Abstract
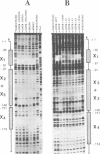
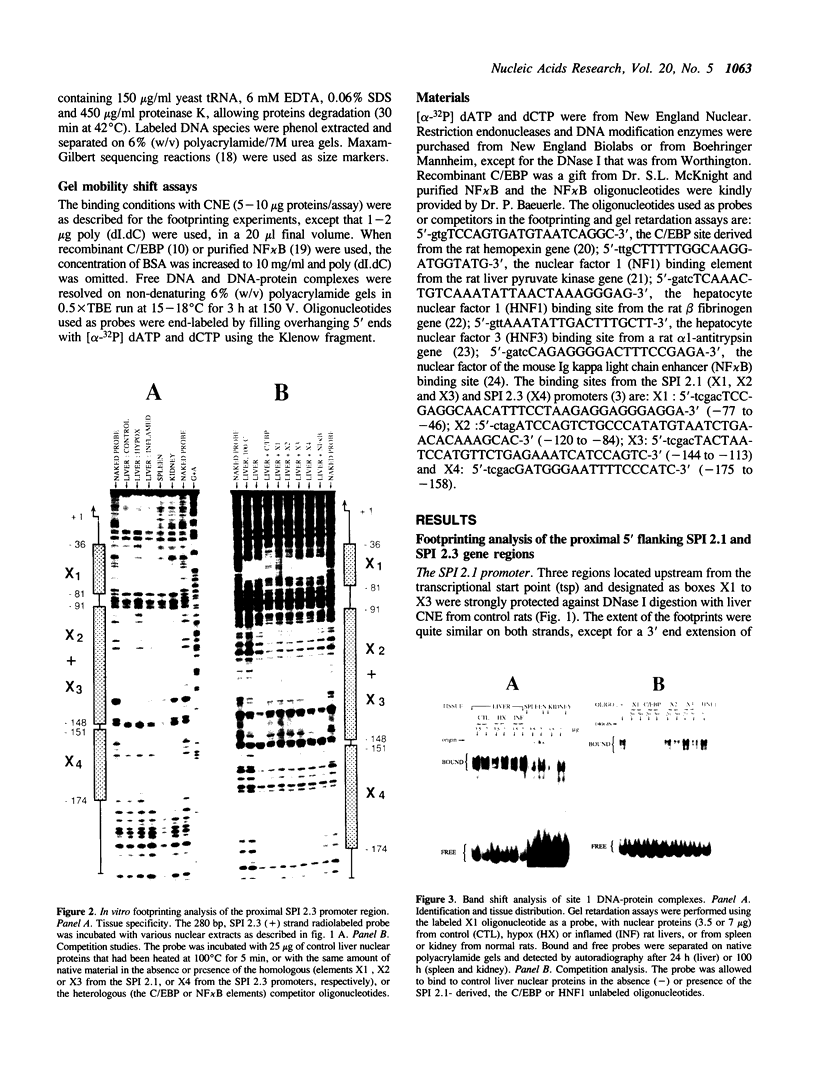
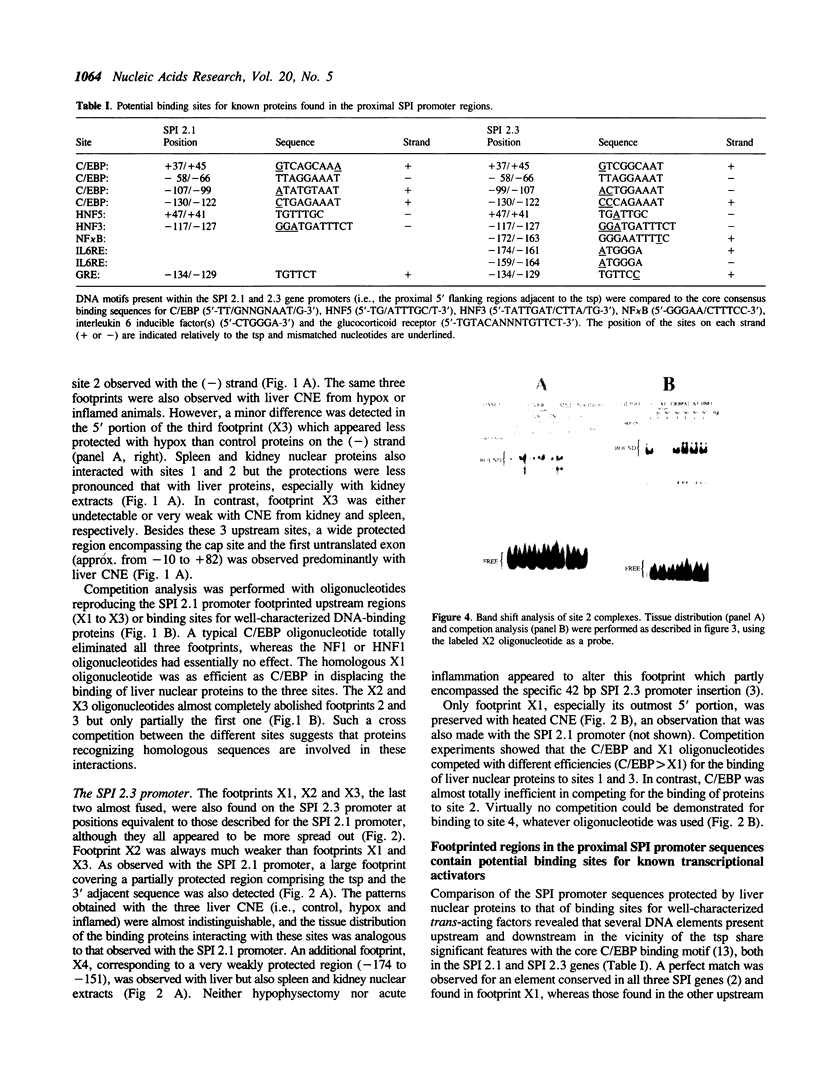
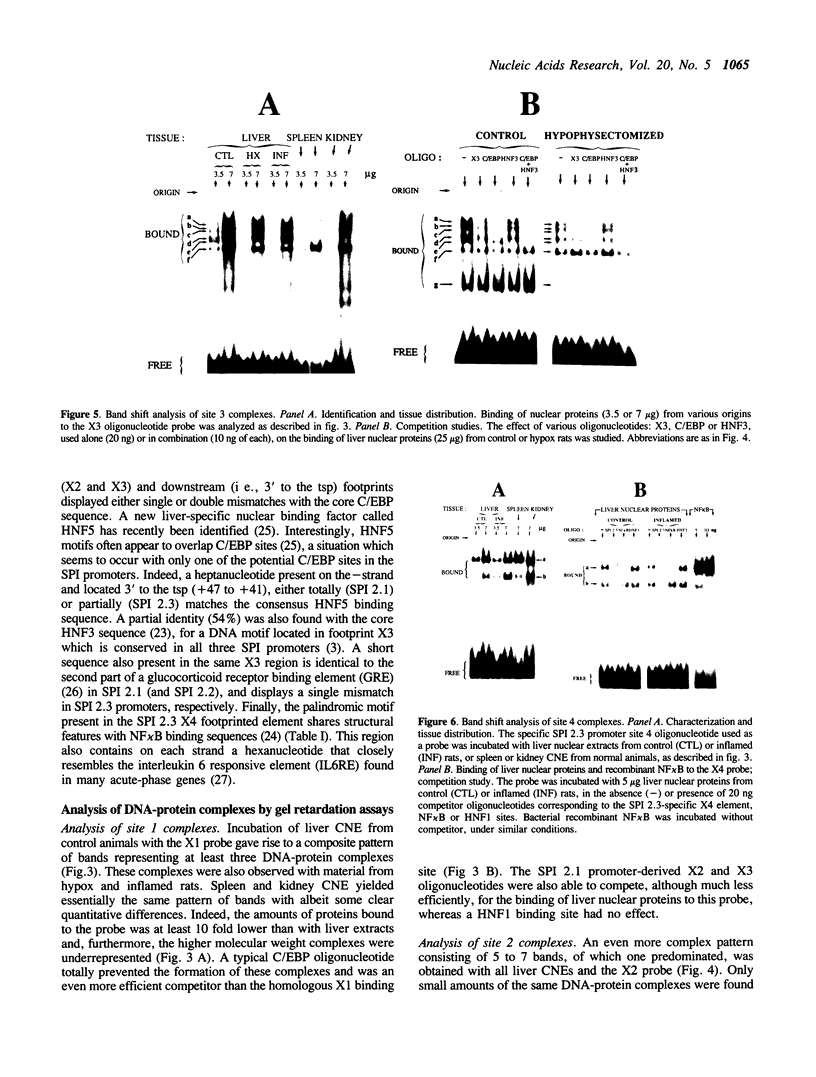
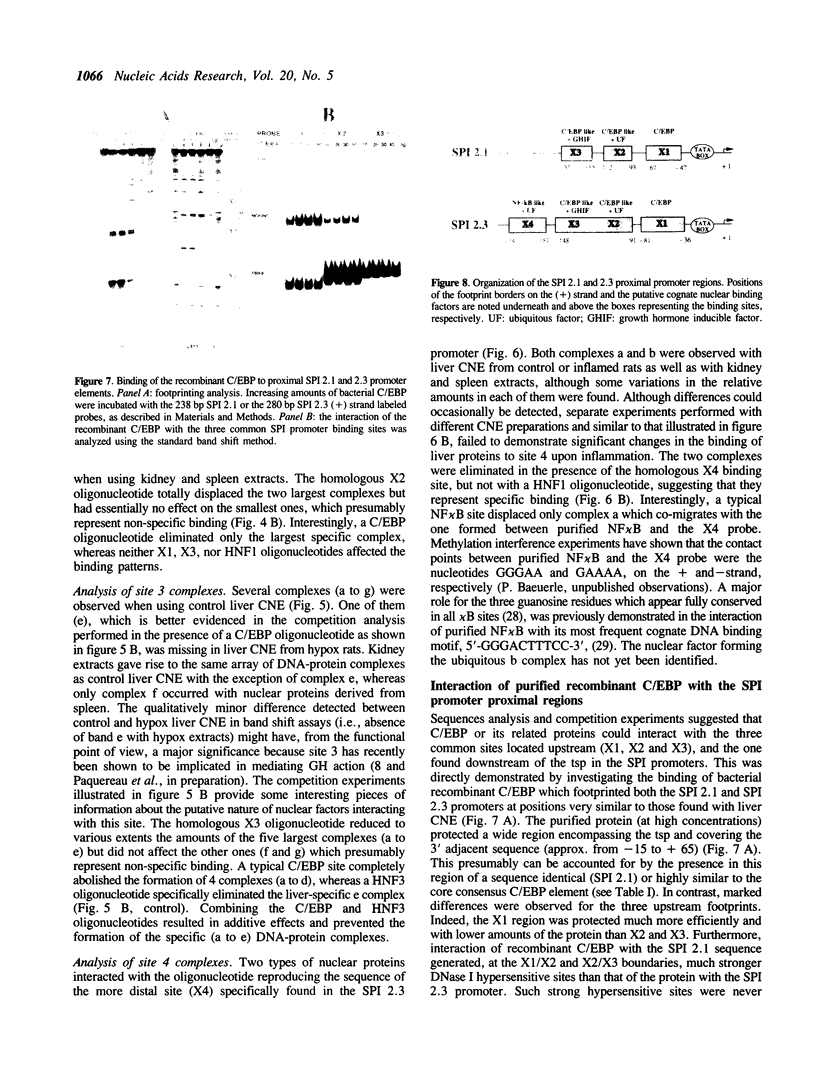
The three serine protease inhibitor (SPI) rat genes expressed preferentially in liver share considerable structural features and, nonetheless, are transcriptionally regulated in completely different manners, more particularly after hypophysectomy or upon acute inflammation. DNase I footprinting and gel mobility shift analyses of the SPI 2.1 and 2.3 proximal promoter regions reveal the presence of three common protein binding sites (1 to 3, 3' to 5') located immediately upstream from the transcription start site. C/EBP, the liver-enriched factor, specifically interacts with site 1 whereas its related proteins (e.g.; DBP, LAP/NFIL6) most likely recognize sites 2 and 3. Another ubiquitous unidentified factor also binds to site 2. A liver-specific protein dependent on growth hormone, whose binding is competed out by an oligonucleotide reproducing an HNF3 motif, interacts exclusively with site 3. The 42 bp sequence which is found only within the SPI 2.3 promoter interacts with two ubiquitous factors, one of which is related to NF kappa B. Acute inflammation does not significantly affect the protein binding patterns observed with the SPI 2.1 or 2.3 proximal promoter sequences. Our results show an apparent discrepancy between the large magnitude of in vivo changes in SPI gene transcription mediated by hormones and the small alterations detected in vitro, in the DNA-protein interactions on the promoters.