Abstract
Objective
Investigate the natural history of PNH clones in patients with acquired aplastic anemia (AA).
Patients and Methods
Twenty-seven patients with AA and a detectable PNH clone were monitored for a median of 5.7 years (range1.5 to 11.5 years). Twenty-two patients received high dose cyclophosphamide (HiCy) therapy. The erythrocyte and granulocyte PNH clone sizes were measured using flow cytometry and analyzed via CellQuest software. PE-conjugated anti-glycophorin A, anti-CD15, FITC-conjugated anti-CD59, and FLAER staining were used to define GPI-AP deficient cells.
Results
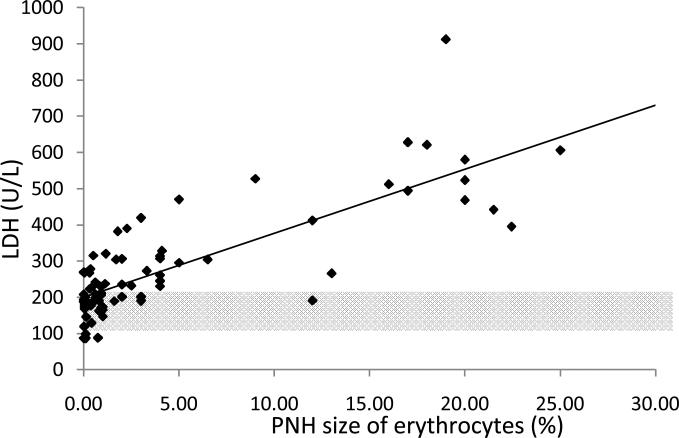
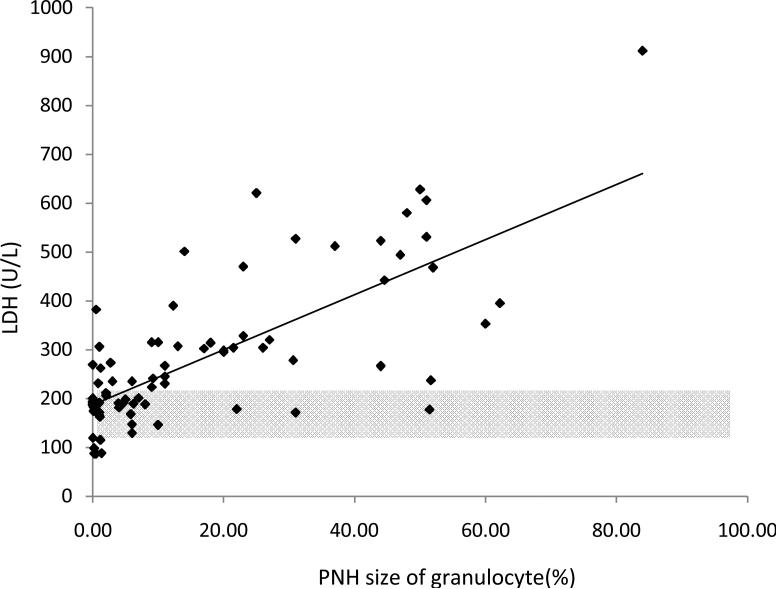
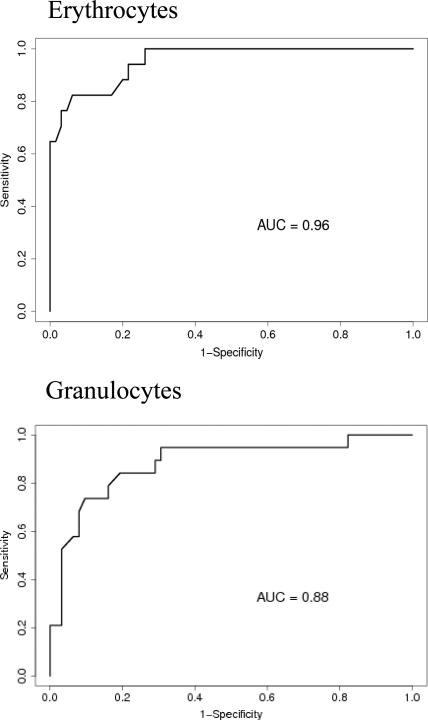
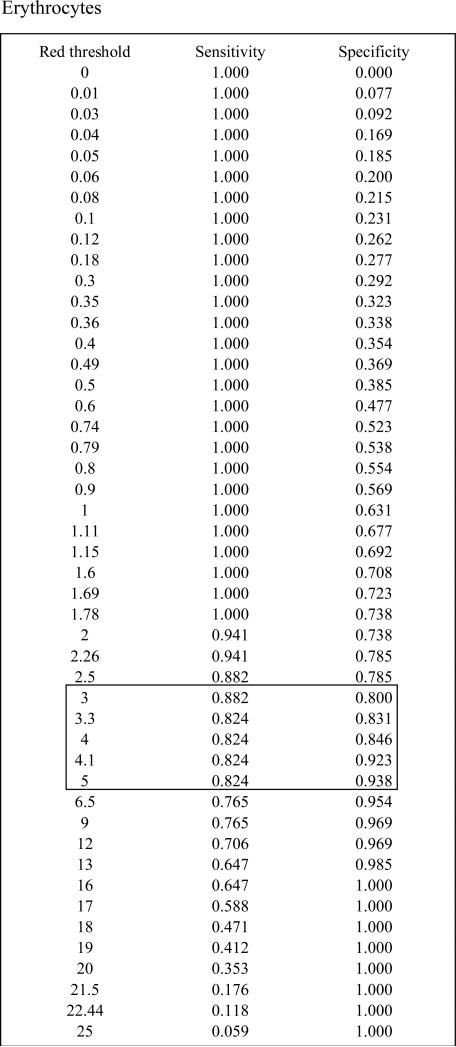
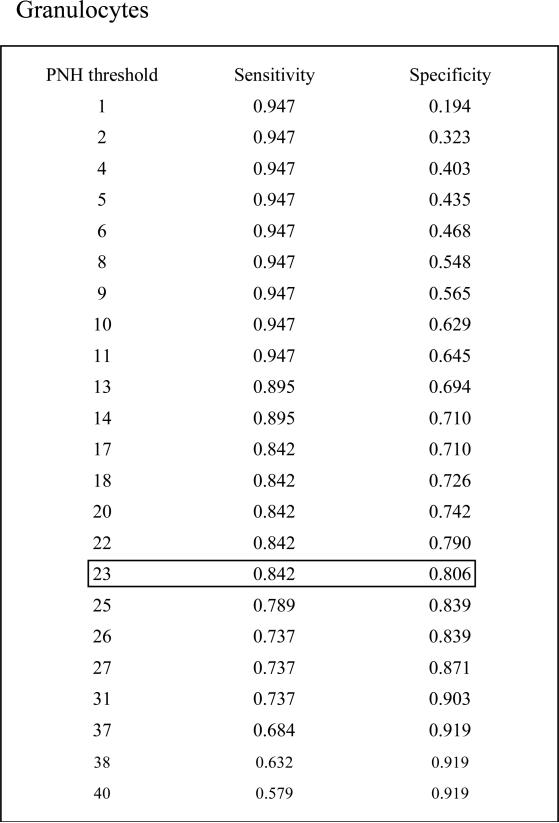
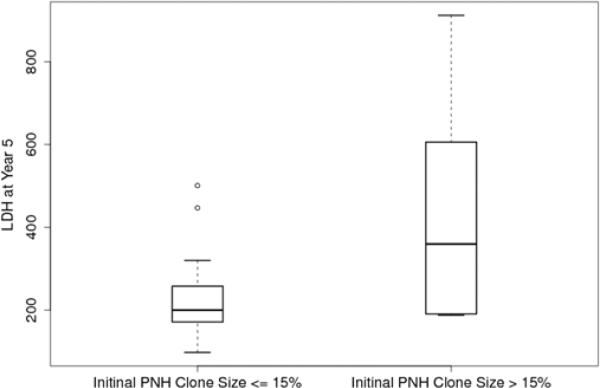
We found a linear relationship between PNH clone size and the development of intravascular hemolysis, assessed by LDH values (Pearson Correlation Coefficient=0.80, P<0.001 for erythrocyte PNH clones; and Pearson Correlation Coefficient=0.73, P<0.0001 for granulocyte PNH clones). An erythrocyte PNH size of 3~5% and granulocyte PNH size of 23% were the thresholds to predict hemolysis as measured by an elevated LDH (ROC analyses with AUC=0.96 for erythrocyte PNH clone sizes and AUC=0.88 for granulocyte PNH clone sizes). Patients with small (≤15%) initial PNH clone sizes were less likely to develop an elevated LDH (mean±SD: 236.9±109.9 vs423.1±248.8; P=0.02). Over time, the PNH clone sizes remained stable in 25.9% of patients; 48.1% experienced a rise in the PNH clone size and 25.9% experienced a decrease.
Conclusion
The risk of developing clinically significant PNH after HiCy therapy appears to be low in AA patients with PNH clones, especially for those with small initial PNH clones and for those who respond to HiCy therapy.
Keywords: Aplastic Anemia (AA), Paroxysmal Nocturnal Hemoglobinuria (PNH), High dose cyclophosphamide (HiCy), Fluoresceinated Aerolysin Variant (FLAER), Lactate Dehydrogenase (LDH).
Introduction
