Abstract
Study Design
Retrospective review of scoliosis progression, pulmonary and cardiac function in a series of patients with Duchenne Muscular Dystrophy (DMD).
Objective
To determine whether operative treatment of scoliosis decreases the rate of pulmonary function loss in patients with DMD.
Summary of Background Data
It is generally accepted that surgical intervention should be undertaken in DMD scoliosis once curve sizes reach 35 degrees to allow intervention before critical respiratory decline has occurred. There are conflicting reports, however, regarding the effect of scoliosis stabilization on the rate of pulmonary function decline when compared to non operative cohorts.
Methods
We reviewed spinal radiographs, echocardiograms, and spirometry, hospital, and operative records of all patients seen at our tertiary referral center from July 1, 1992 to June 1, 2007 Data was recorded to Microsoft Excel and analyzed with SAS and R statistical processing software.
Results
The percent predicted forced vital capacity (PPFVC) decreased 5% /year prior to operation. The mean PPFVC was 54% (sd=21%) prior to operation with a mean postoperative PPFVC of 43% (sd=14%). Surgical treatment was associated with a 12% decline in PPFVC independent of other treatment variables. PPFVC after operation declined at a rate of 1% per year and while this rate was lower, it was not significantly different than the rate of decline present prior to operation (p=0.18). Cardiac function as measured by left ventricular fractional shortening declined at a rate of 1%/year with most individuals exhibiting an LVFS rate of >30 prior to operation.
Conclusion
Operative treatment of scoliosis in DMD using the Luque Galveston method was associated with a reduction of FVC related to operation. The rate of pulmonary function decline after operation was not significantly reduced when compared to the rate of preoperative FVC decline.
Introduction
Duchenne Muscular Dystrophy (DMD) is a fatal neuromuscular disease, affecting 2 to 3 in 10,000 male births. It is inherited as an X-linked recessive condition caused by a frameshift mutation in the dystrophin gene at the Xp21.2 locus of the X chromosome1. Dystrophin is a large cell-membrane protein involved in calcium transport in muscle cells. Affected boys produce little normal dystrophin gene product leading to muscle cell death and replacement with fibrofatty tissue2. The age of onset of scoliosis in boys with DMD is associated with the age at which they lose the ability to ambulate, which generally occurs between the ages of ten and fourteen years. The scoliosis is initially relatively minor in severity and has an apex at the thoracolumbar junction. As involved males become wheelchair confined, the curves progress and evolve to include the entire thoracic and lumbar spine with potentially catastrophic increases in pelvic obliquity 3,4. Death from pulmonary failure typically occurs in the second or third decade of life with respiratory failure responsible for death in approximately 90% of affected individuals. The remaining 10% of deaths occur due to myocardial disease and its sequelae including heart failure and dysrythmia.
As scoliosis occurs commonly in these patients, several studies have been performed describing its occurrence, side effects and natural history. For example, in a group of thirty-three untreated boys in whom serial radiographs were obtained until eighteen months prior to death, the rate of progression averaged 2.1° per month 5,6,7. Oda et al. found that only 15% of forty-six patients with scoliosis had no progression after four-years of follow-up8. Without surgical intervention, scoliotic curves in DMD may progress to Cobb angles exceeding 80° 9-11.
The long-term outcome of patients with DMD is determined primarily by the respiratory deterioration associated with profound muscle weakness. Pulmonary decline in DMD is due primarily to respiratory muscle weakness, but negative mechanical effects of the deformed thorax on the underlying lungs have also been suggested. The effects of spinal fusion on limiting progression of the restrictive pulmonary disease in DMD have been addressed in variable detail with conflicting reports as to the beneficial effects of surgical stabilization on the rate of forced vital capacity loss 12-20. For example, two classic studies and one recent publication note a decrease in the rate of decline in pulmonary function when compared to nonoperative comparison groups10,11,13,. However, other publications refute these findings suggesting that the rate of decline in pulmonary function is not significantly changed in cohorts of patients undergoing operation 18,19,20.
Materials and Methods
After IRB approval, we reviewed the charts and radiographs of DMD patients in our institutional database. A diagnosis of DMD was determined by clinical findings along with massively elevated creatine phosphokinase levels, an absence of dystrophin staining in muscle biopsy specimens by immunocytochemistry, and dystrophin gene mutations. Surgery was advised to nonambulatory DMD patients once early curve progression had been documented and using a traditional Cobb angle threshold of thirty-five degrees. Once these thresholds had been met, posterior spinal fusion was performed from the upper thoracic region to L4, L5, or the sacrum. As our hospitals serve as tertiary pediatric referral centers, many patients were referred to our clinics with moderate to severe deformities due to curve progression. Many of these patients experienced considerable discomfort, seating difficulties, combined with progressive pulmonary decline. The pulmonary function studies were obtained once patients were able to follow instructions necessary to comply with spirometry testing. In select patients, pulmonary function tests were initiated by age four.
We collected data relating to steroid treatment, left ventricular fractional shortening (LVFS), forced vital capacity (FVC), Cobb angle, pelvic obliquity (PO), Estimated Blood Loss (EBL), operative time, length of time intubated, and survival after surgery and entered data into Microsoft Excel. We studied 174 male patients with DMD, ranging from 9 to 35 years, with an average age at initial visit of almost 21 years. 72 subjects were on steroids, 22 subjects were not on steroids, and the steroid treatment status of 80 subjects was unknown. There were 117 subjects who did not have surgery, 55 who had surgery, and 2 with unknown status. Among 55 subjects who were treated with spinal fusion with instrumentation, 43 had post-operative measurements available. Of these, 28 patients were treated with Luque Galveston instrumentation with sublaminar wire fixation, and 15 had surgery performed using hybrid fixation with pedicle screws, hooks, wires and iliac bolts.
