Abstract
Two-dimensional “pencil-beam” navigator, placed on the right hemidiaphragm (RHD), is used for free-breathing late gadolinium enhancement (LGE) of the left atrium (LA) in patients with atrial fibrillation (AF). The pencil-beam navigator creates an inflow artifact in the right PVs and atrial wall that may obscure local PV and LA scars. To reduce this artifact, we propose a large slab RHD projection navigator that measures the respiratory motion while reducing the associated inflow artifact. Eighteen subjects underwent pulmonary vein LGE using the pencil-beam and projection navigator. Subjective inflow and respiratory motion artifact scores (1=severe, 2=moderate, 3=mild, and 4=none) from two blinded readers were compared. The artifact scores were 3.8±0.4 and 2.1±0.7 for the projection and pencil-beam navigators, respectively (p<0.001). Respiratory motion artifact scores were similar between the two techniques (3.0±0.5 vs. 3.1±0.5 for projection vs. pencil-beam navigator). The proposed method greatly reduces the inflow artifact in free-breathing PV LGE while allowing adequate respiratory motion compensation.
Keywords: atrial fibrillation, pulmonary vein imaging, late gadolinium enhancement, respiratory motion tracking, projection navigator
INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, and accounts for approximately one-third of all the hospitalizations for cardiac rhythm disturbances (1). The pulmonary veins (PVs) are an important source of ectopic beats, initiating frequent paroxysms of AF (2). Over the past decade, radiofrequency (RF) ablation of the PVs has emerged as the leading treatment for both paroxysmal and permanent AF (3).
In consideration of the important role of the PVs in generation of AF, evaluation of PV anatomy before and after PV isolation (PVI) is an essential part of pre- and post-procedural ablation therapy assessment. MR angiography is commonly used to demonstrate PV anatomy for guiding RF ablation and assessing its efficacy (4–5). Recently, late gadolinium enhancement (LGE) has been proven to be capable of visualizing the PVs and atrial wall scar due to the RF ablation (6–11). Furthermore, PV LGE can also demonstrate pre-existing scars and atrial tissue characteristics that could potentially predict response to the RF ablation therapy (8).
Detection of LGE in the thin PVs and atrial walls requires imaging with high spatial resolution over a short period of the cardiac cycle. Therefore, PV LGE is acquired during free-breathing using a respiratory navigator (6,12). Typically, two-dimensional (2D) pencil-beam navigator, placed on the dome of the right hemidiaphragm (RHD), allows for the prospective gating of the respiratory motion of the PVs (6,12). A local navigator restore pulse, a 180° 2D pencil-beam excitation with a wider diameter compared to the navigator, is applied immediately after the inversion pulse to improve the navigator signal, which has been degraded by the non-selective RF inversion pulse of the LGE imaging sequence (13). Unfortunately, the local navigator restore pulse will also “restore” PV blood leading to artifacts in the PVs and left atrium (LA) when the restored PV blood flows into the LA (6). This navigator inflow artifact manifests as signal enhancement of the PV and LA blood pool, thereby reducing the ability of PV LGE to detect scars and fibrosis. Therefore, alternative respiratory motion compensation techniques are required for free-breathing PV LGE.
We have developed and evaluated a prospective large-slab RHD projection navigator that does not require a navigator restore pulse during free-breathing PV LGE, thereby reducing the inflow artifact. Images in healthy adult subjects and AF patients were acquired with the proposed RHD projection navigator sequence and compared with the pencil-beam navigator for PV LGE.
MATERIALS AND METHODS
Written informed consent was obtained from all eighteen subjects and the imaging protocol was approved by our Institutional Review Board. All subjects were scanned using a 1.5 T Achieva magnet (Philips Healthcare, Best, NL) with a 5 channel phased coil array.
A prospective RHD projection navigator technique was implemented by modifying the 2D pencil-beam navigator’s sequence for gating and tracking the respiratory motion. All acquisitions and reconstructions were performed online. In the following subsections, the various technical aspects of the proposed RHD projection navigator and the imaging setup for PV LGE are described.
RHD Projection Navigator Sequence
The RHD projection navigator was acquired in a single repetition time (TR = 6 ms) and consisted of a slab selective RF pulse followed by a readout (Figure 1a). A 50 mm thick slab was prescribed to excite the RHD and retrieve its respiratory motion. A Sinc-Gaussian RF pulse with a 20° flip angle and duration of 0.5 ms was used for the excitation. RHD projection navigator uses the identical RF excitation pulse of the imaging sequence to simplify the programming task. The readout length was 1.56 ms with the bandwidth of 164 kHz/pixel and field of view (FOV) of 256 mm. RHD projection navigator’s parameters were designed to have the identical 1 mm resolution of pencil-beam navigator. The Fourier transform of the measured k-space line was calculated to obtain the projection profile of the RHD (Figure 1b). The acquired profile consisted of signals originating from the liver, lung, thorax, chest wall, back, and PVs. To eliminate superposition of the thorax, chest wall, and back signals on the acquired projection profile, two spatially-selective saturation slabs were applied immediately prior to the navigator excitation (Figure 1c).
Figure 1.