Acquired aplastic anemia (AA) and paroxysmal nocturnal hemoglobinuria (PNH) are closely related bone marrow failure disorders1;2. Most cases of AA result from an autoimmune attack directed against hematopoietic stem/progenitor cells3;4. PNH is an acquired clonal hematopoietic stem cell disorder that manifests following the expansion and differentiation of a hematopoietic stem cell harboring a PIG-A mutation. The PIG-A gene product is necessary for the synthesis of glycosylphosphatidylinositol (GPI), a glycolipid moiety that anchors dozens of proteins to the cell surface. Consequently, PNH cells are missing a variety of GPI-anchored proteins (GPI-APs), two of which (CD55 and CD59) are important complement regulatory proteins. The absence of these proteins on PNH cells contributes to many manifestations of the disease such as hemolytic anemia, thrombosis and smooth muscle dystonias. Both PNH and AA can be cured by allogeneic bone marrow transplantation (BMT), but only a minority of patients is offered this approach due to the potential morbidity and mortality of BMT and the availability of other less toxic approaches5. AA can be treated effectively with immunosuppressive therapy 6,7 and high dose cyclophosphamide (HiCy).8 PNH can be controlled by a monoclonal antibody that interferes with terminal complement activation9-11.
PNH often arises from AA. It has been estimated that more than 50% of AA patients harbor small, but expandable PNH populations at diagnosis12;13. The natural history of patients with classical PNH has been well documented14-17; recently, a retrospective study investigated 83 severe aplastic anemia (sAA) patients with PNH clones following standard immunosuppressive therapy such as antithymocyte globulin and cyclosporine (ATG/CsA).18 However, the natural history of PNH clones in AA patients following HiCy therapy has not been documented. We developed a highly sensitive and specific assay to detect PNH blood cells based on the properties of the bacterial toxin proaerolysin19;20. Since 1998, we have used this assay to detect small PNH clones in patients with AA13. The purpose of this study is to determine the fate and clinical relevance of these PNH clones in patients with AA, especially those patients treated with HiCy.
Patients and Methods
Patients and patient selection
The patient selection criteria for this study were as follows: 1) having an initial diagnosis of acquired AA; 2) having at least three positive values (≥0.02%) on PNH FLAER assay in the course of follow-up; 3) having been monitored for at least 18 months after detecting a PNH clone. These patients were treated and monitored at every 3 to 6 months between November, 1998 and March, 2010.
