Abstract
Alterations of connective tissue collagen are prominent features of both chronologically aged and photoaged (aging due to sun exposure) human skin. These age-related abnormalities are mediated in part by CCN family member, CCN1 (cysteine-rich protein 61). CCN1 is elevated in the dermis of both chronologically aged and photoaged human skin in vivo, and promotes aberrant collagen homeostasis by down-regulating type I collagen, the major structural protein in skin, and promoting collagen degradation. Vitamin A and its metabolites have been shown to improve chronologically aged and photoaged skin by promoting deposition of new collagen and preventing its degradation. Here we investigated regulation of CCN1 expression by retinoids in skin equivalent cultures and chronologically aged and photoaged human skin in vivo. In skin equivalent cultures, all-trans retinoic acid (RA), the major bioactive form of vitamin A in skin, significantly increased type I procollagen, and reduced collagenase (matrix metalloproteinases-1, MMP-1). Addition of recombinant human CCN1 to skin equivalent cultures, significantly reduced type I procollagen and increased MMP-1. Importantly, RA significantly reduced CCN1 expression in skin equivalent cultures. Topical treatment with retinol (vitamin A, 0.4%) for seven days significantly reduced CCN1 mRNA and protein expression in both chronologically aged (80+ years) and photoaged human skin in vivo, compared to vehicle-treated skin. These data indicate that the mechanism by which retinoids improve aged skin, through increased collagen production, involves down-regulation of CCN1.
Keywords: Retinoic acid, CCN1, Collagen, MMP-1, Skin Aging, Skin Equivalent Cultures
INTRODUCTION
Type I collagen comprises the bulk of dermal connective tissue, which physically supports human skin (1–3). Alterations of dermal collagen organization, structure and content occur in both chronologically aged and photoaged human skin (1–4). These abnormalities are largely caused by an imbalance of collagen homeostasis, characterized by reduced production of type I collagen and elevated matrix-degrading matrix metalloproteinases (MMPs)1 (5–8). This imbalance of collagen homeostasis is associated with the winkled appearance of photoaged skin and the thinning of aged skin.
Human skin has the capacity to convert vitamin A (all-trans retinol) to its biologically active metabolite all-trans retinoic acid (RA). Treatment of aged or photoaged skin with retinol (ROL) or RA stimulates collagen production in both chronologically aged and photoaged human skin (5, 9, 10). This stimulation improves the appearance and function of skin. The molecular mechanisms by which RA regulates collagen production in aged and photoaged skin are not well understood. Recently, the CCN (cysteine-rich protein 61 connective tissue growth factor nephroblastoma overexpressed) family member (11–13), CCN1 (cystein-rich protein 61) has been shown to inhibit type I procollagen synthesis and induce collagen degradation via up-regulation of collagenase (matrix metalloproteinases-1, MMP-1) (14, 15). CCN1 has been shown to exhibit diverse cellular functions such as regulation of cell proliferation, chemotaxis, inflammation, apoptosis, adhesion, motility, and extracellular matrix (ECM) remodeling (11, 13, 16–18). CCN1 expression is highly induced by various stimuli such as phobal ester TPA, Vitamin D, inflammation, and wound healing (16, 18, 19). On the other hand, CCN1 expression is inhibited by retinoic acid in breast cancer cell lines (19). We have previously reported that CCN1 is predominantly expressed by dermal fibroblasts, and is significantly elevated in photoaged and chronologically aged human skin in vivo (14). Since elevated CCN1 negatively regulates collagen homeostasis and RA improves aberrant collagen homeostasis, we investigated whether RA is able to regulate CCN1 expression in skin equivalent cultures and in human skin in vivo. Our data indicate that treatment of skin equivalent cultures or aged/photoaged human skin in vivo with RA significantly reduced CCN1 and that this reduction was associated with increased type I procollagen production and reduced MMP-1 expression. These data suggest that the mechanism by which retinoids improve collagen homeostasis in aged human skin involves suppression of CCN1 expression.
MATERIALS AND METHODS
Procurement of human tissue samples and retinol treatment in human skin in vivo
Full thickness punch biopsies (4 mm) were obtained from healthy adult human volunteers, as previously described (4, 20). For young and chronologically aged skin, the biopsies were obtained from healthy sun-protected buttock skin according to age: 21–30 years for young group and ≥ 80 years for aged group. For photoaged skin, the biopsies were obtained from the extensor forearm and sun-protected skin was biopsied from underarm of same individual. The age of photoaged subjects involved in this study ranged from 55 to 65 years. The presence of severe photodamage was determined based on clinical criteria, as described previously (5). For retinol treatment, retinol (vitamin A, 0.4%) and its vehicle (70% ethanol, 30% polyethylene glycol, and 0.05% butylated hydroxytoluene) were applied topically to sun-protected buttock skin (chronologically aged group) and photoaged forearm skin for 7 days at single treatment. The retinol and its vehicle were applied under occlusion to prevent loss and exposure to light. All procedures involving human volunteers were approved by the University of Michigan Institutional Review Board, and all volunteers provided written informed consent.