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990 Jun;9(6):1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Borgmeyer U., Nowock J., Sippel A. E. The TGGCA-binding protein: a eukaryotic nuclear protein recognizing a symmetrical sequence on double-stranded linear DNA. Nucleic Acids Res. 1984 May 25;12(10):4295–4311. doi: 10.1093/nar/12.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier A. R., Ron D., Tate J. E., Habener J. F. A family of constitutive C/EBP-like DNA binding proteins attenuate the IL-1 alpha induced, NF kappa B mediated trans-activation of the angiotensinogen gene acute-phase response element. EMBO J. 1990 Dec;9(12):3933–3944. doi: 10.1002/j.1460-2075.1990.tb07614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S., Raymondjean M., Carranca A. G., Herbomel P., Yaniv M. Factors involved in control of tissue-specific expression of albumin gene. Cell. 1987 Aug 14;50(4):627–638. doi: 10.1016/0092-8674(87)90036-5. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Darnell J. E., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989 Apr;9(4):1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Baumhueter S., Crabtree G. R. Purified hepatocyte nuclear factor 1 interacts with a family of hepatocyte-specific promoters. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7937–7941. doi: 10.1073/pnas.85.21.7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P., Chojkier M., Lichtsteiner S., Falvey E., Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990 Sep;4(9):1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- Fey G. H., Gauldie J. The acute phase response of the liver in inflammation. Prog Liver Dis. 1990;9:89–116. [PubMed] [Google Scholar]