Venous peripheral blood was drawn into heparin-containing tubes after informed consent as approved by the Joint Committee on Clinical Investigation of The Johns Hopkins Hospital. Acquired aplastic anemia was diagnosed as per clinical criteria21;22. Severe aplastic anemia (SAA) was defined as 1) <25% bone marrow cellularity and 2) at least two of the following three peripheral blood counts: neutrophils <0.5×109/L; platelets <20×209/L; and anemia with corrected reticulocytes <1%; patients with very severe aplastic anemia (vSAA) met the same criteria except that the neutrophil count was below 0.2×109/L. Non-severe aplastic anemia (nSAA) was defined as marrow hypoplasia with pancytopenia not severe enough to meet the above criteria23.
Methods
Detection of GPI anchor expression deficiency was assessed via flow cytometry as previously described.16, 19 Briefly, erythrocytes and granulocytes were identified on the basis of forward and side scatter and by staining with PE-conjugated anti-phosphorin A and anti-CD15 (Immunotech). The gates used to define CD59- erythrocytes and FLAER-negative granulocytes were set up by using normal erythrocytes and granulocytes as control.17, 19
CellQuest software was used to collect and analyze the flow cytometry study data. PNH clone size was defined as percentage of GPI-AP deficient erythrocytes or granulocytes in whole peripheral blood. Serum lactate dehydrogenase (LDH) values were measured by Johns Hopkins Hospital core laboratory.
Statistical analysis
Association between PNH and LDH was characterized by Pearson correlation and the statistical significance was determined using a mixed effect model with longitudinal PNH size and LDH value data. To evaluate the accuracy of PNH clone size to discriminate normal and abnormal LDH, receiver operating characteristic (ROC) analysis based on logistic regression was used, and sensitivities and specificities were computed for identifying PNH clone size thresholds. Random coefficient models were applied to examine the association of baseline PNH clone size and LDH over time. Two sample t-test for the numeric LDH and Fisher's exact test for binary LDH status were used to compare LDH values at five years after diagnosis between patients with low and higher initial PNH clone sizes. Separate linear regression of PNH size on elapsed time since the initiation of treatment was constructed for each subject, and the slope parameter of the regression line was used to estimate the change rate of PNH size over the course of follow-up. All analyses were carried out using statistical package SAS®, Version 9.1.
Results
Patient characteristics
Twenty-two patients who received HiCy therapy and met selection criteria were screened from 67 AA patients treated on our HiCy protocol.8 Five AA patients who did not receive HiCy therapy were recruited from our AA clinic. Patient characteristics are outlined in Table 1. All patients were initially diagnosed with acquired AA with a median granulocyte count of 0.15×109/L (range: 0.03-2.30×109 /L), a median platelet count of 9×109/L (range: 4-21×109/L), a median corrected reticulocyte count of 0.3 % (range: 0.1-2.6%), and a median bone marrow CD34 count of 0.1% (range: 0.02%-0.24%). All patients had a bone marrow cellularity <20%. Twenty-one patients met criteria for SAA, two patients met criteria for vSAA, and four patients met criteria for nSAA. Based on initial granulocyte PNH clone sizes, patients were arbitrarily divided into three groups: small (≤15%, n=16), medium (15~40%, n=7), and large (≥40%, n=4). There were fifteen patients initially treated with HiCy therapy; seven patients initially treated with common immunosuppressive regimens, then treated with HiCy due to either refractory or relapsed SAA; two patients only received immunosuppressive therapy (one received CsA and another received ATG/CsA), and three patients (all nSAA) did not require treatment during the period of observation. Patient demographics and response to therapy are shown in Table 2.
Table 1.
Patient characteristics (n=27)
| Initial PNH clone size (granulocytes) | |||||
|---|---|---|---|---|---|
| Characteristics | ≤15% | >15% and <40% | ≥40% | Total | |
| Sex | |||||
| Male, n (%) | 6 (22.2) | 2 (7.4) | 1 (3.7) | 9 (33.3) | |
| Female, n (%) | 10 (37) | 5 (18.5) | 3 (11.1) | 18(66.7) | |
| Age at diagnosis, Median (range) | |||||
| Male | 29 (20-41) | 30 (24-36) | 41 (41) | 24(20-41) | |
| Female | 21 (10-62) | 48 (15-68) | 31 (19-32) | 29(10-68) | |
| Therapy | |||||
| IST only | 1 | 1 | 0 | 2 | |
| 1ST→ HiCy | 5 | 0 | 2 | 7 | |
| HiCy only | 7 | 4 | 2 | 13 | |
| HiCy → BMT | 0 | 1 | 0 | 1 | |
| HiCy → Eculizumab | 1 | 0 | 0 | 1 | |
| No therapy | 2 | 1 | 0 | 3 | |
| Follow up years, Median (range) | 6.3 (1.5-11.5) | 3.3 (2.5-7.3) | 5.5 (3.2-9) | 5.1(1.5-11.5) | |
| PNH clone size | |||||
| Increase, n (%) | 6 (22.2) | 5(18.5) | 2 (7.4) | 13 (48.1) | |
| Decrease, n (%) | 3 (11.1) | 2 (7.4) | 2 (7.4) | 7 (26) | |
| Stable, n (%) | 7 (25.9) | 0 (0) | 0 (0) | 7 (26) | |
| Eventually developed PNH symptoms (n) | 3 | 3 | 1 | 7 | |
| Transfusion dependent | 3 | 3 | 1 | ||
| Worsening Fatigue | 1 | 3 | 0 | ||
| Abdominal pain | 1 | 0 | 0 | ||
| Hemoglobinuria | 1 | 0 | 0 | ||
| Thrombosis | 0 | 0 | 0 | ||
Table 2.