Statistical analysis began with summary statistics (mean, standard deviation, and box plots for each measurement, including differences between last pre-operative and first post-operative measurement, and frequencies of categorical data). Growth curve (longitudinal) models were fitted to estimate how Cobb angle, pelvic obliquity, FVC and LVFS changed over time, whether this varied by age at initial visit (compared to a reference patient at 9 years old, the youngest referral in our study), and how surgery affected the clinical course. We used growth curves to estimate both the immediate effects on the trajectory after surgery and the longer-term effects on rate of change, compared to pre-operative and nonoperative patients. This approach allowed for effects of unequal numbers and spacing of pre- and post-surgical assessments. The effects of height and weight on FVC and LVFS trajectories were also investigated. Cobb angle and pelvic obliquity were log-transformed before fitting growth curves to address skewed distributions. Two subset analyses were performed on the growth curve models. The first analysis was restricted to those individuals with information available on whether they took steroids throughout the study and were created to investigate the effect of steroid use on the four outcomes. The second analysis was restricted to those individuals who had surgery and were created to investigate if there was a significant difference between the two surgical techniques used. This difference was only investigated for outcomes that were significantly predicted by surgery (Cobb angle, pelvic obliquity and FVC). Differences between the surgery group pre-surgery and the non-surgery group were also investigated through longitudinal models.
Results
Characteristics of operative patients and their clinical outcomes
The average patient age at operation was 14.2 years (sd=-2.6) and the mean preoperative Cobb angle at measurement closest to surgery was 49° (Table 1). The average preoperative pelvic obliquity was 13°. Surgery resulted in approximately 50% correction of the Cobb angle to a postoperative mean of 24° and a correction of pelvic obliquity to 8°. There were only minor differences in the preoperative values between the Luque Galveston group and the hybrid instrumentation group for both Cobb angle and pelvic obliquity; hybrid instrumentation use was associated with somewhat but not statistically significantly better correction of pelvic obliquity (P=0.41).
Table 1. Clinical measures in surgical cohort: pre-operative and post-operative means.
We reviewed PFTs, echocardiograms and xrays of patients treated with DMD and scoliosis to establish progression rates and effects of treatment on these parameters. Spinal surgery resulted in a 50% reduction of Cobb angle, FVC declined 5%/year with the rate being unaffected by operation. LVFS declined by 1%/year.
| Measurement | Surgical group (n=43) | Luque Galveston technique (n=28) | Hybrid technique (n=15) | P value for technique difference |
|---|---|---|---|---|
| Age at operation (yrs) | 14.2 | 14.4 | 13.7 | 0.33 |
| Cobb angle | ||||
| Pre-operative | 49.2 | 49.2 | 49.3 | 0.99 |
| Post-operative | 25.7 | 26.5 | 24.4 | 0.98 |
| Change | 23.5 | 25.0 | 25.2 | 0.97 |
| Pelvic obliquity | ||||
| Pre-operative | 13.2 | 13.6 | 12.5 | 0.8 |
| Post-operative | 7.7 | 8.8 | 5.8 | 0.45 |
| Change | 5.5 | 4.2 | 8.2 | 0.41 |
| Predicted forced vital capacity | ||||
| Pre-operative | 0.6 | 0.6 | 0.6 | 0.94 |
| Post-operative | 0.4 | 0.4 | 0.4 | 0.15 |
| Change | 0.2 | 0.2 | 0.1 | 0.15 |
| Left ventricular fractional shortening | ||||
| Pre-operative | 32.5 | 32.7 | 32.2 | 0.77 |
| Post-operative | 30.1 | 30.6 | 31.3 | 0.78 |
| Change | 2.4 | 2.4 | -0.3 | 0.38 |
Surgical correction of scoliosis resulted in a mean blood loss of 2005cc and patients remained intubated for 0.6 (sd=0.9) days after operation. The average length of ICU stay was 2.7 days (sd=1.3) and there were no peri-operative deaths.
Main Cobb angle progression
There were 80 patients for whom radiographs were available for measurement of Cobb angle. An average of five radiographs were obtained during the course of treatment with an average interval between visits of 0.73 years. Nonsurgically treated patients showed generally increasing trajectories of Cobb angle during follow-up, but short of the level at which we recommend surgery (Figure 1a). Surgically treated patients showed pre-operative trajectories of increasing Cobb angle, typically followed by a sharp drop after surgery (Figure 1b). These spaghetti plots also demonstrate substantial variation between patients and even within patients, with trajectories rarely being perfectly smooth, and the plots suggest an asymmetric distribution with some extremely high Cobb angles. We fitted growth curve models to log-transformed Cobb angles and obtained estimates of rates of change and the impact of surgery on Cobb angle trajectories; coefficients are reported in Table 2 as percent differences, after anti-log transformation. The estimated Cobb angle at clinic referral for a patient at the reference age, 9 years old, was 15° and this did not change significantly with an increase in age at initial visit (P=0.18). During follow-up, however, we estimated an average increase of 31% per year in Cobb angle (P<0.001) for non-surgical patients and for surgical patients prior to surgery. Surgical patients experienced a 50% drop in Cobb angle at the first post-operative measurement (P<0.001). After this initial post-operative drop, the Cobb angles began increasing again but the rate of increase was significantly slower than the pre-operative rate. Instead of 1.31-fold or 31% per year, the rate dropped to 85% of that or 1.11-fold per year (11% per year). Surgery following an increase to Cobb angle of 35° would lead by our model to a substantially improved trajectory (Figure 2).
Figure 1.