- Friedman A. D., Landschulz W. H., McKnight S. L. CCAAT/enhancer binding protein activates the promoter of the serum albumin gene in cultured hepatoma cells. Genes Dev. 1989 Sep;3(9):1314–1322. doi: 10.1101/gad.3.9.1314. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Grange T., Roux J., Rigaud G., Pictet R. Cell-type specific activity of two glucocorticoid responsive units of rat tyrosine aminotransferase gene is associated with multiple binding sites for C/EBP and a novel liver-specific nuclear factor. Nucleic Acids Res. 1991 Jan 11;19(1):131–139. doi: 10.1093/nar/19.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese E. M., Boothby M. R., Smas C. M., Glimcher L. H. A lipopolysaccharide-induced DNA-binding protein for a class II gene in B cells is distinct from NF-kappa B. Mol Cell Biol. 1989 Aug;9(8):3184–3192. doi: 10.1128/mcb.9.8.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Hattori M., Abraham L. J., Northemann W., Fey G. H. Acute-phase reaction induces a specific complex between hepatic nuclear proteins and the interleukin 6 response element of the rat alpha 2-macroglobulin gene. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2364–2368. doi: 10.1073/pnas.87.6.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. E., Hastie N. D. Accelerated evolution in the reactive centre regions of serine protease inhibitors. Nature. 1987 Mar 5;326(6108):96–99. doi: 10.1038/326096a0. [DOI] [PubMed] [Google Scholar]
- Jantzen H. M., Strähle U., Gloss B., Stewart F., Schmid W., Boshart M., Miksicek R., Schütz G. Cooperativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell. 1987 Apr 10;49(1):29–38. doi: 10.1016/0092-8674(87)90752-5. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Johnson P. F. Transcriptional activators in hepatocytes. Cell Growth Differ. 