Atomic force microscopy (AFM) imaging
Human skin equivalent culture and human skin biopsies were embedded in OCT, and cryo-sections (10 μm) were attached onto the microscope cover glass (1.2 mm diameter, Fisher Scientific Co., Pittsburgh, PA). These AFM samples were allowed to air dry for at least 24 hours before imaging them to AFM analysis. AFM images were carried out using a Multimode Nanoscope IIIa AFM (Veeco Instrument Inc., CA) equipped with a 120μm × 120μm × 3.2μm (X,Y,Z dimension) tube J scanner. An initial scan position of the skin was determined by light optical image. AFM images were obtained with tapping mode using a silicon etched cantilever (NSC15/AIBS, MikroMasch, San Jose, CA) with a full tip cone angle ~ 40° and the tip radius of curvature ~ 10 nm. AFM images were acquired at a scan rate of 1.0 Hz, 512×512 pixels resolution, integral and proportional gain settings of 0.4 and 0.6 respectively. The image quality was optimized by dynamically lowering the scan rate and setpoint, and increasing the gains and drive amplitude.
Immunohistology
Immunohistology was performed as described previously (14, 21). Briefly, skin OCT-embedded cryo-sections (7μm) were fixed in paraformaldehyde. Subsequently, the slides were incubated for 1 hour at room temperature with normal rabbit serum followed by incubation of goat polyclonal antibodies against CCN1 (sc-8560, Santa Cruz Biotechnology, CA). To verify antibody specificity, antibodies were incubated with antigenic peptides (Santa Cruz Biotechnology, CA) for 30 minutes prior to addition to skin sections. All sections were lightly counterstained with haematoxylin, and were mounted with mounting media (Vector, Laboratories, CA). The intensity of positive staining was quantified by computerized image analysis (Image-pro Plus software, version 4.1, Media Cybernetics, MD).
Quantitative Real-time RT-PCR
Total RNA was extracted from skin equivalent cultures and human skin using Micro RNA isolation kit (Stratagene, La Jolla, CA). Total RNA (100ng) was reverse transcribed, with random primers, using Taqman Reverse Transcription kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed on a 7300 Sequence Detector (Applied Biosystems) using Taqman Universal PCR Master Mix Reagents (Applied Biosystems). Primers and probes for real-time PCR are as follows: CCN1, sense primer, 5′-TCAAAGACCTGTGGAACTGGTATC-3′; anti-sense primer, 5′-CACAAATCCGGGTTTCTTTCA-3′; probe, 5′-CAATGACAACCCTGAGTGCCGCCT-3′; CRABP-2, sense primer, 5′-CAAGACCTCGTGGACCAGAGA-3′; anti-sense primer, 5′-ACCCTGGTGCACACAACGT-3′; probe, 5′-TCCGCCGTCATGGTCAGGATCAGTTC-3′. Primers and probes for 36B4, type I procollagen and MMP-1 were described previously (1, 21–23). Target genes and 36B4 mRNA levels were quantified and normalized to 36B4, a housekeeping control.
Skin equivalent cultures
Human skin equivalent cultures (EpiDerm™-FT-400) were kindly provided by MatTek Corporation (Ashland, MA). Skin equivalent cultures consist of type I collagen and two major cell types of human skin, keratinocytes and fibroblasts derived from same donors. Upon receipt, the tissues were cultured in 2.0 ml EFT-400 medium, and equilibrated at 37°C, 5% CO2 for 24 hours. At the end of equilibration, the tissues were treated with all-trans-retinoic acid (10 μM, Sigma) for 7 days (fresh retinoic acid-containing medium was replaced every other day) or recombinant human CCN1 (10 μg/ml, PeproTech, NJ) for 24 hours. Throughout the experiment, the skin equivalent cultures were cultured at air-liquid interface with the lower dermis submerged in culture media and the epidermis exposed to air. At the end of the treatment, both media and cells were collected for analysis.
Western analysis and enzyme-linked immunosorbent assay (ELISA)
Western blot was performed as described previously (14, 24). Briefly, whole cell extract was resolved on 10% SDS-PAGE, transferred to PVDF membrane, and blocked with PBST (0.1% Tween 20 in PBS) containing 5% milk. Primary antibodies (CCN1, Santa Cruz Biotechnology, CA; Type I procollagen, Southern Biotechnology, AL; MMP-1, Chemicon, CA) were incubated with PVDF membrane for one hour at room temperature. Blots were washed three times with PBST solution and incubated with appropriate secondary antibody for one hour at room temperature. After washing three times with PBST, the blots were developed with ECF (Vistra ECF Western Blotting System, GE Healthcare, Piscataway, NJ), and were scanned by STORM PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The intensities of specific bands were quantified and normalized using β-actin as a marker for loading control. Secreted type I procollagen from skin equivalent cultures was determined by procollagen type I C-peptide (PIP) in vitro enzyme-linked immunosorbent assay (Takara Bio Inc, WI) according to manufacturer’s instructions, and previous publication (25).
Statistical analysis
Comparisons between groups were determined with the Student’s t-test. All p values are two-tailed, and considered significant when p<0.05.