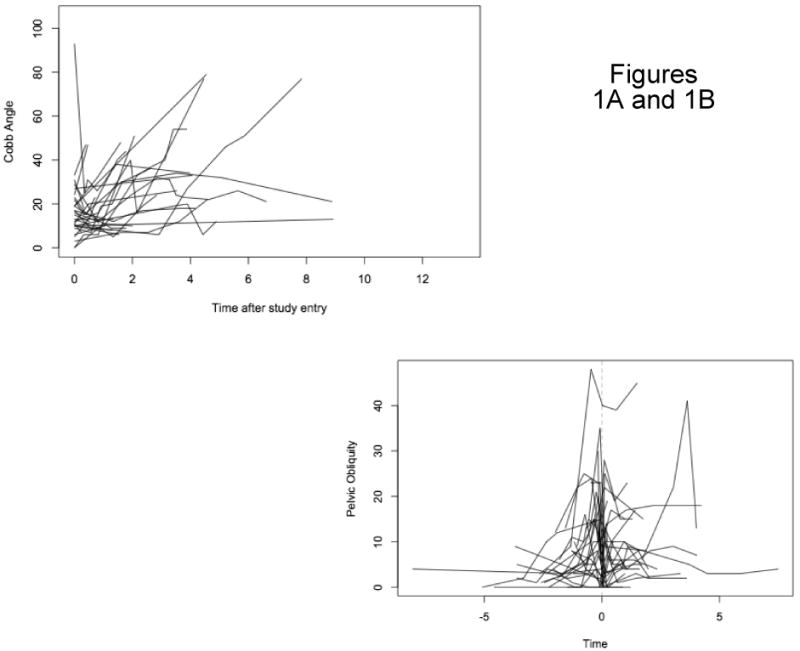
Figure 1A: Cobb Angle Non-Surgery Patients
Figure 1B: Pelvic Obliquity Surgery Patients At Point of Surgery
Table 2. Estimated Cobb angle trajectories and the effects of age and surgery on trajectory, measured as percent change.
| Trajectory parameter | Estimate | 95% CI | P value |
|---|---|---|---|
| Predicted first measurement for 9 yr old. | 14.7 | (10.6, 20.1) | <0.001 |
| Percent change per year older at baseline | 2% | (-1%, 6%) | 0.18 |
| Percent change per year of follow-up | 31% | (18%, 45%) | <0.001 |
| Percent change immediately after surgery | -50% | (-56%, -43%) | <0.001 |
| Percent change per year in post-op period (P value compared to pre-op change per yr) | 11% | (0%, 23%) | <0.001 |
Figure 2. Predicted Cobb Trajectories.
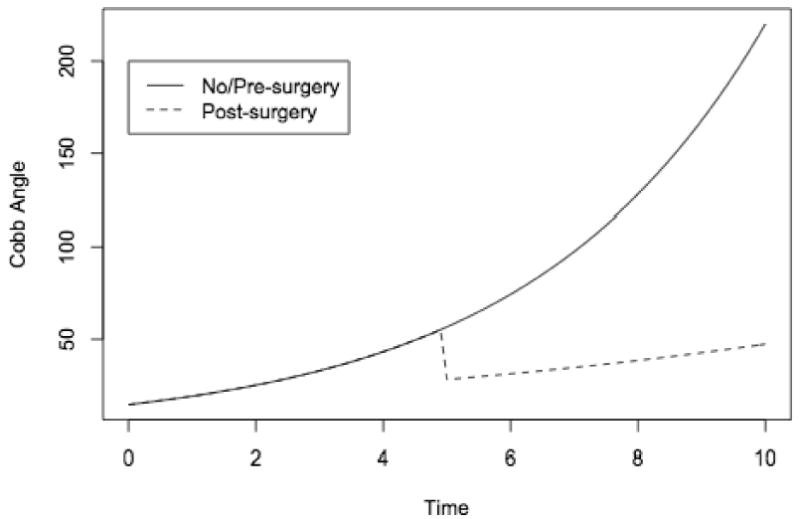
Our models allowed for differences between patients in Cobb angle at initial visit; we also examined the effects of surgery in separate models restricted to surgical patients, and in models allowing for differences between nonsurgical and surgical patients in rate of change from initial visit, but the effects of surgery were not materially changed in those models.
Pelvic Obliquity
There were 78 patients in whom radiographs were available for pelvic obliquity measurements, with an average number of 5.1 radiographs per individual and an average interval between visits of 0.73 years. Spaghetti plots of the trajectories of pelvic obliquity for nonsurgical patients (Figure 3a) and surgical patients (Figure 3b) showed patterns similar to those for Cobb angle, with increasing angles prior to surgery and a drop in angle at the first post-operative measurement. Growth curve models, fitted to the log-transformed pelvic obliquity data, confirmed the visual observations (Table 3). The model estimated that a reference patient, aged 9 years old at first measurement, would have a pelvic obliquity of 5° and would experience an increase in pelvic obliquity of 14% per year (P<0.001). Age at first measurement was not associated with a difference in initial pelvic obliquity. A patient who had surgery using either Luque Galveston or hybrid techniques was estimated to have a 20% reduction in his pelvic obliquity (P<0.001). Although surgery was followed by an immediate reduction in pelvic obliquity, there was not a statistically significant change in the rate of increase in pelvic obliquity during the post-operative follow-up period compared to the pre-operative period or to non-surgical patients, and the mean trajectory resumed its gradual rise (Figure 4). We found no significant differences in rate of change before surgery between pre-operative and nonoperative patients (not shown).
Figure 3.