1990 Jan;1(1):47–52. [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Le Cam A., Pages G., Auberger P., Le Cam G., Leopold P., Benarous R., Glaichenhaus N. Study of a growth hormone-regulated protein secreted by rat hepatocytes: cDNA cloning, anti-protease activity and regulation of its synthesis by various hormones. EMBO J. 1987 May;6(5):1225–1232. doi: 10.1002/j.1460-2075.1987.tb02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cam G., Le Cam A. Synthesis of the growth hormone-regulated rat liver anti-protease GHR-P63 is inhibited by acute inflammation. FEBS Lett. 1987 Jan 1;210(1):1–5. doi: 10.1016/0014-5793(87)81286-3. [DOI] [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin J., Schibler U. The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell. 1987 Dec 24;51(6):963–973. doi: 10.1016/0092-8674(87)90583-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mueller C. R., Maire P., Schibler U. DBP, a liver-enriched transcriptional activator, is expressed late in ontogeny and its tissue specificity is determined posttranscriptionally. Cell. 1990 Apr 20;61(2):279–291. doi: 10.1016/0092-8674(90)90808-r. [DOI] [PubMed] [Google Scholar]
- Pages G., Rouayrenc J. F., Le Cam G., Mariller M., Le Cam A. Molecular characterization of three rat liver serine-protease inhibitors affected by inflammation and hypophysectomy. Protein and mRNA analysis and cDNA cloning. Eur J Biochem. 1990 Jun 20;190(2):385–391. doi: 10.1111/j.1432-1033.1990.tb15587.x. [DOI] [PubMed] [Google Scholar]
- Pagès G., Rouayrenc J. F., Rossi V., Le Cam G., Mariller M., Szpirer J., Szpirer C., Levan G., Le Cam A. Primary structure and assignment to chromosome 6 of three related rat genes encoding liver serine protease inhibitors. Gene. 1990 Oct 15;94(2):273–282. doi: 10.1016/0378-1119(90)90398-b. [DOI] [PubMed] [Google Scholar]
- Poli V., Silengo L., Altruda F., Cortese R. The analysis of the human hemopexin promoter defines a new class of liver-specific genes. Nucleic Acids Res. 1989 Nov 25;17(22):9351–9365. [PMC free article] [PubMed] [Google Scholar]