Clinical characteristics of the cohort.
| Patient | Sex/Age (yrs) | Initial Diagnosis | Follow-up (mos) | PNH size (%) (initial/current)* | LDH (mg/dl) (initial/current) | Therapy | Post-treatment AA status |
|---|---|---|---|---|---|---|---|
| 1 | F/41 | SAA | 114 | 0.5 /0.2 | 87/ 98 | HiCy | Remission |
| 2 | F/22 | SAA | 129 | 0.16/50 | 186/628 | HiCy | Remission for 108 mos, then transformed to PNH |
| 3 | F/68 | vSAA | 86 | 22/0.18 | 178/172 | HiCy | Remission |
| 4 | F/15 | SAA | 87 | 24 /31 | 382/527 | HiCy | Remission for 84 mos, then transformed to PNH |
| 5 | M/37 | SAA | 78 | 12.3 / 14 | 390/501 | HiCy | Remission |
| 6 | M/9 | SAA | 109 | 66.5/96.7 | 536/507 | HiCy | Remission for 60 mos, then asymptomatic PNH expansion |
| 7 | F/19 | SAA | 63 | 0.1/38 | 181/447 | HiCy | Remission |
| 8 | F/19 | SAA | 52 | 0.12/23 | 193/285 | HiCy | Remission for 52 mos, then AA relapse |
| 9 | M/22 | SAA | 50 | 5/0.8 | 198/162 | HiCy | Remission |
| 10 | M/36 | SAA | 49 | 31/3.9 | 171/190 | HiCy | Remission |
| 11 | M/20 | SAA | 37 | 0.06/1 | 200/ 269 | HiCy | Remission |
| 12 | F/32 | SAA | 38 | 51.67/40.7 | 237/191 | HiCy | Remission with asymptomatic PNH expansion |
| 13 | M/20 | SAA | 18 | 0.34/ 0.46 | 180/ 172 | HiCy | Remission |
| 14 | M/24 | SAA | 30 | 17/ 84 | 302/ 912 | HiCy | AA relapse after 3 mos remission, then transformed to PNH after 18 mos |
| 15 | F/52 | SAA | 39 | 23/72 | 314/229/188 | HiCy, then BMT | Refractory, then remission post BMT |
| 16 | F/34 | SAA | 138 | 0.14/3 | 235/182 | CSA, then HiCy | AA relapse after 6 mos remission, then remission again post HiCy |
| 17 | F/18 | vSAA | 123 | 6/ 26 | 147/ 266 | Prednisone, then HiCy | Refractory, then remission post HiCy |
| 18 | F/10 | SAA | 134 | 0.02 / 0 | 178/208 | ATG/CSA, then HiCy | AA relapse after 30 mos remission, then remission again post HiCy |
| 19 | F/31 | SAA | 82 | 57 / 62.2 | 267/ 395 | ATG/CSA, then HiCy | Refractory, then remission post HiCy |
| 20 | M/41 | SAA | 81 | 0.12/1 | 174/ 171 | ATG/CSA, then HiCy twice | AA relapse after 7 mos remission, then relapse again 72 mos after HiCy, then remission again post second HiCy |
| 21 | F/62 | SAA | 60 | 15/6.2 | 189/189 | CSA, then HiCy | Refractory, then remission post HiCy |
| 22 | F/19 | SAA | 49 | 60/1 | 353/ 306 | CSA, then HiCy | Refractory, then remission post HiCy |
| 23 | F/46 | NSAA | 131 | 0.5 /6 | 223/ 235 | CSA | Remission |
| 24 | F/27 | SAA | 38 | 17/50 | 307/ 600 | ATG/CSA | Remission for 30 mos, then asymptomatic PNH expansion |
| 25 | M/39 | NSAA | 84 | 0.3/1.5 | 206/231 | No treatment | Self recovery |
| 26 | F/17 | NSAA | 56 | 15/4.2 | 419/ 189 | No treatment | Self recovery |
| 27 | F/48 | NSAA | 36 | 25/51 | 512/ 606 | No treatment | Asymptomatic PNH expansion |
Remission was classified as HCT ≥30%/independence of transfusion/ANC≥500/cumm/Platelet count≥20K/cumm. PNH transformation was classified as presenting classical PNH symptoms and developing apparent hemolysis with LDH value >1.5x of normal upper limit.
initial PNH size means the PNH size before treatment, and current PNH size means the PNH size in the most recent visit.
Relationship between PNH size and serum LDH value
In order to determine the minimum PNH clone size that is associated with the onset of intravascular hemolysis, we plotted all available serum LDH values with paired PNH clone sizes, which were measured at the same time point for individual patients (Fig.1A). We found a significant linear relationship between PNH clone sizes and LDH values in both erythrocytes and granulocytes (Pearson Correlation Coefficient=0.804, P<0.001 for erythrocytes; and Pearson Correlation Coefficient=0.72788, P<0.0001 for granulocytes). Using a receiver operating characteristic curve (ROC) analysis, we demonstrated that the area under the ROC curve (AUC) for erythrocytes was 0.96 and for granulocytes was 0.88, which indicated that the size of the PNH clone in both erythrocytes and granulocytes had the predictive value to discriminate patients with normal and abnormal LDH values (Fig.1B). The subsequent sensitivity and specificity analysis demonstrated that a PNH clone size of 3~5% for erythrocytes and of 23% for granulocytes had threshold effects to distinguish normal and abnormal LDH, which in turn would classify a patient as with or without hemolysis. Next, we investigated the probability of developing intravascular hemolysis, using LDH as a surrogate, based on initial PNH size at diagnosis (Figure 2). We compared serum LDH values five years after diagnosis of AA among the patients who initially presented with a small percentage of PNH granulocyte (n=15), to patients who presented with medium and large percentages of PNH granulocytes (n=9). We found that the AA patients in the small size group had significantly lower LDH levels than those in other two groups (mean±SD=236.9±109.9 vs 423±248.8; P=0.02). Furthermore, using random coefficient models, we found that LDH values over time have a significant relationship with the baseline PNH sizes. Higher LDH values over the period of follow-up are significantly associated with larger granulocyte PNH clone size at diagnosis (P=0.03). Finally, we arbitrary chose a LDH value of 1.5x the upper limit of normal LDH value (330mg/dL) as a surrogate for clinically relevant intravascular hemolysis. This is the LDH value that has been used in prior PNH studies for eligibility to receive eculizumab9;10. Fisher's exact test showed that patients with high initial PNH clone size had a greater chance to develop hemolysis (P=0.06). Among the AA patients with granulocyte PNH size ≤15%, only two of fifteen patients (13.3%) acquired LDH values greater than 330mg/dL five years after diagnosis. In contrast, among those AA patients with granulocyte PNH size >15% at diagnosis, five of nine patients (55.6%) had LDH values > 330mg/dL at five years.