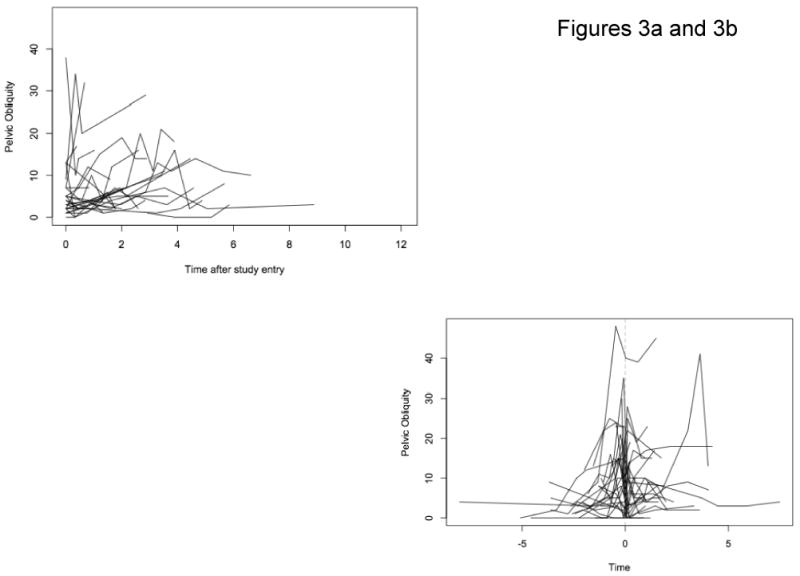
Figure 3a: Pelvic Obliquity Non-Surgery Patients
Figure 3b: Pelvic Obliquity Surgery Patients At Point of Surgery
Table 3. Estimated pelvic obliquity trajectories and the effects of age and surgery on trajectory, measured as percent change.
| Trajectory parameter | Estimate | 95% CI | P value |
|---|---|---|---|
| Predicted first measurement for 9 yr old. | 3.33 | (2.02, 5.49) | <0.001 |
| Percent change per year older at baseline | 2% | (-2%, 6%) | 0.26 |
| Percent change per year of follow-up | 14% | (8%, 21%) | <0.001 |
| Percent change immediately after surgery | -21% | (-37%, -1%) | 0.04 |
| Percent change per year in post-op period (P value compared to pre-op change per yr) | 14% | (13%, 15%) | 0.94 |
Figure 4. Predicted Pelvic Obliquity Trajectories.
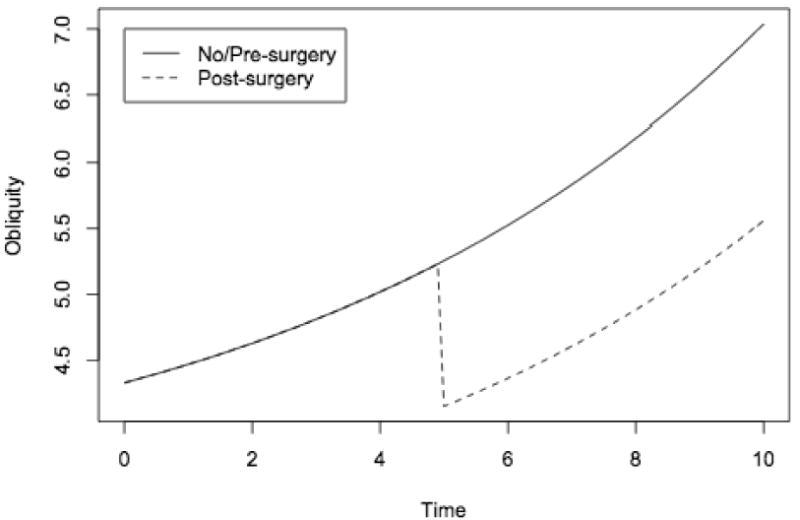
Cardiac Function
Although respiratory failure is the primary cause of death in DMD patients, some patients die secondary to myocardial failure. In our case series, due to the large number of missing observations in the left ventricle ejection fraction measurement (293 out of 443, or 66%), left ventricular fractional shortening (LVFS) was the measurement used to examine cardiac function. In brief, LVFS is the two dimensional cross sectional change seen between systole and diastole and is a directly measured indicator of myocardial function. One hundred and five patients had adequate echocardiographic records, with LVFS measurements ranging from 0 to 50. There was an average of approximately 4 echocardiograms per subject with an average of 1.1 years between visits. Spaghetti plots for non-surgical patients (Figure 5a) and non-surgical patients (Figure 5b) show variation but little striking trend. In most individuals LVFS was maintained in a normal range at the age when most children with DMD undergo scoliosis surgery.
Figure 5.
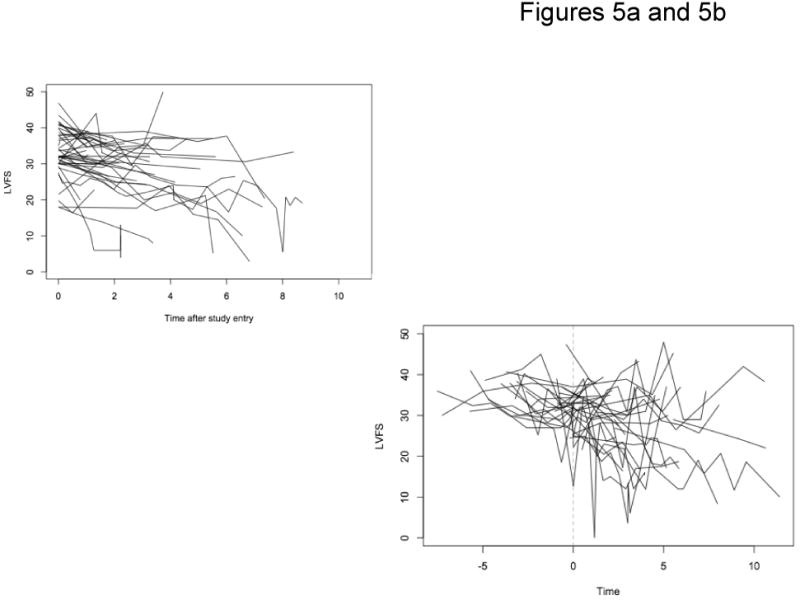
Figure 5a: LVFS Non-Surgery Patients
Figure 5b: LVFS Surgery Patients At Point of Surgery
We used growth curve models to characterize the effects of age, time (in years) since diagnosis with DMD, surgery, time (in years) after surgery, height and weight on LVFS trajectories. The estimated mean LVFS at baseline for a reference patient 9 years old was 39.6, decreasing about 0.6 for every year older at first measurement (P<0.001). Over the course of follow-up, a patient's mean LFVS decreased by 0.84 per year (P<0.001). Height and weight were not associated with LFVS in this group. There was no significant change in LFVS at the first measurement following surgery, but there was a modest reduction in the rate of cardiac function decline over time compared to the pre-operative rate (P=0.04).