- Raymondjean M., Cereghini S., Yaniv M. Several distinct "CCAAT" box binding proteins coexist in eukaryotic cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):757–761. doi: 10.1073/pnas.85.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Vaulont S., Puzenat N., Kahn A., Raymondjean M. Analysis by cell-free transcription of the liver-specific pyruvate kinase gene promoter. Mol Cell Biol. 1989 Oct;9(10):4409–4415. doi: 10.1128/mcb.9.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. C., Cantwell C. A., Johnson P. F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991 Sep;5(9):1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- Ye R. D., Wun T. C., Sadler J. E. cDNA cloning and expression in Escherichia coli of a plasminogen activator inhibitor from human placenta. J Biol Chem. 1987 Mar 15;262(8):3718–3725. [PubMed] [Google Scholar]
- Yoon J. B., Berry S. A., Seelig S., Towle H. C. An inducible nuclear factor binds to a growth hormone-regulated gene. J Biol Chem. 1990 Nov 15;265(32):19947–19954. [PubMed] [Google Scholar]
- Yoon J. B., Towle H. C., Seelig S. Growth hormone induces two mRNA species of the serine protease inhibitor gene family in rat liver. J Biol Chem. 1987 Mar 25;262(9):4284–4289. [PubMed] [Google Scholar]
- Zabel U., Baeuerle P. A. Purified human I kappa B can rapidly dissociate the complex of the NF-kappa B transcription factor with its cognate DNA. Cell. 1990 Apr 20;61(2):255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]
- Zabel U., Schreck R., Baeuerle P. A. DNA binding of purified transcription factor NF-kappa B. Affinity, specificity, Zn2+ dependence, and differential half-site recognition. J Biol Chem. 1991 Jan 5;266(1):252–260. [PubMed] [Google Scholar]