Abstract
Asthma in children with sickle cell anemia (SCA) is associated with increased morbidity and mortality. However, the definition of asthma in SCA is based on a physician's impression. In a retrospective cohort of children with SCA, relationships between a physician diagnosis of asthma and total and allergen specific IgE levels were evaluated. In children with SCA, elevated total and specific IgE levels were significantly associated with a diagnosis of asthma (P<0.05), further supporting the concept that asthma is a separate co-morbid condition of SCA.
Keywords: sickle cell disease, IgE, asthma
Background
Sickle cell anemia (SCA) and asthma are two common chronic diseases in children. The presence of asthma in individuals with SCA increases rates of acute chest syndrome (ACS) 1, 2 and pain episodes 1 and increases the risk of premature death 1-3. Despite the strong association between asthma and SCA-related morbidity and mortality, the definition of asthma among children with SCA is not clear, as SCA without associated asthma can produce recurrent respiratory symptoms.
One strategy to provide evidence that asthma is a distinct clinical entity among children with SCA is to evaluate whether asthma risk factors are associated with physician diagnosis of asthma. Phillips et al. demonstrated that there is a familial pattern of inheritance of asthma in patients with SCA 4. These data suggest, but do not confirm, that children with SCA and asthma-like symptoms can have a lung disease due to asthma and not just symptoms from SCA alone.
An elevated level of immunoglobulin E (IgE) is considered a risk factor for asthma. The level of total IgE has been shown to be associated with physician-diagnosed asthma and the presence of airway hyper-responsiveness 5. Burrows et al. demonstrated that the prevalence of asthma increased as the level of total serum IgE increased 6. Further, Naqvi et al. showed that higher IgE levels were associated with an increase in symptom-based and pulmonary function-based asthma severity in children 7.
Among children with asthma, the presence of allergen-specific IgE is common. Several studies evaluated a positive result of Phadiatop, a laboratory analysis to detect allergen-specific IgE antibodies to inhalant allergens, as a potential risk factor for asthma. Lilja et al. tested children at risk of developing asthma at 6 months, 18 months, and 5 years of age with Phadiatop. Seventy-five percent of the children positive at 6 months developed asthma at 18 months. By the age of five, all children in the study had asthma 8. Further, positive tests for food allergens are associated with more severe asthma symptoms 9. Studies have shown that a positive skin test reaction to Alternaria is associated with the presence of asthma, new onset of asthma, and greater severity of asthma 10.
We conducted a retrospective cohort analysis to test the hypothesis that a physician diagnosis of asthma is associated with elevated total and allergen specific IgE levels in children with SCA. Demonstration of relationships between total and allergen specific IgE levels and a clinical diagnosis of asthma would provide additional evidence that asthma is a separate co-morbid condition associated with SCA, rather than a lung disease phenotype mimicking asthma.
Design and Methods
This study was approved by the Institutional Review Board of Washington University School of Medicine and the Ethics Committee for the Centre Hospitalier Intercommunal Créteil (CHIC) 2. The de-identified data analyzed was obtained from children with SCA who were followed for a minimum of 6 months at the CHIC from 1980 to 2007 2. At least annually, patients were followed at the CHIC for interval and medication histories, a physical exam, evaluation of SCA-related morbidity and other co-morbid conditions, and laboratory testing consisting of complete blood analyses 2.
A pain episode was defined as an event in which pain in the head, chest, back, abdomen, or extremities resulted in hospitalization 2. ACS was defined as a fever, respiratory signs, or thoracic pain associated with a new radiographic finding of the lungs 2. The diagnosis of asthma was made when a child with SCA had at least 3 episodes of bronchiolitis under two years of age or when wheezing was heard at hospitalization or at the time of a clinic visit. Asthma diagnosis was confirmed based on an audit by a pediatric pulmonologist documenting more than one episode of wheezing 2.
Four prominent asthma risk factors, total serum IgE level 5, 6, peripheral blood eosinophil count 11, respiratory allergy (aeroallergen specific IgE levels) 6, and food allergy (food specific IgE levels) 9 were assessed. IgE levels were age-adjusted. An elevated total IgE level was defined as two standard deviations above the population mean after age adjustment 12. An elevated eosinophil count was defined as the absolute number of eosinophils > 350 per cubic millimeter 13. Respiratory sensitization was determined by the presence of a positive Phadiatop test, which evaluated specific inhalant allergens including acarids of house dust, herbaceous plant, various grass pollens, molds (Alternaria and Aspergillus), cat, and dog. Food sensitization was determined by the presence of a positive Fx5 test for the allergens milk, egg, groundnut, soya, wheat, and fish.