Fig. 1A.
The correlation between PNH-like clone size and LDH value. (Pearson Correlation Coefficient=0.80; P<0.001 for erythrocytes; and Pearson Correlation Coefficient=0.73; P<0.0001 for granulocytes. The partial transparent gray color areas represent normal LDH ranges.)
Fig. 1B.
Receiver operating characteristic curve (ROC) analysis. We define LDH ≥ 330 mg/dl (1.5x of normal upper limit) as abnormal. The aim is to evaluate the predictive value of PNH to discriminate normal and abnormal LDH. The area under the ROC curve (AUC) is 0.96 for erythrocyte PNH and is 0.88 for granulocyte PNH. The erythrocyte PNH size of 3~5% and granulocyte PNH size of 23% are the optimal threshold at which the PNH clone sizes can best classify patients as with and without hemolysis.
Fig. 2.
Box plot comparison of LDH values at year 5 after diagnosis by initial PNH size. The LDH at 5 years after diagnosis is significantly higher for the patients whose initial granulocyte PNH size was larger than 15% (mean±SD=423.1±248.8, n=9) compared to those with granulocyte size PNH smaller than 15 (mean=236.9±109.9, n=15) (P= 0.02).
Trends of PNH clone size
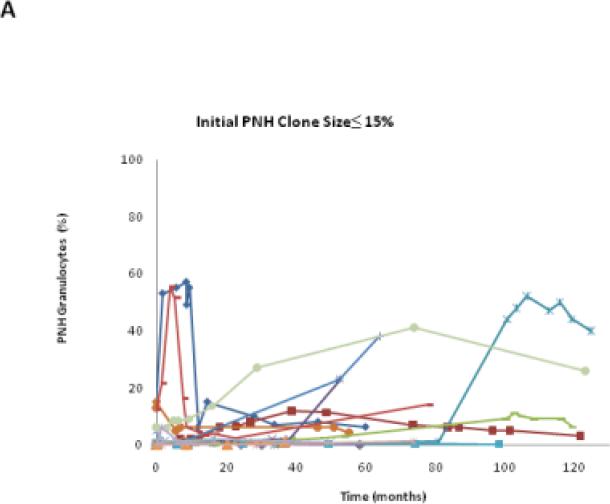
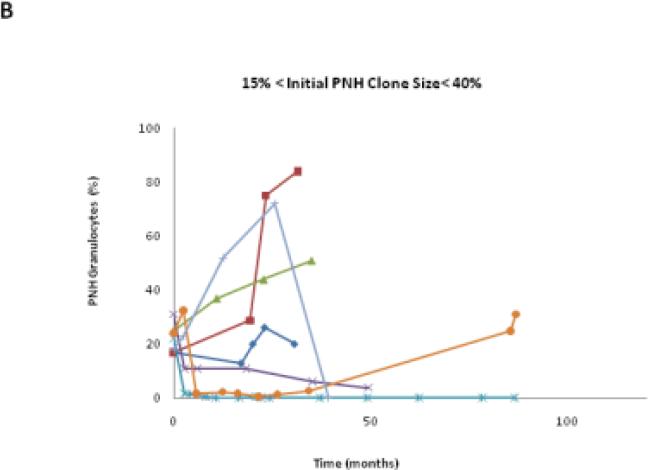
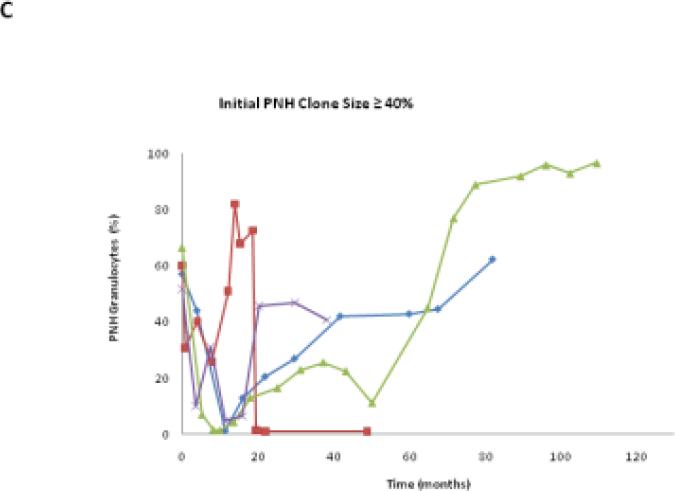
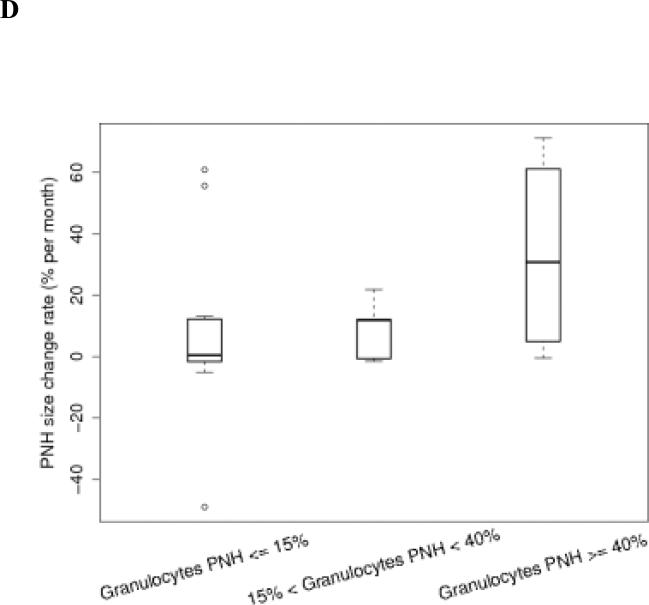
We classified the PNH clone size as stable if the percentage change of PNH granulocytes was < 2.5% of the original value. In this study, 25.9% (7/27) patients remained stable, 48.1% (13/27) patients increased their clone size, and 25.9% (7/27) patients decreased their clone size (Table 1). Each patient had his/her unique PNH clone size expansion pattern, even within his/her own group (Fig.3A to C). Among patients in these three groups, those with initial PNH clones size of ≤15% were the most stable over time (Table 1 and Fig.3D).