Respiratory Function
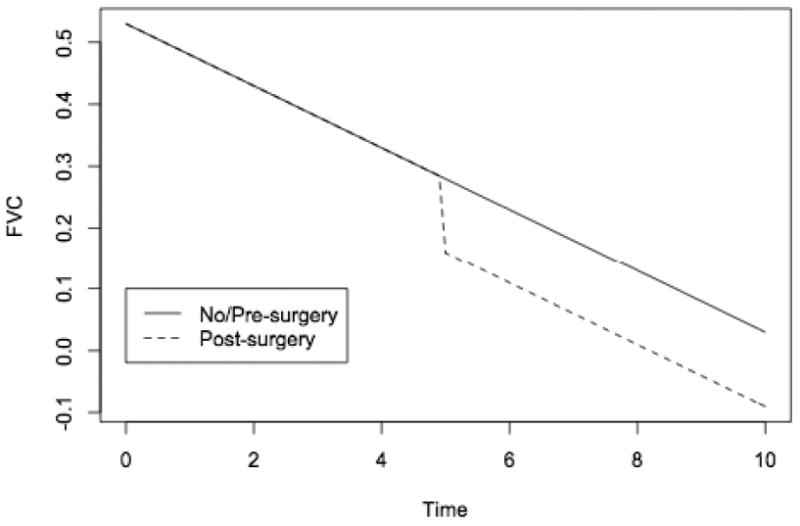
FVC expressed as percent of predicted value (%FVC) was used as a measure of pulmonary function. One hundred and nine patients had forced vital capacity measurements; the mean number of PFT measurements was 5.1, with an average of 0.8 years between visits. Figure 6a shows the trajectories of percent predicted FVC over time for nonsurgical patients, and Figure 6b for surgical patients pre- and post-operatively. Growth curve models characterized the trajectories before surgery; effects of age, height and weight; and effects of surgery on FVC immediately after surgery and over the follow-up period. The mean baseline percent predicted forced vital capacity was approximately 110% (Table 5). This decreased by two percentage points for every year older a patient was at first measurement (P<0.001), and by 0.2 percentage points for every centimeter taller, after accounting for age (P<0.001), but was unrelated to weight (P=0.76). Following the initial referral for DMD, the mean FVC decreased almost five percentage points per year of follow-up. For patients who underwent operative treatment, there was an eleven percent reduction in forced vital capacity immediately following surgical intervention which did not occur in the non-operative group. The average rate of postoperative FVC decline of 4% per year was not significantly different than the average preoperative or non-operative rate of FVC decline of 5% per year; the estimated trajectory for surgical patients thus showed a drop in pulmonary function after surgery, followed by continued decline (Figure 7).
Figure 6.
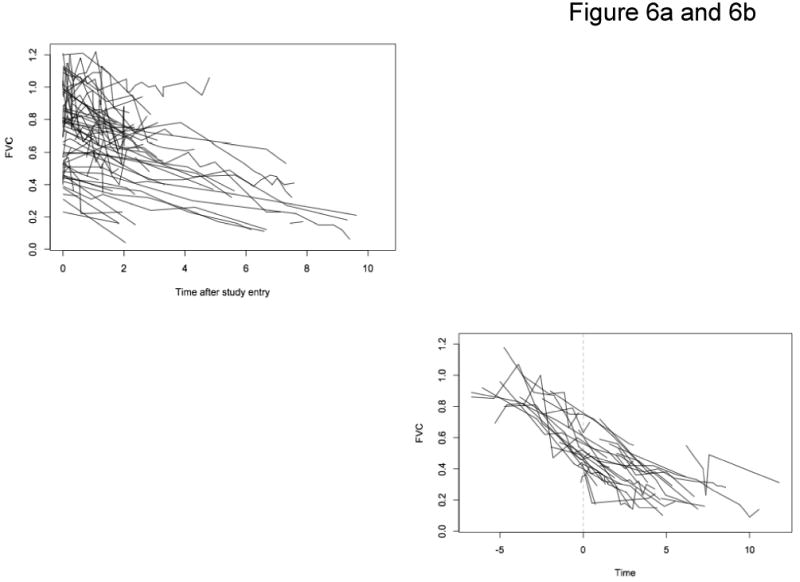
Figure 6a: FVC Non-Surgery Patients
Figure 6b: FVC Surgery Patients At Point of Surgery
Table 5. Estimated predicted forced vital capacity trajectories and the effects of age, height, weight and surgery on trajectory (1=average for age, ×100 to give % of predicted).
| Trajectory parameter | Estimate | 95% CI | P value |
|---|---|---|---|
| Predicted first measurement for 9 yr old. | 1.10 | (1.0, 1.2) | <0.001 |
| Change per year older at baseline | -0.02 | (-0.03, -0.01) | <0.001 |
| Change per cm taller | -0.001 | (-0.001, -0.002) | <0.001 |
| Change per kg heavier | 0.000 | (-0.001, 0.001) | 0.76 |
| Change per year of follow-up | -0.05 | (-0.05, -0.04) | <0.001 |
| Change immediately after surgery | -0.12 | (-0.16, -0.08) | <0.001 |
| Change per year in post-op period (P value compared to pre-op change per yr) | -0.04 | (-0.03, -0.05) | 0.19 |
Figure 7. Predicted Forced Vital Capacity Trajectories.
Steroid treatment and progression of the disease
Subset analysis was performed on individuals who received steroid treatment to see if treatment had a significant effect on any of the above measurements. However, steroid use did not significantly affect the Cobb angle (p = 0.49), LVFS (p = 0.93), FVC (0.64) or pelvic obliquity (p = 0.3).