RESULTS
RA responsiveness in skin equivalent cultures
Retinoids have been shown to regulate collagen homeostasis by stimulation of type I collagen production and reducing collagen degradation in chronologically aged (9, 10) and photoaged human skin (5, 20, 26, 27). The molecular mechanisms by which retinoids regulate collagen homeostasis in human skin have been difficult to investigate largely due to lack of appropriate in vitro models. For example, primary keratinocytes or dermal fibroblasts are minimally responsive to RA treatment in monolayer culture (28, 29). This lack of responsiveness is due, at least in part, to low levels of nuclear retinoid receptors, which mediate expression of retinoid-regulated genes (30, 31). Therefore, we evaluated the use of skin equivalent cultures as a model to investigate retinoid regulation of collagen homeostasis. Skin equivalent cultures are composed of stratified, differentiated keratinocytes (model epidermis) on top of a type I collagen lattice, in which dermal fibroblasts are embedded (model dermis).
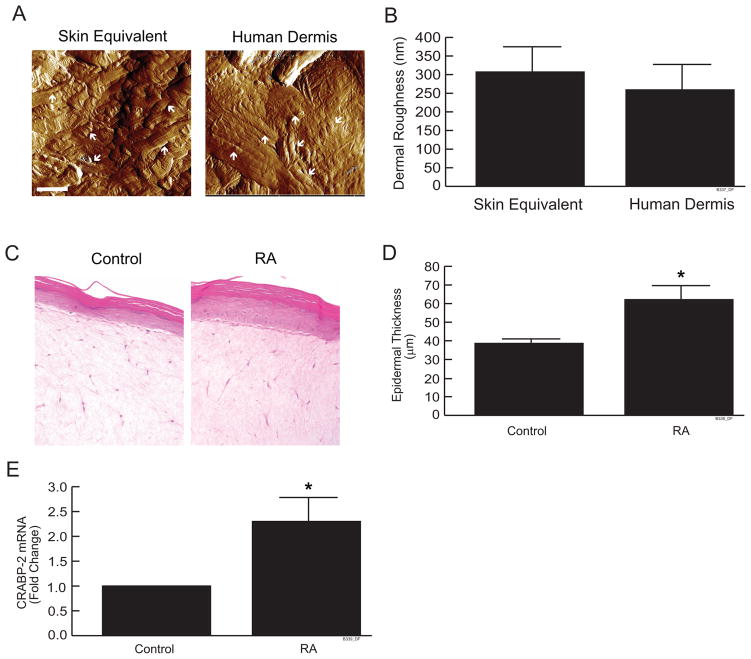
We used atomic force microscopy (AFM) to characterize the structure and surface topology of collagen fibrils in skin equivalent cultures and human dermis. AFM images show that the collagenous extracellular matrix in skin equivalent cultures and human dermis are morphologically similar (Fig. 1a). Collagen fibrils are formed into a sheet in both skin equivalent cultures and human dermis. The measurement of dermal surface roughness shows that no significant difference was found between skin equivalent cultures and human dermis (Fig. 1b).
Figure 1.
Organization of collagen fibrils and RA responsiveness in skin equivalent cultures. (a) Skin equivalent cultures and human skin were embedded in OCT and cryo-sections (7 μm) were analyzed by atomic force microscopy (AFM). AFM images were obtained using a Multimode Nanoscope IIIa AFM (Veeco Instrument Inc., CA), as described in Experimental Procedures. Images are representative of three experiments. Bar = 1μm. Arrows indicate collagen bundles in skin equivalent cultures and human skin (b) Skin dermal roughness was determined by Nanoscope IIIa software. Data are expressed as mean±SEM, N=4. (c) Skin equivalent cultures were treated with all-trans retinoic acid (10μM) for 7 days. Cultures were embedded in OCT and cryo-sections (7 μm) were stained with H&E. Lines indicate dermal-epidermal boundary. Images are representative of three experiments. (d) Average vertical height of the stratified layers of keratinocytes was quantified by computerized image analysis. Data are expressed as mean±SEM, N=3, *p<0.05. (e) CRABP-2 mRNA levels were quantified by real-time RT-PCR and were normalized to mRNA for 36B4, a ribosomal protein used as an internal control for quantitation. Data are expressed as mean±SEM, N=3, *p<0.05.
Treatment of skin equivalent cultures with RA significantly increased the number of keratinocyte layers (Fig. 1c). Thickness of the keratinocyte layers was significantly increased (50%, Fig. 1d), similar to the effect of topical application of RA to human skin in vivo. (9, 20, 32). Consistent with these data, RA treatment significantly increased (2.3-fold) CRABP-2 (cellular RA-binding protein II) mRNA levels (Fig. 1e). The CRABP-2 gene is directly regulated by nuclear retinoic acid receptors, and is a well characterized marker of nuclear retinoic acid receptor activation in human skin (27, 31, 33–35). These histological and molecular changes in response to RA treatment indicate that skin equivalent cultures are a useful model for investigating mechanisms by which retinoids regulate collagen homeostasis in human skin.