Given that absolute IgE values are not normally distributed and are heavily skewed, we transformed the values using a log normal distribution and performed a t-test to evaluate the difference in mean IgE levels in the presence or absence of physician diagnosis of asthma 5, 6 (Figures 1 and 2). A chi-square test was performed to evaluate the difference in specific IgE level in the presence or absence of a physician-diagnosis of asthma. Incidence rates of ACS episodes and painful events were assessed using negative binomial regression correcting for over-dispersion after adjustments for factors known to affect pain and ACS: average hemoglobin concentration, white blood cell count, fetal hemoglobin level, and age. Version 9.1 of SAS system for Windows was used for statistical analyses. A threshold of statistical significance of 0.05 was used for all analyses.
Figure 1.
IgE levels in children with sickle cell anemia with and without asthma.
The absolute IgE values for this cohort of children with sickle cell anemia are heavily skewed to the left. Given the distribution of the IgE values, the values were log transformed for all analyses.
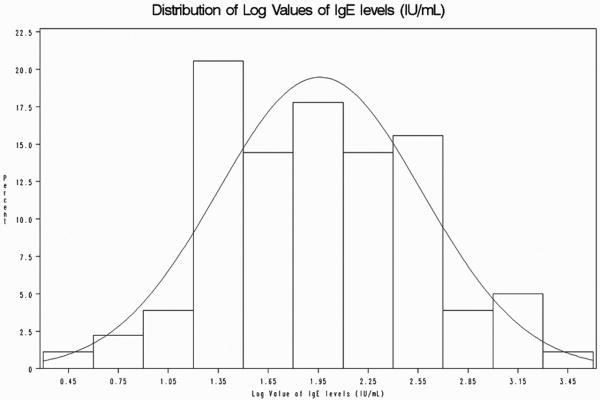
Figure 2.
Logarithmic IgE values in children with sickle cell anemia with and without asthma.
Logarithmic transformation of the IgE values normalized the data. The log normal IgE values were used to perform a t-test to evaluate the difference in mean IgE levels in the presence or absence of physician diagnosis of asthma.
Results
Demographics
The medical records of 324 children with SCA (Hb SS) followed for at least six months were reviewed. The medical records indicated that 297 children were evaluated for asthma. In the cohort, 25 children (8%) were diagnosed with asthma. The demographic features for children with and without asthma are listed in Table 1.
Table 1.
Demographics of children with sickle cell anemia with and without asthma in this retrospective cohort (n= 297).
| Variables | Asthma | Non-asthma | P |
|---|---|---|---|
| Number of children, n (% of group) | 25 (8%) | 272 (92%) | |
| Age, years | 4.0 (4.2) | 4.0 (4.8) | 0.35 |
| Gender-male, n (% of group) | 14 (56%) | 140 (51%) | 0.66 |
| Hemoglobin (g/dl) | 9.1 (1.4) | 9.0 (1.7) | 0.30 |
| White blood cell count (/mm3) | 10916 (3796) | 11915 (4627) | 0.21 |
| Hemoglobin F (%) | 9.2 (5.6) | 8.6 (7.2) | 0.47 |
| Eosinophil count (/mm3) | 529 (378) | 414 (487) | 0.08 |
There were no significant differences in the demographic variables between children with and without asthma. All values are mean (standard deviation) unless otherwise indicated.
IgE Levels in children with SCA
The mean IgE level for children with asthma was compared to children without asthma, a statistically significant difference was noted, 623 IU/mL and 207 IU/mL, respectively, P=0.005. Overall, the mean total IgE levels are higher in patients with SCA independent of asthma status when compared to the general population. In general, the expected normal IgE level is 62 IU/mL for the mean age adjusted IgE level in the French population; whereas, in this study population the mean total IgE level is 234.8 IU/mL 14. There was no significant difference in the percentage of children with age-adjusted IgE level elevation among children with and without asthma, 72% (13 of 18) and 70% (148 of 211), respectively, P=0.85.
Asthma and allergen-specific IgE Levels in children with SCA
Children with a physician diagnosis of asthma demonstrated more sensitization to respiratory and food allergens when compared to children without asthma. Seventy-nine percent of children (15 of 19) with a clinical diagnosis of asthma had positive Phadiatop results as compared to 43% of children without a clinical diagnosis of asthma (92 of 215), P=0.03. Among children with asthma, 61% (11 of 18) had positive Fx5 test results compared to 35% (63 of 179) of children without asthma, P=0.002.
IgE Levels and SCA-morbidity in children
IgE levels, total and specific for respiratory and food allergens, were not associated with adjusted pain or ACS rates in this cohort. The pain rate was not significantly different between children with and without an elevated total IgE level (0.57 episodes per year, 95% Confidence Interval (CI) 0.46 to 0.71 and 0.66 episodes per year, 95% CI 0.48 to 0.90, respectively P=0.47). The rate of ACS was not significantly different for children with and without an elevated total IgE (0.17 episodes per year, 95% CI 0.13 to 0.21 and 0.15 episodes per year, 95% CI 0.11 to 0.22, respectively P=0.65).