Fig. 3.
The PNH size change trends in each patient group. A,B,C show the granulocyte PNH size change of individual patient in the course of this study. D shows the PNH size expansion rates of each patient group after receiving HiCy treatment.
Using separate linear regression analyses, we determined that the average monthly PNH clone size change rates following HiCy therapy for each patient group were 7.47±27.32%, 8.75±9.8%, and 33.07±33.74% respectively (Fig. 3D). These data suggests that patients with large initial PNH clones (size ≥ 40%) are more likely to experience expansion of their PNH clones (p=0.08). In addition, we found that PNH clone size expanded more rapidly following relapse of AA (P=0.029) (Table 3). Interestingly, no patient in this study had clinical signs or symptoms of thrombosis during the period of observation.
Table 3.
PNH clone size change in five patients received HiCy only treatment, who first went in remission and then relapsed
| Patient ID | PNH clone size (%)change with time | |
|---|---|---|
| during remission | during relapse | |
| 2 | 0.84 (0.16 to 1) in 86 mos | 49 (1 to 50) in 35 mos |
| 4 | 1.02 (1.68+ to 2.7) in 30 mos | 28.3 (2.7 to 31) in 53 mos |
| 7 | 0.3 (0.1 to 0.4) in 53 mos | 37.6 (0.4 to 38) in 56 mos |
| 8 | 1.88 (0.12 to 2) in 38 mos | 21 (2 to 23) in 14 mos |
| 14 | 3 mos* | 55.3 (28.7 to 84) in 12 mos |
| Mean±SD | 1.01±0.7 in 51.8±24.7 mos | 38.2±14.2 in 34±20.8 mos |
P=0.029
no PNH size data available during those 3 months of remission period.
this patient's initial (treatment naïve) PNH granulocyte clone size was 24% (see table 2) and her initial post HiCy treatment PNH granulocyte clone size was 1.68%. Other 4 patients didn't have obvious PNH size change right after HiCy treatments, which might be due to their original PNH clone sizes were very small already.
Influences of therapy on PNH granulocyte clone size
There were fifteen patients who received HiCy as initial therapy. Fourteen of them (93.3%) achieved remissions. One patient, a non-responder, was observed to have continuous PNH size expansion following HiCy therapy (23% to 52%). She eventually received an Allogeneic BMT for refractory SAA 30 months after HiCy and remains in remission without detectable PNH granulocytes. Late expansion of the PNH clone was observed in seven of these fifteen patients (46.6%), who were initially managed with HiCy. Five of these patients eventually required intermittent supportive care such as blood transfusion, but only one patient developed symptomatic hemolysis necessitating eculizumab therapy (Table 3). The median time to granulocyte PNH clone expansion after HiCy was 38 (range: 18 to 86) months.
There were nine patients who initially were treated with immunosuppressive therapies other than HiCy: four patients received ATG/CsA, four patients received oral CsA alone, and one patient received oral prednisone. Two patients (one had nSAA and was managed with CsA alone, and another one had SAA and was treated with ATG/CsA) achieved a remission following immunosuppressive therapy and did not require further medical intervention. Interestingly, that latter patient kept a normal CBC profile and was asymptomatic while her PNH clone size expanded to 50% after 37 months observation. Two nSAA patients in the small PNH group spontaneously recovered their own hematopoiesis during the observation periods of 84 months and 56 months respectively, neither had expansion of their PNH clones. Expansion of the PNH clone was observed in seven of nine (77.8%) patients who were initially managed with immunosuppressive therapy at a median of 3 (range: 2 to 87) months after immunosuppressive therapy. Seven of these patients eventually received HiCy salvage therapy because of refractory pancytopenia. After HiCy salvage, all of them became transfusion independent and four had no further expansion of their PNH clones.
A nSAA patient who remained transfusion independent with a hematocrit >35% never required any medical intervention; her granulocyte PNH size increased from 25% to 51% and her LDH level increased to over 500 U/L after 36 months of observation.
Discussion
Detecting PNH cells at diagnosis in AA is increasingly common with the advent of newer and more sensitive flow cytometric assays to detect PNH cells;13,20,25 however, the clinical relevance of these often diminutive populations is not well studied. We and others have previously shown that more than 50% of patients with acquired aplastic anemia harbor PNH cells at diagnosis.19,20,25 The purpose of this study is to uncover the natural history of these PNH cells over time, especially for those patients who received HiCy therapy.