RA increases type I procollagen and reduces MMP-1 in skin equivalent cultures
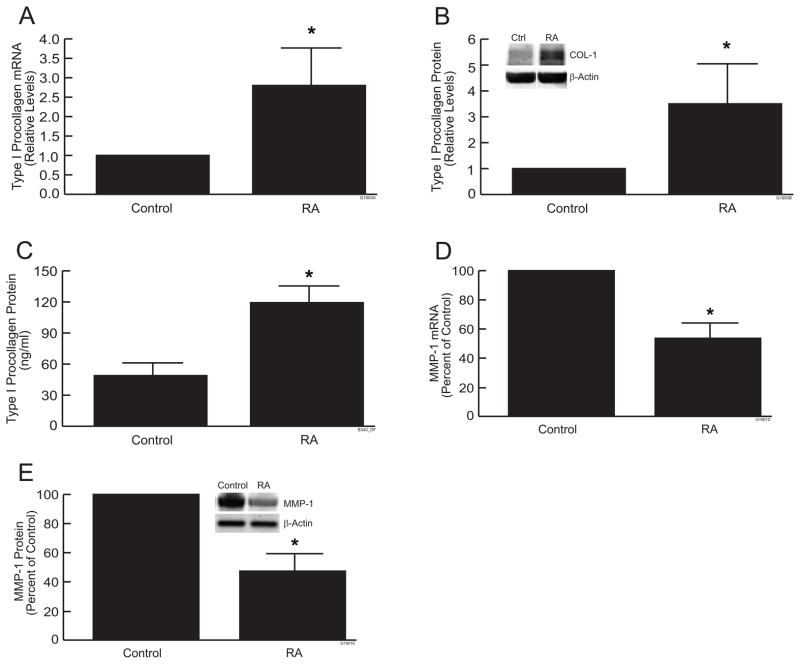
Importantly, RA treatment significantly increased type I procollagen mRNA (Fig. 2a) and protein levels (Fig. 2b) in skin equivalent cultures, compared to vehicle control. Treatment with RA (10μM) for 24 hours increased type I procollagen mRNA and protein levels nearly 3-fold. In addition, RA treatment increased the level of extracellular, secreted type I procollagen more than 2-fold (Fig 2c). RA treatment also significantly reduced expression of MMP-1, which initiates cleavage of type I collagen fibrils. RA reduced MMP-1 mRNA (Fig 2d) and protein (Fig 2e) approximately 50%, in skin equivalent cultures, compared to vehicle control. These data indicate that RA regulates collagen homeostasis, in skin equivalent cultures, as observed in human skin in vivo by up-regulation of type I procollagen and down-regulation of MMP-1.
Figure 2.
RA increases type I procollagen and reduces MMP-1 in skin equivalent cultures. Skin equivalent cultures were treated with all-trans retinoic acid (10μM) for 7 days. (a) Type I procollagen mRNA; (b) Type I procollagen protein (Western), (c) Type I procollagen protein (ELISA); (d) MMP-1 mRNA; (e) MMP-1 protein (Western). mRNA levels were quantified by real-time RT-PCR and were normalized to mRNA for 36B4, a ribosomal protein used as an internal control for quantitation. Western analyses were normalized using β-actin as loading control. Inset shows representative Western blots. Secreted type I procollagen (c) in culture medium was determine by ELISA. Data are expressed as mean±SEM, N=3, *p<0.05.
RA reduces CCN1 expression which negatively regulates collagen homeostasis in skin equivalent cultures
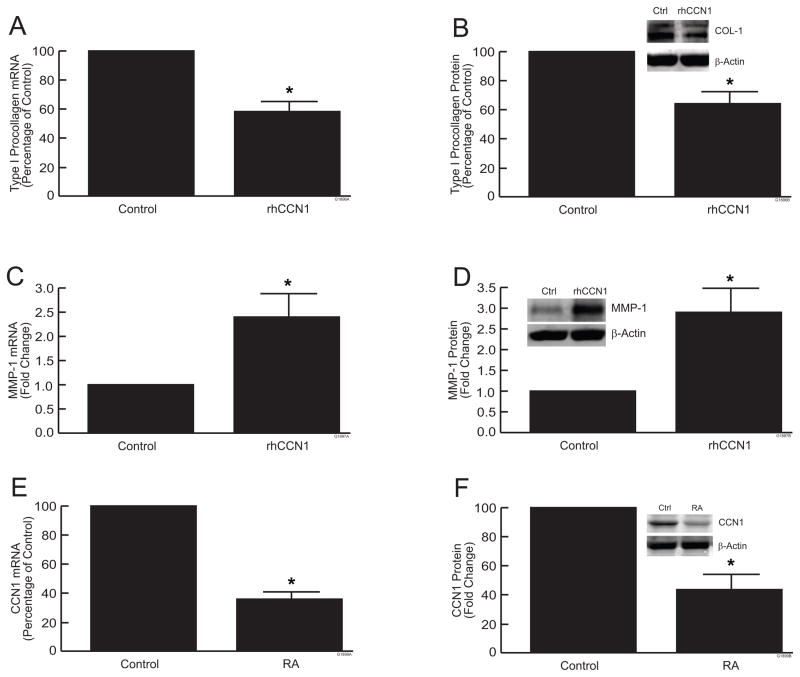
We have previously reported that over-expression of CCN1 in human skin fibroblast monolayer cultures alters collagen homeostasis (14). To further investigate the functions of CCN1, skin equivalent cultures were treated with recombinant human CCN1 (rhCCN1, 5μg/ml). Treatment of cultures for 24 hours with rhCCN1 significantly reduced the levels of type I procollagen mRNA (Fig. 3a) and protein (Fig. 3b) approximately 40%, compared to vehicle control. In addition, CCN1 treatment of skin equivalent cultures increased MMP-1 mRNA (Fig. 3c) and protein (Fig. 3d) approximately 2.5-fold, compared vehicle control in skin equivalent cultures. These data indicate that collagen metabolism by fibroblasts in skin equivalent cultures is regulated by CCN1.
