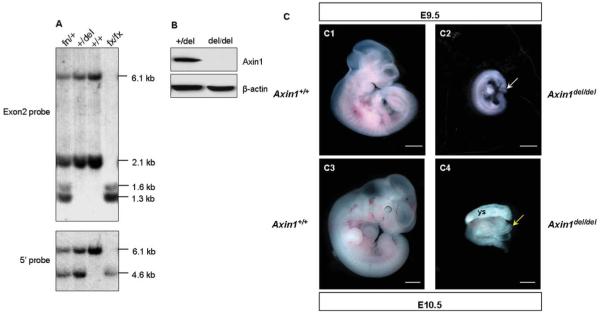
To demonstrate that the loxP flanked exon 2 of the Axin1 gene could be deleted in vivo and is essential for Axin1 function, the Axin1fx/fx mice were mated with TgCMV-Cre transgenic mice (Schwenk et al., 1995), in which Cre recombinase is expressed under the control of the human cytomegalovirus (CMV) promoter that is active in most cells and tissues. The exon 2-deleted mouse was confirmed by PCR and Southern blot analysis using exon 2 probes (Fig. 2A). Both 6.1 kb and 2.1 kb bands representing the wild-type allele were detected in Axin1fx/+, Axin1+/del (del denotes exon 2 deleted), and wild-type mice, but absent in Axin1fx/fx mice. About 1.3 kb and 1.6 kb bands representing in targeted allele were observed in Axin1fx/+ and Axin1fx/fx mice but were absent in Axin1+/del and wild-type mice. The genotypes of the mice were confirmed by hybridizing the same membrane to the 5′ external probe (Fig. 2A). Axin1+/del mice heterozygous for the Axin1 gene are viable, fertile, and did not display any obvious visible abnormalities. To analyze the effect of a homozygous deletion of exon 2 of the Axin1 gene, Axin1+/del mice were intercrossed. The Axin1del/del homozygous mouse was confirmed by PCR analysis. Next, we performed Western blot analysis using cell lysates extracted from whole embryos (Fig. 2B). A strong Axin1 band was observed in the Axin1+/del extracts, but no signal was observed in Axin1del/del extracts. These data demonstrated that, as expected, the Axin1 protein is not produced in Axin1del/del mutant embryos.
FIG. 2.
Axin1del/del embryos died during early embryonic development. A: Southern blot analysis using 5′ probe and exon 2 probe confirmed the deletion of exon 2 of the Axin1 gene in Axin1+/del mice. The genomic DNA was digested with BamHI and BglII. The 5′ probe detected 6.1-kb and 4.6-kb fragments for wild-type allele and flox allele, respectively. The exon 2 probe detected 6.1-kb and 2.1-kb fragments for wild-type allele and 1.3-kb and 1.6-kb fragments for flox allele. B: Western blot analysis on Axin1 in Axin1+/del and Axin1del/del embryos. Total protein extract from E9.5 embryos were blotted for Axin1 and β-actin (the loading control). C: Axin1del/del mice display an embryonic lethal phenotype. E9.5 and E10.5 Axin1del/del embryos are much smaller than their wild-type littermates (C1–C4). Axin1del/del embryos show underdeveloped head folds (C2, white arrow) and open head folds (C4, yellow arrow). Ys, yolk sac. Bars: 0.5 mm.
Loss of Axin1 results in early embryonic lethality. The Axin1−/− homozygous embryos died at E9.5, displaying a wide spectrum of abnormalities including incomplete closure or malformation of head folds, crooked neural tube, cardia bifida, and duplication of embryonic axis (Perry et al., 1995; Zeng et al., 1997). The Axin1del/del mice exhibit recessive embryonic defects very similar to those caused by the null allele Axin1Tg1 (Chia et al., 2009; Perry et al., 1995; Zeng et al., 1997). Twenty-seven embryos from intercrossed among Axin1+/del mice were examined at E10.5 and seven Axin1del/del homozygotes were found, consistent with the expected Mendelian ratio. Eighteen embryos from intercrossed among Axin1+/del mice were also examined at E9.5 and four Axin1del/del homozygotes were found as the expected frequency. All embryos of the homozygotes were severely abnormal. They were significantly smaller than their wild-type or heterozygous littermates (Fig. 2C) and displayed underdeveloped head folds (Fig. 2C2) and open head folds (Fig. 2C4). The embryos of heterozygotes were indistinguishable from that of wild-type littermates (data not shown). Therefore, the deleted allele should represent a null allele of Axin1.
In summary, we have successfully generated a conditional null Axin1 allele and showed that deletion of exon 2 of the Axin1 gene leads to recessive lethal phenotype very similar to those of Axin1−/− mice. These mice provide a powerful tool to determine the physiological role of Axin1 during late embryonic development and postnatal life and a mouse model to investigate organ and tissue-specific function of Axin1.
METHODS
Generation of Mice Carrying the Floxed and Knockout Axin Alleles
A bacterial artificial chromosome (BAC) clone containing the Axin1 genomic region was obtained by screening the RPCI-22 129/SvEvTac mouse BAC library (BACPAC Resources, Children’s Hospital of Oakland, Oakland, CA). A 14.1-kb NotI-XhoI fragment containing exons 1 and 2 was subcloned into pBluescript plasmid vector for the construction of the targeting vector. A replacement targeting vector was constructed with a diphtheria toxin (DTA) expression cassette, a 2.6-kb NotI/NheI fragment as 5′ homologous arm, a loxP site, a 2.6-kb NheI/PmeI fragment containing exon 2 of the Axin1 gene, a second loxP site (in the same orientation as the first one), an Frt-flanked Neo/thymidine kinase (Neo-TK) positive selection cassette, a 7.4-kb PmeI/SpeI fragment as the 3′ homology arm and another PGK-DTA expression cassette as negative selection marker.