Luque Rod Vs. Hybrid Fixation
Subset analysis was performed on individuals who had surgery to determine if the type of spinal instrumentation used had a significant effect on Cobb Angle, FVC or pelvic obliquity. While there was a trend towards better correction of Cobb angle and pelvic obliquity with Hybrid techniques of instrumentation, the difference in trends did not meet statistical significance. However, there was a 14% greater reduction in FVC in the Luque Rod group compared to the Hybrid group (p = 0.04; Table 6).
Table 6. Estimated predicted forced vital capacity trajectories for surgery patients only, and the effects of age, height, and surgery type on trajectory (1=average for age, ×100 to give % of predicted).
| Trajectory parameter | Estimate | 95% CI | P value |
|---|---|---|---|
| Predicted first measurement for 9 yr old. | 1.08 | (0.72, 1.45) | <0.001 |
| Change per year older at baseline | 0.02 | (0.00, 0.03) | 0.01 |
| Change per cm taller | -0.001 | (-0.001, -0.000) | <0.001 |
| Change per year of follow-up | -0.05 | (-0.05, -0.04) | <0.001 |
| Change immediately after surgery, hybrid procedure | -0.04 | (-0.05, -0.03) | <0.001 |
| Change immediately after surgery, Luque rod procedure (P value compared to hybrid procedure | -0.18 | (-0.31, -0.05) | 0.04 |
Discussion
Our analysis examined the trajectories of four key clinical measures for nonoperative patients and for operative patients during the period prior to surgery, and also assessed the immediate and long-term effects of surgery on the trajectories for the operative patients. As we would expect, because operative patients were selected through a process dependent on the degree of the Cobb angle measure, the preoperative cohort had absolute measurements of Cobb angle and pelvic obliquity that were almost 50% higher than the nonoperative cohort. Cobb angle increased 31% per year and pelvic obliquity increased 14% per year for all patients who had not had surgery, but there was no significant difference in rate of change per year between the preoperative and nonoperative subjects. We also found no differences between preoperative and nonoperative patients in their rate of decline in cardiac contractility as reflected by fractional ventricular shortening, about 1 unit per year. There were no significant differences at baseline or in the rate of change in forced vital capacity, about 5 percentage points decline per year, when comparing the pre-surgical and non-surgical cohorts. These findings indicate that, while the operative cohort may have been clinically more advanced at initial visit, all patients in our cohort were experiencing comparable rates of physical and functional decline in the absence of corrective surgery.
The trajectories we describe fall within the range of values reported in the majority of previous studies9-11,21. Our reported 31% per year rate of progression of Cobb angle is similar to that reported by Galasko et al who reported a progression rate of 3 degrees per year in ambulatory children and a 7 degree per year progression rate once individuals became wheelchair bound13. We note a one percent decrease in LVFS per year after the diagnosis with DMD and one half percent loss in LVFS per year of age in our patient series. While other authors have commented on the nature of cardiac dysfunction present (dilated cardiomyopathy) or segmental wall motion abnormality, we are not aware of previous publications which predict the rate of left ventricle ejection fraction or left ventricular fractional shortening loss. With regard to lung function, Kurz et al noted that FVC decreased 4% for every year of age after diagnosis and for every 10 degrees of scoliosis which had occurred 16. More recently Velasco et al reported an 8% rate of FVC loss per year prior to operation in their patient cohort, however, the rate of pulmonary capacity loss was based on two preoperative values only. Similarly their postoperative rate of FVC decline (3%) was based upon two spirometry measurements, one of which was the preoperative value used to calculate the preoperative rate of decline11. While our rates of pulmonary function decline before and after operation do not correlate with the rates of decline seen in the study by Velasco et al, we note that the 8% decline quoted in their series is higher than most other clinical series. As the majority of pulmonary function loss is related to progressive muscle disease and weakening of the diaphragm, FVC is not likely to be changed by the alterations in chest wall shape that occurs with scoliosis correction.
We found that surgery was associated with substantial corrections both to Cobb angle (50% reduction) and pelvic obliquity (21% reduction), and for Cobb angle, with a reduction in rate of progression after surgery, from 31% per year to just 11% per year. The rate of pelvic obliquity progression was not effectively reduced postoperatively compared to the nonoperative patients. As pelvic obliquity is a major source of sitting imbalance in neuromuscular scoliosis, it is disappointing to note that the Luque Galveston technique was ineffective in improving and maintaining pelvic obliquity reduction. Previous authors have noted similar results and have demonstrated the superiority of iliac and iliosacral screw fixation methods used to correct pelvic obliquity in neuromuscular scoliosis 22,23.
In our Luque Galveston instrumented patient cohort we note that there was a 12% decrease in percent predicted forced vital capacity attributable to the surgical intervention itself. The decrease forced vital capacity is concerning as in this fragile cohort of patients surgical intervention has been previously advocated for the positive effects on pulmonary function predicted by linear regression models16. We have determined that the decrease in FVC due to surgery is not an artifact of surgery being performed on the subjects with more severe cases of DMD (and hence a lower FVC), as there are no significant differences between the preoperative group and the non-operative group either at study entry or in change in FVC over time.
Recent publications document decreases in FVC and increases in residual volume following posterior spinal fusion in idiopathic scoliosis 24,25,26. As deleterious effects are seen even in children and adolescents with normal diaphragmatic muscle undergoing scoliosis correction for idiopathic scoliosis, it is reasonable to expect similar changes in scoliosis patients with neuromuscular disease.
While we did not note a significant effect of steroids on any of the cardiac, pulmonary, or radiologic markers of scoliosis severity, there was no standardization of steroid administration in this series. For example, the steroid positive group may have been treated with Prednisone or Deflazicort during this time frame and the drug dose or duration of administration was not standardized. The ratio of steroid treated patients to no steroid treatment in this study was approximately three to one. Our results do not concur with other authors' reports regarding steroid effects. For example, a retrospective review of patients treated with or without Deflazacort noted prolonged ambulation, significantly greater FVC (88%vs 39%) in the steroid positive group compared to the non treated group27. These results must be interpreted against the backdrop of significant inter-individual differences in range of FVC seen in children with DMD as reported by Rideau et al28.