Figure 3.
CCN1 regulates collagen homeostasis and Retinoic Acid reduces CCN1 expression in skin equivalent cultures. Skin equivalent cultures were treated with recombinant human CCN1 (5μg/ml) for 24 hours. (a) Type I procollagen mRNA levels; (b) type I procollagen protein levels; (c) MMP-1 mRNA levels; (d) MMP-1 protein levels. mRNA levels were quantified by real-time RT-PCR and were normalized to mRNA for 36B4, a ribosomal protein used as an internal control for quantitation. Western analyses were normalized using β-actin as loading control. Inset shows representative Western blots.
(e,f) Cultures were treated with all-trans retinoic acid (10μM) for 7 days. (e) CCN1 mRNA levels; (f) CCN1 protein levels. mRNA levels were quantified by real-time RT-PCR and were normalized to mRNA for 36B4, a ribosomal protein used as an internal control for quantitation. Western analyses were normalized using β-actin as loading control. Inset shows representative Western blots. Data are expressed as mean±SEM, N=3, *p<0.05.
Given the ability of RA and CCN1 to regulate collagen homeostasis in skin equivalent cultures, we next investigated whether RA could alter CCN1 expression. Importantly, treatment of skin equivalent cultures with RA (10μM) for 7 days reduced expression of CCN1 mRNA (Fig. 3e) protein (Fig. 3f) by approximately 60%. Taken together, the above data indicate that RA regulates both collagen homeostasis and CCN1 expression in skin equivalent cultures.
CCN1 is elevated in chronologically aged and photoaged human skin, and reduced by retinoid treatment in vivo
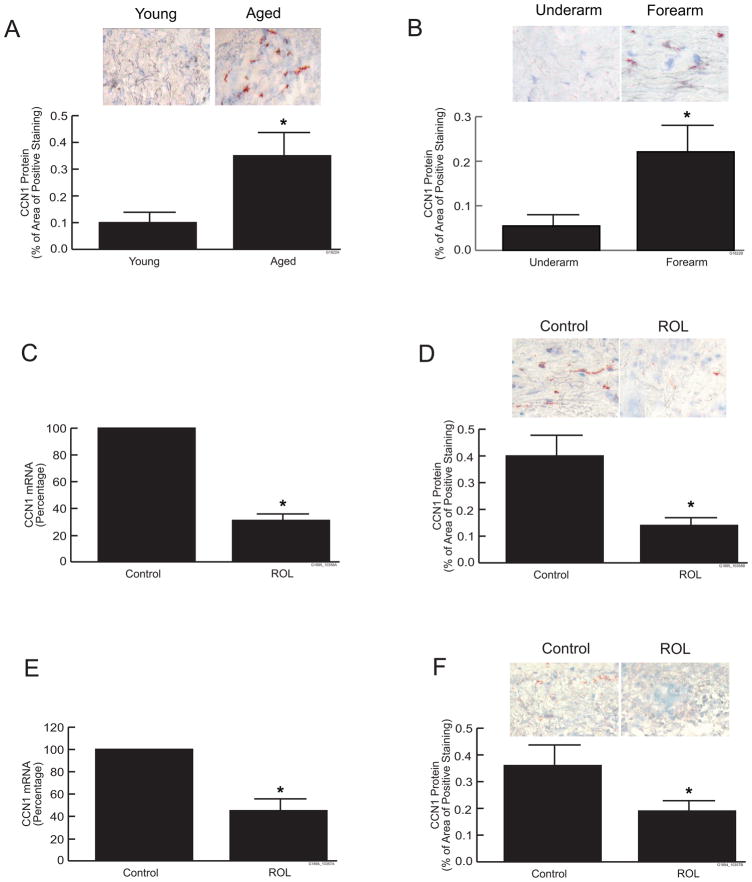
We have previously reported that CCN1 is elevated in aging skin determined by RT-PCR and Western analysis (14). To further investigate the expression and localization of CCN1 in skin aging, we determined CCN1 expression and localization in naturally aged and photoaged human skin by immunohistology. Immunohistochemical staining of CCN1 protein was significantly elevated in the dermis of both naturally aged (80+years), sun protected buttocks skin (Fig. 4a) and photoaged extensor forearm skin (Fig. 4b), compared to young or subject-matched underarm (sun-protected) skin, respectively. Levels of CCN1 protein were elevated 3 to 4-fold in the dermis of both naturally aged and photoaged skin, compared to their respective controls. Since elevated CCN1 negatively regulates collagen homeostasis, we investigated the effect retinoid treatment on CCN1 expression in human skin in vivo. Retinol (0.4%) or vehicle was topically applied under occlusion for seven days to aged (80+ years) buttocks skin or photoaged extensor forearm skin. Application of retinol significantly reduced elevated CCN1 in both naturally aged and photoaged human skin, compared to vehicle-treated control skin. Retinol reduced dermal expression of CCN1 mRNA and protein by 50–70% in both naturally aged (Figs. 4c and 4d) and photoaged forearm (Figs. 4e and 4f) skin in vivo, compared to vehicle-treated control skin.