In this study, laboratory evidence of intravascular hemolysis, demonstrated by a rise in LDH greater than 1.5 times the upper limit of normal value, correlated closely with the granulocyte and red cell PNH clone size. Most patients with greater than 23% PNH granulocytes (or greater than 3~5% PNH erythrocytes) had laboratory evidence of hemolysis. The PNH clone size in granulocyte population is usually larger than in erythrocyte population for following reasons: 1) many of these patients were receiving erythrocyte transfusions, 2) erythrocytes have a longer half-life than granulocytes, and 3) PNH erythrocytes are more vulnerable to complement mediated destruction. AA patients with small initial PNH clones were unlikely to develop laboratory evidence of hemolyisis over time. Only 15% of patients who initially had fewer than 15% PNH granulocytes developed an abnormal LDH (LDH> 1.5x control) within 5 years of diagnosis. In contrast, most patients with larger PNH clones at diagnosis experienced a rise in LDH within 5 years. Furthermore, following HiCy therapy the patients with initial PNH clone size larger than 40% were prone to expansion of their PNH clones (Fig.3D). Only one patient developed clinically significant intravascular hemolysis that required treatment with eculizumab and none of the patients developed thrombosis over the period of observation.
There are now a few studies that have investigated the natural history of PNH clones in AA patients.17, 18, 24,26 The major difference between our study and previous reports is that the majority patients in this study received HiCy therapy8. In this study, we found that expansion or contraction of the PNH clone size appeared to be stochastic. Roughly 50% of AA patients demonstrated an increase in their PNH clone with time; whereas, 25% experienced a decrease in clone size and another 25% remained stable. These finding are in agreement with Sugimori et al., who used flow cytometry to follow the fate of small PNH clones in 75 Japanese patients with AA over a 5 year period24. They found that the PNH clone increased in 17%, persisted in 59%, and disappeared in 24% of patients; however, the median starting size of the PNH clone in Sugimori's study was 0.178 (range, 0.003 to 94.2%). We chose to exclude patients with less than 0.02% of granulocyte PNH size since we and others have shown that healthy control subjects can have 0.002 to 0.003% PNH granulocytes that are the consequence of non-clonal PIG-A mutations that arise in colony forming cells.25,27 Nevertheless, both our study and that of Sugimori et al demonstrated that AA patients with very small PNH clones at diagnosis are less likely to experience expansion of the PNH clone than those with larger PNH clones. Our study is also in agreement with a more recent report from Sheinberg et. al18, demonstrating a correlation between PNH size and LDH value. Similar to the Sugimori study, the patients reported by Sheinberg and his colleagues were also predominantly treated with antithymocyte globulin and cyclosporine.
It is impossible to know what, if any, influence HiCy had on the expansion or regression of the PNH clone; however, while expansion of the PNH clone may occur after HiCy and other immunosuppressive therapies28, progression to clinically relevant PNH is not common within 10 years in patients who respond to treatment.8,18,29 Interestingly, expansion of the PNH clone appeared to accelerate at the time of AA relapse (Table 3), which suggests that the PNH clone preferentially survives the immune mediated killing 30;31.
In conclusion, the risk of developing clinically significant PNH after HiCy therapy appears to be low in AA patients with detectable PNH clones. Patients with very small PNH clones at diagnosis (granulocyte PNH size≤ 15%) are especially unlikely to experience expansion of their PNH clones or a rise in their LDH values.
Acknowledgements
This study was supported by the National Institutes of Health (grant P01CA70970 and T32HL007525).
Reference List
- 1.Brodsky RA, Jones RJ. Aplastic anaemia. Lancet. 2005;365:1647–1656. doi: 10.1016/S0140-6736(05)66515-4. [DOI] [PubMed] [Google Scholar]
- 2.Brodsky RA. Narrative review: paroxysmal nocturnal hemoglobinuria: the physiology of complement-related hemolytic anemia. Ann.Intern.Med. 2008;148:587–595. doi: 10.7326/0003-4819-148-8-200804150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Melenhorst JJ, van Krieken JHJM, Dreef E, Landegent JE, Willemze R, Fibbe WE. T cells selectively infiltrate bone marrow areas with residual haemopoiesis of patients with acquired aplastic anaemia. Br J Haematol. 1997;99:517–519. doi: 10.1046/j.1365-2141.1997.4353245.x. [DOI] [PubMed] [Google Scholar]
- 4.Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. Increased expression of Fas antigen on bone marrow CD34+ cells of patients with aplastic anaemia. Br J Haematol. 1995;91:245–252. doi: 10.1111/j.1365-2141.1995.tb05277.x. [DOI] [PubMed] [Google Scholar]
- 5.Brodsky RA. Stem cell transplantation for paroxysmal nocturnal hemoglobinuria. Haematologica. 2010;95:855–856. doi: 10.3324/haematol.2010.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003;101:1236–1242. doi: 10.1182/blood-2002-04-1134. [DOI] [PubMed] [Google Scholar]
- 7.Marsh J, Schrezenmeier H, Marin P, Ilhan O, Ljungman P, McCann S, Socie G, Tichelli A, Passweg J, Hows J, Raghavachar A, Locasciulli A, Bacigalupo A. Prospective randomized multicenter study comparing cyclosporin alone versus the combination of antithymocyte globulin and cyclosporin for treatment of patients with nonsevere aplastic anemia: a report from the European Blood and Marrow Transplant (EBMT) Severe Aplastic Anaemia Working Party. Blood. 1999;93:2191–2195. [PubMed] [Google Scholar]