Study limitations
Our study group consists of a consecutive cohort of patients with retrospective chart review. While our patient series is relatively large, many nonoperative patients did not have all four clinical measures available. In addition steroid use was not consistent and there were patients with Deflazacort or Prednisone treatment and the dosing regimens may have been variable throughout the period of study. Additionally, study conditions were not optimized to ensure high statistical power in predicting the effects of steroid treatment. We did not note a significant effect of steroids on the rate of curve progression, FVC loss, pelvic obliquity progression or change in Left Ventricular fractional shortening. These parameters could be addressed in prospective randomized studies.
The purpose of this review was to report on scoliosis process measures --Cobb angle over time, pelvic obliquity over time, pulmonary function, cardiac function and longevity as specific outcomes in the treatment of patients with Duchennes dystrophy. The study was not designed prospectively to address the overall utility of scoliosis surgery on the quality of life for a child and family afflicted by Duchennes, thus it did not include non-operative, preoperative, and postoperative patient based outcomes instruments to measure the effects of operation. Further, while the surgical treatment methods were contemporary during the period of observation, numerous authors have noted improved acute scoliosis correction and maintenance of correction using screws and hooks in the thoracic and lumbar spines and screw fixation to the ilium.
While many efforts were undertaken to record the effects of sagittal plane alignment on pulmonary function, lateral radiographs of the spine were not routinely obtained at scheduled clinic visits on most patients. In general, lateral radiographs were only obtained at the time of the initial clinic inpatient visit and at the time of admission for operation. Hence in most individuals (>90%), we were not able to assess the effects of sagittal plane alignment on pulmonary function or the propensity for curve progression. A notable strength of our study, compared to previous work, was the use of growth-curve models to characterize the trajectories throughout the entire pre- and post-surgical period, and to compare operative to nonoperative patients. Using these models, we were able to use all available data while adjusting appropriately for unequal numbers and spacing of measurements; for differences in age, height and weight of patients; and for variance of measurements between and within patients at initial clinical visit and during the course of clinical follow-up.
We believe that patients can be counseled regarding the beneficial effects of scoliosis surgery in reducing the ultimate severity of scoliosis in DMD. As previous studies demonstrate catastrophic deformity resulting from unrelenting curve progression in at risk individuals, significant improvements may result in sitting balance and comfort level. However, we cannot substantiate in our series review a decrease in the rate of pulmonary function loss after scoliosis surgery performed at our institutions using sublaminar wires and pelvic rod fixation. More refined health related quality of life measures and patient based satisfaction instruments should be utilized in prospective randomized studies to determine the merit of surgical intervention in DMD scoliosis, with the ultimate goal of improving the quality of life in Duchenne Muscular Dystrophy via modern approaches to deformity reduction and correction.
As a result of our review of the consecutive patient series at our institution(s) we recommend the following.
If progressive scoliosis is seen in patients with DMD, surgical correction is offered as a means of diminishing the rate of deformity progression.
We recommend the use of pelvic fixation with iliac screws and advocate for rigid thoracic and lumbar fixation with pedicle screws and hooks rather than wire only constructs.
We inform patients and their families that there may be a reduction in pulmonary function (FVC) after operation and that scoliosis surgery may not arrest or diminish the rate of pulmonary function loss when compared to unoperated individuals.
Cobb angles progressed at a rate of 31% per year in patients diagnosed with Duchennes Dystrophy
Percent Predicted forced vital capacity declined at a rate of 5 percent per year in non-surgically treated patients.
The rate of decline in FVC was not significantly reduced by spinal fusion.
Cardiac function (LVFS) declined at a rate of one percent per year and was typically not critically diminished at age twelve when most patients undergo spinal fusion.
Table 4. Estimated left ventricular fractional shortening trajectories and the effects of age and surgery on trajectory, measured as percent change.
| Trajectory parameter | Estimate | 95% CI | P value |
|---|---|---|---|
| Predicted first measurement for 9 yr old. | 40.0 | (35.2, 44.0) | <0.001 |
| Change per year older at baseline | -0.59 | (-0.84, -0.34) | <0.001 |
| Change per cm taller | -0.008 | (-0.03, 0.02) | 0.54 |
| Change per kg heavier | 0.008 | (-0.03, 0.05) | 0.70 |
| Change per year of follow-up | -0.84 | (-1.25, -0.43) | <0.001 |
| Change immediately after surgery | -1.20 | (-3.55, 1.15) | 0.32 |
| Change per year in post-op period (P value compared to pre-op change per yr) | -0.20 | (-0.82, 0.42) | 0.04 |
Footnotes
No funds were received in support of this work
References