Figure 4.
Retinoid treatment reduces CCN1 expression in chronologically aged and photoaged human skin in vivo. Full-thickness human skin punch biopsies were obtained from (a) young (21–30 years) and aged (80+years) buttock skin, or (b) sun-protected underarm and subject-matched photoaged extensor forearm skin. CCN1 protein levels were determined by immunohistology, and quantified by computerized image analysis. Figures show representative immunohistology. Data are expressed as means ± SEM, n=9, *p<0.05. (c and d), Aged (80+years) buttocks skin was topically treated with vehicle or retinol (ROL, 0.4%) for seven days. (c) CCN1 mRNA levels, (d) CCN1 protein levels. (e and f), Photodamaged forearm skin was topically treated with vehicle or retinol (ROL, 0.4%) for seven days. (e) CCN1 mRNA levels, (f) CCN1 protein levels. CCN1 mRNA levels were quantified by real-time RT-PCR and were normalized to mRNA for 36B4, a ribosomal protein used as an internal control for quantitation. CCN1 protein levels were determined immunohistology, and quantified by computerized image analysis. Inset shows representative immunohistology. Data are expressed as means ± SEM, n=4–10, *p<0.05.
DISCUSSION
Chronologically aged and photoaged skin (photoaging) share common molecular features such as altered homeostasis of type I collagen, characterized by decreased collagen synthesis and elevated collagen degradation. Alterations of connective tissue collagen reduce the structural integrity and increase the fragility of skin, thereby significantly impairing skin function. Alterations of dermal collagen homeostasis are mediated, at least in part, by CCN family gene, CCN1 (14, 15). CCN1 is predominantly expressed in dermal fibroblasts, and elevated in chronologically aged, photoaged, and UV-irradiated human skin dermis in vivo (14, 15). Elevated CCN1 negatively regulates collagen homeostasis by inhibiting type I collagen production and promoting collagen degradation.
Retinoids have been used as therapeutic agents for numerous skin diseases, including psoriasis, acne, and photodamage (36). However, the precise mechanism(s) by which RA improves clinical features and skin function in chronologically aged or photoaged skin is not well understood. Our data suggest that one mechanism by which RA improves skin connective tissue may involve suppression of CCN1 in aged human skin.
Since retinoids have been shown to increase collagen production in chronologically aged and photoaged skin (5, 9, 10, 20), we investigated the effect of retinoids on CCN1 expression in human skin in vivo, and in skin equivalent cultures in vitro. Our data indicate that retinoid treatment significantly reduces CCN1 mRNA and protein levels in aged or photoaged human skin in vivo, and in skin equivalent cultures. This reduction is associated with increased type I procollagen production and reduced MMP-1 expression in skin equivalent cultures.
The mechanism(s) by which retinoids reduce CCN1 expression in chronologically aged or photoaged human skin remains to be determined. One possibility is that retinoid reduces CCN1 through inhibition of AP-1 transcription factor. CCN1 has been shown to be transcriptionally activated by a variety of extracellular stimuli. We have found that transcriptional regulation of CCN1 in response to UV irradiation is primarily controlled by AP-1 transcription factor, in primary human dermal fibroblasts (15). AP-1 transcriptional activity is elevated in both chronologically aged and photoaged human skin, and is critically important in mediating skin connective tissue damage. Importantly, elevated AP-1 is suppressed by applications of retinoid in aged and photoaged human skin in vivo (5, 10, 27, 37, 38), suggesting that retinoids down-regulate CCN1 expression by inhibiting AP-1 transcription factor. Interestingly, we have reported that elevated CCN1 also activates transcription factor AP-1, (14), suggesting that a positive feedback mechanism may contribute to sustained elevation of CCN1 in aged and photoaged human skin.