- 8.Brodsky RA, Chen AR, Dorr D, Fuchs EJ, Huff CA, Luznik L, Smith BD, Matsui WH, Goodman SN, Ambinder RF, Jones RJ. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136–2141. doi: 10.1182/blood-2009-06-225375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillmen P, Young NS, Schubert J, Brodsky RA, Socié G, Muus P, Röth A, Szer J, Elebute MO, Nakamura R, Browne P, Risitano AM, Hill A, Schrezenmeier H, Fu CL, Maciejewski J, Rollins SA, Mojcik CF, Rother RP, Luzzatto L. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N.Engl.J.Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, Gaya A, Coyle L, de Castro C, Fu CL, Maciejewski JP, Bessler M, Kroon HA, Rother RP, Hillmen P. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2009;113:6522–6527. doi: 10.1182/blood-2009-03-195966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tichelli A, Gratwohl A, Nissen C, Speck B. Late clonal complications in severe aplastic anemia. Leuk.Lymphoma. 1994;12:167–175. doi: 10.3109/10428199409059587. [DOI] [PubMed] [Google Scholar]
- 13.Mukhina GL, Buckley JT, Barber JP, Jones RJ, Brodsky RA. Multilineage glycosylphosphatidylinositol anchor deficient hematopoiesis in untreated aplastic anemia. Br J Haematol. 2001;115:476–482. doi: 10.1046/j.1365-2141.2001.03127.x. [DOI] [PubMed] [Google Scholar]
- 14.Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253–1258. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 15.de Latour RP, Mary JY, Salanoubat C, Terriou L, Etienne G, Mohty M, Roth S, de Guibert S, Maury S, Cahn JY, Socié G, French Society of Hematology. French Association of Young Hematologists Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008;112:3099–3106. doi: 10.1182/blood-2008-01-133918. [DOI] [PubMed] [Google Scholar]
- 16.Moyo VM, Mukhina GL, Garrett ES, Brodsky RA. Natural history of paroxysmal nocturnal hemoglobinuria using modern diagnostic assays. Br J Haematol. 2004;126:133–138. doi: 10.1111/j.1365-2141.2004.04992.x. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura J, Kanakura Y, Ware RE, Shichishima T, Nakakuma H, Ninomiya H, Decastro CM, Hall S, Kanamaru A, Sullivan KM, Mizoguchi H, Omine M, Kinoshita T, Rosse WF. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore) 2004;83:193–207. doi: 10.1097/01.md.0000126763.68170.46. [DOI] [PubMed] [Google Scholar]
- 18.Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075–1080. doi: 10.3324/haematol.2009.017889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brodsky RA, Mukhina GL, Nelson KL, Lawrence TS, Jones RJ, Buckley JT. Resistance of Paroxysmal Nocturnal Hemoglobinuria Cells to the Glycosylphosphatidylinositol-Binding Toxin Aerolysin. Blood. 1999;93:1749–1756. [PubMed] [Google Scholar]
- 20.Brodsky RA, Mukhina GL, Li S, Nelson KL, Chiurazzi PL, Buckley JT, Borowitz MJ. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am.J.Clin.Pathol. 2000;114:459–466. doi: 10.1093/ajcp/114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camitta BM, Rappeport JM, Parkman R. and Nathan DG. Selection of patients for bone marrow transplantation in severe aplastic anemia. Blood. 1975;45:355–363. [PubMed] [Google Scholar]
- 22.Bacigalupo A, Hows JM, Gluckman E, Nissen C, Marsh J, Van Lint MT, Congiu M, De Planque MM, Ernst P, McCann S, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA Working Party. Br J Haematol. 1988;70:177–182. doi: 10.1111/j.1365-2141.1988.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 23.Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, Keidan J, Laurie A, Martin A, Mercieca J, Killick SB, Stewart R, Yin JA, British Committee for Standards in Haematology Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70. doi: 10.1111/j.1365-2141.2009.07842.x. [DOI] [PubMed] [Google Scholar]
- 24.Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, Mizoguchi H, Omine M, Nakao S. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308–1314. doi: 10.1182/blood-2005-06-2485. [DOI] [PubMed] [Google Scholar]
- 25.Sugimori C, Mochizuki K, Qi Z, Sugimori N, Ishiyama K, Kondo Y, Yamazaki H, Takami A, Okumura H, Nakao S. Origin and fate of blood cells deficient in glycosylphosphatidylinositol-anchored protein among patients with bone marrow failure. Br J Haematol. 2009;147:102–112. doi: 10.1111/j.1365-2141.2009.07822.x. [DOI] [PubMed] [Google Scholar]
- 26.Shichishima T, Ikeda K, Takahashi N, Kameoka J, Tajima K, Murai K, Tamai Y, Shichishima-Nakamura A, Akutsu K, Noji H, Okamoto M, Kimura H, Harigae H, Oyamada T, Kamesaki T, Takeishi Y, Sawada K. Low concentration of serum haptoglobin has impact on understanding complex pathophysiology in patients with acquired bone marrow failure syndromes. Int J Hematol. 2010;91:602–610. doi: 10.1007/s12185-010-0559-z. [DOI] [PubMed] [Google Scholar]
- 27.Hu R, Mukhina GL, Piantadosi S, Barber JP, Jones RJ, Brodsky RA. PIG-A mutations in normal hematopoiesis. Blood. 2005;105:3848–3854. doi: 10.1182/blood-2004-04-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dingli D, Luzzatto L, Pacheco JM. Neutral evolution in paroxysmal nocturnal hemoglobinuria. Proc.Natl.Acad.Sci.U.S.A. 2008;105:18496–18500. doi: 10.1073/pnas.0802749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brodsky RA, Sensenbrenner LL, Jones RJ. Complete remission in acquired severe aplastic anemia following high-dose cyclophosphamide. Blood. 1996;87:491–494. [PubMed] [Google Scholar]
- 30.Savage WJ, Barber JP, Mukhina GL, Hu R, Chen G, Matsui W, Thoburn C, Hess AD, Cheng L, Jones RJ, Brodsky Glycosylphosphatidylinositol-anchored protein deficiency confers resistance to apoptosis in PNH. Experimental Hematology. 2009;37:42–51. doi: 10.1016/j.exphem.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanaoka N, Kawaguchi T, Horikawa K, Nagakura S, Mitsuya H, Nakakuma H. Immunoselection by natural killer cells of PIGA mutant cells missing stree-inducible ULBP. Blood. 2006;107:1184–1191. doi: 10.1182/blood-2005-03-1337. [DOI] [PubMed] [Google Scholar]