- 1.Kunkel LM, Hejtmancik JF, Caskey CT, et al. Analysis of deletions in DNA from patients with Becker and Duchenne muscular dystrophy. Nature. 1986;322:73–7. doi: 10.1038/322073a0. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman EP, Fischbeck KH, Brown RH, et al. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N Engl J Med. 1988;318:1363–8. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- 3.Karol LA. Scoliosis in patients with Duchenne muscular dystrophy. J Bone Joint Surg Am. 2007;89 1:155–62. doi: 10.2106/JBJS.F.00506. [DOI] [PubMed] [Google Scholar]
- 4.McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74:S70–92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- 5.Smith AD, Koreska J, Moseley CF. Progression of scoliosis in Duchenne muscular dystrophy. J Bone Joint Surg Am. 1989;71:1066–74. [PubMed] [Google Scholar]
- 6.Sussman M. Duchenne muscular dystrophy. J Am Acad Orthop Surg. 2002;10:138–51. doi: 10.5435/00124635-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Rideau Y, Gatin G, Bach J, et al. Prolongation of life in Duchenne's muscular dystrophy. Acta Neurol (Napoli) 1983;5:118–24. [PubMed] [Google Scholar]
- 8.Oda T, Shimizu N, Yonenobu K, et al. Longitudinal study of spinal deformity in Duchenne muscular dystrophy. J Pediatr Orthop. 1993;13:478–88. doi: 10.1097/01241398-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro F, Specht L. The diagnosis and orthopaedic treatment of inherited muscular diseases of childhood. J Bone Joint Surg Am. 1993;75:439–54. doi: 10.2106/00004623-199303000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro F, Sethna N, Colan S, et al. Spinal fusion in Duchenne muscular dystrophy: a multidisciplinary approach. Muscle Nerve. 1992;15:604–14. doi: 10.1002/mus.880150512. [DOI] [PubMed] [Google Scholar]
- 11.Velasco MV, Colin AA, Zurakowski D, et al. Posterior spinal fusion for scoliosis in duchenne muscular dystrophy diminishes the rate of respiratory decline. Spine. 2007;32:459–65. doi: 10.1097/01.brs.0000255062.94744.52. [DOI] [PubMed] [Google Scholar]
- 12.Brooke MH, Fenichel GM, Griggs RC, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39:475–81. doi: 10.1212/wnl.39.4.475. [DOI] [PubMed] [Google Scholar]
- 13.Galasko CS, Williamson JB, Delaney CM. Lung function in Duchenne muscular dystrophy. Eur Spine J. 1995;4:263–7. doi: 10.1007/BF00301031. [DOI] [PubMed] [Google Scholar]
- 14.Hahn A, Bach JR, Delaubier A, et al. Clinical implications of maximal respiratory pressure determinations for individuals with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 1997;78:1–6. doi: 10.1016/s0003-9993(97)90001-0. [DOI] [PubMed] [Google Scholar]
- 15.Inkley SR, Oldenburg FC, Vignos PJ., Jr Pulmonary function in Duchenne muscular dystrophy related to stage of disease. Am J Med. 1974;56:297–306. doi: 10.1016/0002-9343(74)90611-1. [DOI] [PubMed] [Google Scholar]
- 16.Kurz LT, Mubarak SJ, Schultz P, et al. Correlation of scoliosis and pulmonary function in Duchenne muscular dystrophy. J Pediatr Orthop. 1983;3:347–53. doi: 10.1097/01241398-198307000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Rideau Y, Glorion B, Delaubier A, et al. The treatment of scoliosis in Duchenne muscular dystrophy. Muscle Nerve. 1984;7:281–6. doi: 10.1002/mus.880070405. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins JG, Bohn D, Edmonds JF, et al. Evaluation of pulmonary function in muscular dystrophy patients requiring spinal surgery. Crit Care Med. 1982;10:645–9. doi: 10.1097/00003246-198210000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy JD, Staples AJ, Brook PD, et al. Effect of spinal surgery on lung function in Duchenne muscular dystrophy. Thorax. 1995;50:1173–8. doi: 10.1136/thx.50.11.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller RG, Chalmers AC, Dao H, et al. The effect of spine fusion on respiratory function in Duchenne muscular dystrophy. Neurology. 1991;41:38–40. doi: 10.1212/wnl.41.1.38. [DOI] [PubMed] [Google Scholar]
- 21.Rideau Y, Jankowski LW, Grellet J. Respiratory function in the muscular dystrophies. Muscle Nerve. 1981;4:155–64. doi: 10.1002/mus.880040213. [DOI] [PubMed] [Google Scholar]
- 22.Miladi LT, Ghanem IB, Draoui MM, et al. Iliosacral screw fixation for pelvic obliquity in neuromuscular scoliosis. A long-term follow-up study. Spine (Phila Pa 1976) 1997;22:1722–9. doi: 10.1097/00007632-199708010-00007. [DOI] [PubMed] [Google Scholar]
- 23.Peelle MW, Lenke LG, Bridwell KH, et al. Comparison of pelvic fixation techniques in neuromuscular spinal deformity correction: Galveston rod versus iliac and lumbosacral screws. Spine (Phila Pa. doi: 10.1097/01.brs.0000238973.13294.16. [DOI] [PubMed] [Google Scholar]
- 24.Upadhyay SS, Ho EK, Gunawardene WM, et al. Changes in residual volume relative to vital capacity and total lung capacity after arthrodesis of the spine in patients who have adolescent idiopathic scoliosis. J Bone Joint Surg Am. 1993;75:46–52. doi: 10.2106/00004623-199301000-00007. 1976) 2006;31:2392-8; discussion 9. [DOI] [PubMed] [Google Scholar]
- 25.Kim YJ, Lenke LG, Bridwell KH, et al. Pulmonary function in adolescent idiopathic scoliosis relative to the surgical procedure. J Bone Joint Surg Am. 2005;87:1534–41. doi: 10.2106/JBJS.C.00978. [DOI] [PubMed] [Google Scholar]
- 26.Yuan N, Fraire J, Margetis M, Skaggs D, Tolo V, Keens T. The Effect of Scoliosis Surgery on Lung Function in the Immediate Postoperative Period. Spine. 2005;30(19):2182–2185. doi: 10.1097/01.brs.0000181060.49993.4a. [DOI] [PubMed] [Google Scholar]
- 27.Biggar WD, Gingras M, Fehlings DL, et al. Deflazacort treatment of Duchenne muscular dystrophy. J Pediatr. 2001;138:45–50. doi: 10.1067/mpd.2001.109601. [DOI] [PubMed] [Google Scholar]
- 28.Rideau Y, Jankowski LW, Grellet J. Respiratory function in the muscular dystrophies. Muscle Nerve. 1981;4:155–64. doi: 10.1002/mus.880040213. [DOI] [PubMed] [Google Scholar]