Treatment of skin equivalent cultures with RA resulted in increased epidermal cell layers and CRABP-2 expression, which are characteristic responses of human epidermis to retinoid treatment in vivo. Skin equivalent cultures have been shown to be a useful model to study functional interactions between epidermis and dermis (39–41). We find that neither CCN1 nor type I collagen is regulated by retinoid in primary fibroblasts cultured in monolayer or three dimensional collagen lattices (data not shown). An increasing body of evidence indicates that interactions between epidermal keratinocytes and dermal fibroblasts plays a pivotal role in dermal fibroblasts behavior and functions (42–45). Our data suggest that such interactions may be involved in regulation of CCN1 expression by retinoid, in dermal fibroblasts, and skin equivalent cultures appear to be a useful model to investigate the nature of these interactions. In summary, our data suggest that the mechanism by which retinoids improve collagen homeostasis in aged and photoaged human skin involves suppression of CCN1 expression.
Acknowledgments
We thank Suzan Rehbine for the procurement of tissue specimens, Laura Van Goor for graphic support, and Diane Fiolek for graphic and administrative assistance. We thank MatTek Corporation (Ashland, MA) for providing human skin equivalent cultures, and also thank Mitch Klausner (MatTek Corporation) and Patrick J Hayden (MatTek Corporation) for technical suggestions. We thank Electron Microbeam Analysis Laboratory (EMAL), University of Michigan College of Engineering for AFM analysis. This work was supported by the National Institute of Health (grant ES014697 to T Quan), and Dermatology Foundation Research grant (to T Quan).
Footnotes
Abbreviations: MMPs, matrix metalloproteinases; CCN, cysteine-rich protein 61 connective tissue growth factor nephroblastoma overexpressed; CCN1, cysteine-rich protein 61; ROL, retinol; RA, all-trans-retinoic acid; UV, ultraviolet; ECM, extracellular matrix; MMP-1, matrix metalloproteinase-1; PCR, polymerase chain reaction; CRABP-2, cellular RA-binding protein II; rhCCN1, recombinant human CCN1; AFM, atomic force microscopy
References
- 1.Fisher GJ, Quan T, Purohit T, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174:101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fligiel SE, Varani J, Datta SC, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- 4.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths CE, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) N Engl J Med. 1993;329:530–535. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- 6.Talwar HS, Griffiths CE, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–290. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- 7.Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varani J, Schuger L, Dame MK, et al. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J Invest Dermatol. 2004;122:1471–1479. doi: 10.1111/j.0022-202X.2004.22614.x. [DOI] [PubMed] [Google Scholar]
- 9.Cho S, Lowe L, Hamilton TA, Fisher GJ, Voorhees JJ, Kang S. Long-term treatment of photoaged human skin with topical retinoic acid improves epidermal cell atypia and thickens the collagen band in papillary dermis. J Am Acad Dermatol. 2005;53:769–774. doi: 10.1016/j.jaad.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 10.Varani J, Warner RL, Gharaee-Kermani M, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 11.Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 12.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 13.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 14.Quan T, He T, Shao Y, et al. Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am J Pathol. 2006;169:482–490. doi: 10.2353/ajpath.2006.060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan T, Qin Z, Xu Y, et al. Ultraviolet irradiation induces CYR61/CCN1, a mediator of collagen homeostasis, through activation of transcription factor AP-1 in human skin fibroblasts. J Invest Dermatol. 2010;130:1697–1706. doi: 10.1038/jid.2010.29. [DOI] [PubMed] [Google Scholar]
- 16.Bai T, Chen CC, Lau LF. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol. 2010;184:3223–3232. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 18.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai MS, Bogart DF, Li P, Mehmi I, Lupu R. Expression and regulation of Cyr61 in human breast cancer cell lines. Oncogene. 2002;21:964–973. doi: 10.1038/sj.onc.1205131. [DOI] [PubMed] [Google Scholar]
- 20.Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce Ap-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Investig Dermatol Symp Proc. 1998;3:61–68. [PubMed] [Google Scholar]
- 21.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J Invest Dermatol. 2002;119:499–506. doi: 10.1046/j.1523-1747.2002.01834.x. [DOI] [PubMed] [Google Scholar]
- 22.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002;118:402–408. doi: 10.1046/j.0022-202x.2001.01678.x. [DOI] [PubMed] [Google Scholar]
- 23.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol. 2004;165:741–751. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quan T, He T, Voorhees JJ, Fisher GJ. Ultraviolet irradiation blocks cellular responses to transforming growth factor-beta by down-regulating its type-II receptor and inducing Smad7. J Biol Chem. 2001;276:26349–26356. doi: 10.1074/jbc.M010835200. [DOI] [PubMed] [Google Scholar]