The pain rate was not significantly different between children with and without a positive Phadiatop results (0.58 episodes per year, 95% CI 0.45 to 0.73 and 0.62 episodes per year, 95% CI 0.48 to 0.78, respectively P=0.47). The rate of ACS was not significantly different between children with and without positive Phadiatop results (0.17 episodes per year, 95% CI 0.13 to 0.22 and 0.15 episodes per year, 95% CI 0.11 to 0.19,respectively P=0.38).
The pain rate was not significantly different between children with and without positive Fx5 test results (0.55 episodes per year, 95% CI 0.39 to 0.78 and 0.60 episodes per year, 95% CI 0.47 to 0.76, respectively P=0.71). The ACS rate was not significantly different between children with and without positive Fx5 test results (0.16 episodes per year, 95% CI 0.11 to 0.24 and 0.17 episodes per year, 95% CI 0.14 to 0.22, respectively P=0.77).
Discussion
A physician diagnosis of asthma among children with SCA is associated with an increase in morbidity and mortality, yet few studies have documented objective laboratory findings associated with asthma. The prevalence of asthma in our study population was 8%, similar to previous studies evaluating the prevalence of asthma in children of African descent in France 15, 16. We show that total and specific IgE levels are associated with a diagnosis of asthma in children with SCA. Our findings provide evidence that asthma in children with SCA is similar to asthma in children without SCA.
No association existed between total IgE levels or specific IgE levels with pain and ACS rates. This finding was unexpected given the previously documented association between asthma and the rate of pain and ACS in children with SCA. We can only postulate as to why there was no evidence to support our hypothesis that elevated IgE levels are associated with increased rate of SCA morbidity. One possibility is that the association between SCA morbidity in asthma is independent of the IgE pathway. Alternatively, the previous association is spurious, namely that the relationship between SCA morbidity and asthma is false. Our study design could not distinguish between these two possibilities. Future studies that include transgenic SCA mouse models with experimentally induced asthma or large clinic studies are warranted to explore specific and total IgE level relationships with SCA morbidities 17.
As expected in this retrospective cohort study, several limitations exist. A significant limitation is the low number of children with a physician diagnosis of asthma (n=25). Despite the small number, we were able to validate the established relationship between asthma and total and specific IgE levels in children with SCA. However, we were unable to demonstrate a relationship between pain and ACS rates with specific or total IgE levels. Another limitation was our inability to pursue other causes of increased IgE levels. Despite not being able to document other causes of an elevated IgE level, most children with the other diagnoses known to be associated with elevated IgE levels, such as cystic fibrosis and inflammatory bowel disease, have significant clinical histories 18, 19. No documentation of these co-morbidities was identified in the medical records.
The pathogenesis of a marked increase in IgE levels in SCA is unknown. The possibilities include that IgE levels may be a non-specific response to the chronic inflammatory state of SCA, as elevated IgE levels has been associated with other chronic inflammatory diseases such as inflammatory bowel disease18. A second possibility is that children with SCA have an increased baseline white blood cell count with corresponding absolute eosinophilia. An increase in the eosinophil count may account for the increase in total IgE levels; although, this possibility does explain the higher IgE level among children with asthma when compared to children without asthma, it may also explain the elevation in IgE levels in children without asthma. Another possibility involves the role of T-helper 2 (Th2) cytokines in IgE production. Interleukin-4 (IL-4) and interleukin-13 (IL-13) are pivotal in regulating the IgE response20. The IL-4 and IL-13 pathway have a common component, because the two cytokines share a receptor subunit 20. We could not address the etiologies for elevated IgE levels; however, future work focused on the pathogenesis of elevated IgE levels in this patient population should interrogate these two interrelated pathways.
In summary, we provide further evidence that asthma is a distinct co-morbid condition of SCA by demonstrating total and allergen specific IgE levels are associated with a clinical diagnosis of asthma. Additionally, the mean IgE levels in this patient population are higher than in the general population. However, the mechanism for the increase in IgE levels in children with SCA is not known. Future studies focusing on the etiology of elevated IgE levels in this patient population are warranted.
Table 2.