The targeting vector was linearized with AclI and electroporated into SV129 mouse ES cells, which were selected with 175 mg/ml of G418. G418-resistant ES clones were screened by Southern blot analysis of BamHI/BglII-digested ES cells genomic DNA with a 5′ external probe. The targeting frequencies were 2 of 120. The two independent targeted ES clones were microinjected into C57BL/6J host blastocysts. Three chimeras were bred to C57BL/6 mice, and F1 agouti off-spring mice were genotyped by PCR and Southern blot hybridization to validate the germline transmission. The Frt-flanked neo gene was deleted in vivo by breeding the Axin1fn/+ or Axin1fn/fn mice with the FLPeR mice (Farley et al., 2000). Exon 2 of the Axin1 gene was deleted by crossing the Axin1fx/fx mice with TgCMV-Cre transgenic mice (Schwenk et al., 1995). The C57BL/6, FLPeR (Farley et al., 2000), and TgCMV-Cre (Schwenk et al., 1995) mice were obtained from The Jackson Laboratory.
Genotyping
Genomic DNA was isolated from mouse tail and analyzed by Southern blotting according to standard protocols. Mice carrying wild-type and Axin1fx alleles were genotyped in a single PCR reaction using a forward primer 3F (TGAACTCTTGATCAGGTCTTG) and reverse primer 2R (TATGATCTTTGGTCCTTTCTG), which amplify the wild-type (372-bp) and Axin1fx (463-bp) alleles. PCR reactions for genotyping were carried out under the following conditions: 94°C for 5 min, 35 cycles at 94°C, and 59°C each for 30 s, followed by extension at 72°C for 1 min and final extension at 72°C for 5 min.
Western Blot
Embryos were dissected at E9.5 and homogenized in a homogenizer with RIPA buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, and 1% sodium deoxycholate) with protease inhibitor cocktail (Sigma). Protein concentrations were measured using Coomassie Plus Protein Assay kit (Pierce, Rockford, IL). About 30 micrograms protein samples were separated in a denaturing 10% SDS–PAGE gel (Invitrogen) and transferred to nitrocellulose membrane. The membranes were washed, blocked (5% milk in PBS-T), and incubated in the appropriate antibodies overnight at 4°C. Antibodies for Western blot analysis: rabbit anti-Axin1 (Zymed Laboratories, South San Francisco, CA) at a dilution of 1:500 and mouse anti-β-actin (Sigma) at a dilution of 1:5000. Secondary antibodies were horseradish peroxidase conjugated. Visualization was done with Pico or Femto chemiluminescent agents (Pierce).
Acknowledgments
Contract grant sponsor: National Institute of Health, Contract grant number: R01 AR051189, Contract grant number: R01 AR054465, Contract grant number: R01 AR055915, Contract grant sponsor: New York State Department of Health and Empire State Stem Cell Board, Contract grant number: N08G-070
LITERATURE CITED
- Arnold HK, Zhang X, Daniel CJ, Tibbitts D, Escamilla-Powers J, Farrell A, Tokarz S, Morgan C, Sears RC. The Axin1 scaffold protein promotes formation of a degradation complex for c-Myc. EMBO J. 2009;28:500–512. doi: 10.1038/emboj.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia IV, Costantini F. Mouse Axin and Axin2/conducting proteins are functionally equivalent in vivo. Mol Cell Biol. 2005;25:4371–4376. doi: 10.1128/MCB.25.11.4371-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia IV, Kim MJ, Itoh K, Sokol SY, Costantini F. Both the RGS domain and the six C-terminal amino acids of mouse Axin are required for normal embryogenesis. Genetics. 2009;181:1359–1368. doi: 10.1534/genetics.109.101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (Flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Rui H, Wang J, Lin S, He Y, Chen M, Li Q, Ye Z, Zhang S, Chan SC, Chen YG, Han J, Lin SC. Axin is a scaffold protein in TGF-β signaling that promotes degradation of Smad7 by Arkadia. EMBO J. 2006;25:1646–1658. doi: 10.1038/sj.emboj.7601057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/b-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry WL, III, Vasicek TJ, Lee JJ, Rossi JM, Zeng L, Zhang T, Tilghman SM, Costantini F. Phenotypic and molecular analysis of a transgenic insertional allele of the mouse Fused locus. Genetics. 1995;141:321–332. doi: 10.1093/genetics/141.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Xu Z, Lin S, Li Q, Rui H, Luo W, Zhou HM, Cheung PY, Wu Z, Ye Z, Li P, Han J, Lin SC. Axin stimulates p53 functions by activation of HIPK2 kinase through multimeric complex formation. EMBO J. 2004;23:4583–4594. doi: 10.1038/sj.emboj.7600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic A, Lee E, Mayer L, Kirschner MW. Control of β-catenin stability: Reconstitution of the cytoplasmic steps of the Wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WLI, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Neo SY, Wang X, Han J, Lin SC. Axin forms a complex with MEKK1 and activates c-Jun NH2-terminal kinase/stress-activated protein kinase through domains distinct from Wnt signaling. J Biol Chem. 1999;274:35247–35254. doi: 10.1074/jbc.274.49.35247. [DOI] [PubMed] [Google Scholar]