- 25.Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–24. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang S, Fisher GJ, Voorhees JJ. Photoaging and topical tretinoin: therapy, pathogenesis, and prevention. Arch Dermatol. 1997;133:1280–1284. [PubMed] [Google Scholar]
- 27.Wang Z, Boudjelal M, Kang S, Voorhees JJ, Fisher GJ. Ultraviolet irradiation of human skin causes functional vitamin A deficiency, preventable by all-trans retinoic acid pre-treatment. Nat Med. 1999;5:418–422. doi: 10.1038/7417. [DOI] [PubMed] [Google Scholar]
- 28.Elder JT, Astrom A, Pettersson U, et al. Retinoic acid receptors and binding proteins in human skin. J Invest Dermatol. 1992;98:36S–41S. doi: 10.1111/1523-1747.ep12462180. [DOI] [PubMed] [Google Scholar]
- 29.Elder JT, Astrom A, Pettersson U, et al. Differential regulation of retinoic acid receptors and binding proteins in human skin. J Invest Dermatol. 1992;98:673–679. doi: 10.1111/1523-1747.ep12499896. [DOI] [PubMed] [Google Scholar]
- 30.Fisher GJ, Talwar HS, Xiao JH, et al. Immunological identification and functional quantitation of retinoic acid and retinoid X receptor proteins in human skin. J Biol Chem. 1994;269:20629–20635. [PubMed] [Google Scholar]
- 31.Di W, Li XY, Datta S, et al. Keratinocyte-specific retinoid regulation of human cellular retinoic acid binding protein-II (hCRABPII) gene promoter requires an evolutionarily conserved DR1 retinoic acid-responsive element. J Invest Dermatol. 1998;111:1109–1115. doi: 10.1046/j.1523-1747.1998.00455.x. [DOI] [PubMed] [Google Scholar]
- 32.Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996;10:1002–1013. doi: 10.1096/fasebj.10.9.8801161. [DOI] [PubMed] [Google Scholar]
- 33.Astrom A, Pettersson U, Chambon P, Voorhees JJ. Retinoic acid induction of human cellular retinoic acid-binding protein-II gene transcription is mediated by retinoic acid receptor-retinoid X receptor heterodimers bound to one far upstream retinoic acid-responsive element with 5-base pair spacing. J Biol Chem. 1994;269:22334–22339. [PubMed] [Google Scholar]
- 34.Siegenthaler G, Tomatis I, Chatellard-Gruaz D, Jaconi S, Eriksson U, Saurat JH. Expression of CRABP-I and -II in human epidermal cells. Alteration of relative protein amounts is linked to the state of differentiation. Biochem J. 1992;287 ( Pt 2):383–389. doi: 10.1042/bj2870383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virtanen M, Sirsjo A, Vahlquist A, Torma H. Keratins 2 and 4/13 in reconstituted human skin are reciprocally regulated by retinoids binding to nuclear receptor RARalpha. Exp Dermatol. 2010;19:674–681. doi: 10.1111/j.1600-0625.2010.01079.x. [DOI] [PubMed] [Google Scholar]
- 36.Orfanos CE, Zouboulis CC, Almond-Roesler B, Geilen CC. Current use and future potential role of retinoids in dermatology. Drugs. 1997;53:358–388. doi: 10.2165/00003495-199753030-00003. [DOI] [PubMed] [Google Scholar]
- 37.Fisher GJ, Datta S, Wang Z, et al. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000;106:663–670. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher GJ, Talwar HS, Lin J, et al. Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J Clin Invest. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Afaq F, Zaid MA, Khan N, Dreher M, Mukhtar H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp Dermatol. 2009;18:553–561. doi: 10.1111/j.1600-0625.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basset-Seguin N, Culard JF, Kerai C, et al. Reconstituted skin in culture: a simple method with optimal differentiation. Differentiation. 1990;44:232–238. doi: 10.1111/j.1432-0436.1990.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 41.Martin R, Pierrard C, Lejeune F, Hilaire P, Breton L, Bernerd F. Photoprotective effect of a water-soluble extract of Rosmarinus officinalis L. against UV-induced matrix metalloproteinase-1 in human dermal fibroblasts and reconstructed skin. Eur J Dermatol. 2008;18:128–135. doi: 10.1684/ejd.2008.0349. [DOI] [PubMed] [Google Scholar]
- 42.Bauer BS, Tredget EE, Marcoux Y, Scott PG, Ghahary A. Latent and active transforming growth factor beta1 released from genetically modified keratinocytes modulates extracellular matrix expression by dermal fibroblasts in a coculture system. J Invest Dermatol. 2002;119:456–463. doi: 10.1046/j.1523-1747.2002.01837.x. [DOI] [PubMed] [Google Scholar]
- 43.Ghaffari A, Kilani RT, Ghahary A. Keratinocyte-conditioned media regulate collagen expression in dermal fibroblasts. J Invest Dermatol. 2009;129:340–347. doi: 10.1038/jid.2008.253. [DOI] [PubMed] [Google Scholar]
- 44.Harrison CA, Gossiel F, Bullock AJ, Sun T, Blumsohn A, Mac Neil S. Investigation of keratinocyte regulation of collagen I synthesis by dermal fibroblasts in a simple in vitro model. Br J Dermatol. 2006;154:401–410. doi: 10.1111/j.1365-2133.2005.07022.x. [DOI] [PubMed] [Google Scholar]
- 45.Nowinski D, Hoijer P, Engstrand T, Rubin K, Gerdin B, Ivarsson M. Keratinocytes inhibit expression of connective tissue growth factor in fibroblasts in vitro by an interleukin-1alpha-dependent mechanism. J Invest Dermatol. 2002;119:449–455. doi: 10.1046/j.1523-1747.2002.01841.x. [DOI] [PubMed] [Google Scholar]