Total and specific IgE levels in children with sickle cell anemia with and without asthma.
| Variables | Asthma (n= 25) | Non-asthma (n=272) | P |
|---|---|---|---|
| Total IgE, mean (SD) | 623 (941) | 207 (339) | 0.005 |
| Food sensitization present n, (% of group) † | 11 (61%) | 63 (35%) | 0.03 |
| Respiratory sensitization present n, (% of group) †† | 15 (79%) | 92 (43%) | 0.002 |
Mean IgE levels are shown and compared using a student t-test for children with and without asthma. The mean total IgE level significantly differed between children with and without asthma (p=0.005). A chi-square test was performed to evaluate the difference in specific IgE level in the presence or absence of a physician diagnosis of asthma. The proportion of children with food allergy or respiratory allergy is significantly different among asthma and non-asthma groups (P=0.03 and 0.002, respectively).
Food sensitization presence defined by positive Fx5c tests.
Respiratory sensitization presence defined by positive Phadiatop.
Acknowledgments
Doris Duke Charitable Foundation, Grant # 2004061, Burroughs Wellcome Translational Research Award #: 1006671, Developmental Cardiology and Pulmonary Training Program, Grant #: T32 HL007873-11, and NHLBI grant number 2R01HL079937-05
References
- 1.Boyd JH, Macklin EA, Strunk RC, et al. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108(9):2923–7. doi: 10.1182/blood-2006-01-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernaudin F, Strunk RC, Kamdem A, et al. Asthma is associated with acute chest syndrome, but not with an increased rate of hospitalization for pain among children in France with sickle cell anemia: a retrospective cohort study. Haematologica. 2008 doi: 10.3324/haematol.13090. [DOI] [PubMed] [Google Scholar]
- 3.Boyd JH, Macklin EA, Strunk RC, et al. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92(8):1115–8. doi: 10.3324/haematol.11213. [DOI] [PubMed] [Google Scholar]
- 4.Phillips KL, An P, Boyd JH, et al. Major gene effect and additive familial pattern of inheritance of asthma exist among families of probands with sickle cell anemia and asthma. Am J Hum Biol. 2008;20(2):149–53. doi: 10.1002/ajhb.20703. [DOI] [PubMed] [Google Scholar]
- 5.Sears MR, Burrows B, Flannery EM, et al. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325(15):1067–71. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 6.Burrows B, Martinez FD, Halonen M, et al. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320(5):271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 7.Naqvi M, Choudhry S, Tsai HJ, et al. Association between IgE levels and asthma severity among African American, Mexican, and Puerto Rican patients with asthma. J Allergy Clin Immunol. 2007;120(1):137–43. doi: 10.1016/j.jaci.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Lilja G, Oman H, Johansson SG. Development of atopic disease during childhood and its prediction by Phadiatop Paediatric. Clin Exp Allergy. 1996;26(9):1073–9. [PubMed] [Google Scholar]
- 9.Simpson AB, Glutting J, Yousef E. Food allergy and asthma morbidity in children. Pediatr Pulmonol. 2007;42(6):489–95. doi: 10.1002/ppul.20605. [DOI] [PubMed] [Google Scholar]
- 10.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113(2):227–34. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033–9. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 12.Havnen J, Amlie PA, Hvatum M, et al. IgE concentrations in allergic asthma in children. Arch Dis Child. 1973;48(11):850–5. doi: 10.1136/adc.48.11.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338(22):1592–600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 14.Burney P, Malmberg E, Chinn S, et al. The distribution of total and specific serum IgE in the European Community Respiratory Health Survey. J Allergy Clin Immunol. 1997;99(3):314–22. doi: 10.1016/s0091-6749(97)70048-4. [DOI] [PubMed] [Google Scholar]
- 15.Delmas MC, Guignon N, Leynaert B, et al. Prevalence of asthma among children in France. Arch Pediatr. 2009;16(9):1261–9. doi: 10.1016/j.arcped.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Momas I, Dartiguenave C, Fauroux B, et al. Prevalence of asthma or respiratory symptoms among children attending primary schools in Paris. Pediatr Pulmonol. 1998;26(2):106–12. doi: 10.1002/(sici)1099-0496(199808)26:2<106::aid-ppul6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Nandedkar SD, Feroah TR, Hutchins W, et al. Histopathology of experimentally induced asthma in a murine model of sickle cell disease. Blood. 2008;112(6):2529–38. doi: 10.1182/blood-2008-01-132506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levo Y, Shalit M, Wollner S, et al. Serum IgE levels in patients with inflammatory bowel disease. Ann Allergy. 1986;56(1):85–7. [PubMed] [Google Scholar]
- 19.Tacier-Eugster H, Wuthrich B, Meyer H. Atopic allergy, serum IgE and RAST specific IgE antibodies in patients with cystic fibrosis. Helv Paediatr Acta. 1980;35(1):31–7. [PubMed] [Google Scholar]
- 20.Kelly-Welch A, Hanson EM, Keegan AD. Interleukin-13 (IL-13) pathway. Sci STKE. 2005;2005(293):cm8. doi: 10.1126/stke.2932005cm8. [DOI] [PubMed] [Google Scholar]