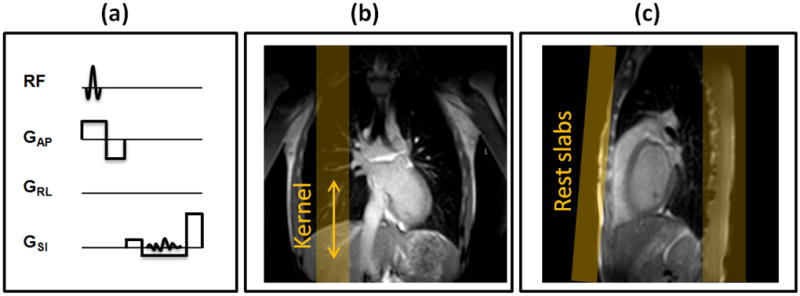
Right hemidiaphragm (RHD) projection navigator and its imaging prescription for pulmonary vein late gadolinium enhancement: (a) a slab selective excitation and a readout is used for monitoring motion in superior-inferior direction (b) a kernel (region of interest) is defined on the edge of the RHD to measure the respiratory motion and (c) two spatially-selective saturation (rest) slab are positioned to suppress the signal from the chest wall and the back.
RHD Projection Navigator Processing
The acquired RHD projection profile varies due to the respiratory motion. The first acquired projection profile was set as the reference p0(x). To track the respiratory motion of the RHD, a region of interests, so called kernel k(x), was manually determined in a scout image. Kernel with length w was placed at the edge of the RHD covering liver and lung. k(x) was then measured from p0(x), and overlaid Δx pixels on the proceeding acquired projection profiles pi (x) (i represents heart beat number) to calculate the cross-correlations as:
where x represents the acquired pixels in the projection profile and kernel, respectively. The amount of Δx where the cross-correlation was maximized was then chosen as the RHD displacement for the ith heart beat.
The RHD projection navigator was prescribed using the scout (localizer), commonly acquired at the beginning of the imaging, to localize the anatomy. The first acquired projection profile, i.e. reference, is defined as the received signal from the entire excited slab. An 80mm portion of this projection is defined as the kernel and its position is defined during the prescription of the RHD projection navigator. During the training phase, RHD displacement is monitored to calculate the maximum position which corresponds to the end-expiration for updating the kernel. The center of gating window was also defined in the training phase, as the RHD position where the cross-correlation was maximized, minus the 25% of the gating window size. The arbitrary 25% shift was selected to match the navigator processing available in the commercial Philips software, presumably to compensate for the respiratory drift. Figure 2 displays the RHD projection profile using the RHD projection and pencil-beam navigators, respectively.
Figure 2.
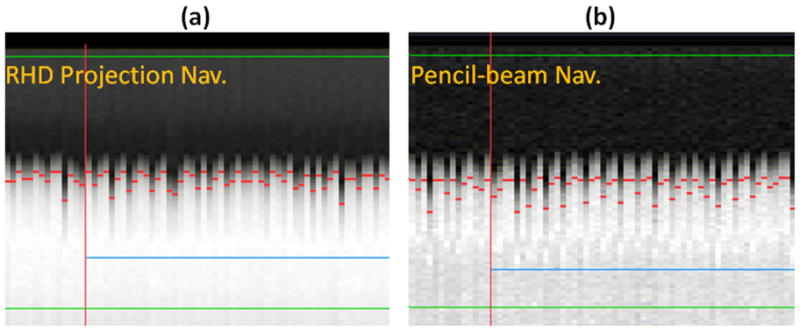
Example navigator signals of the right hemi-diaphragm (RHD) acquired with the projection (a) and pencil-beam (b) navigators.
Free Breathing PV LGE Imaging Sequence
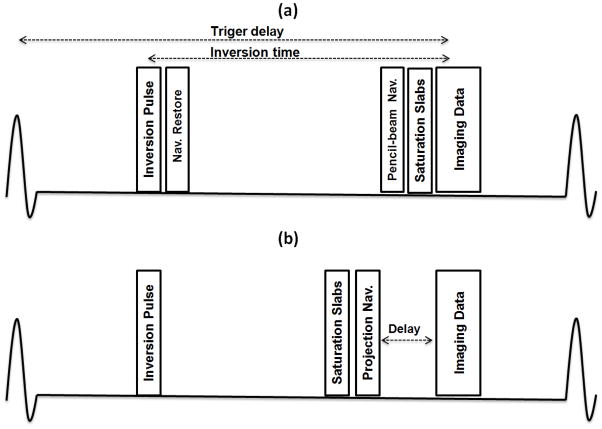
Figure 3 demonstrates the free breathing PV LGE imaging sequence that was used to track the respiratory motion and acquire PV images using either the pencil-beam or proposed RHD projection navigator. In the pencil-beam acquisition, a non-selective inversion pulse was followed by a 2D pencil-beam RF pulse to restore the magnetization in the region where the navigator was positioned. The navigator restore pulse had a larger excitation diameter (factor 1.2) to compensate for the imperfect excitation profile of the 2D RF pulse.
Figure 3.
Pulmonary vein late gadolinium enhancement sequence diagram using (a) standard 2D pencil-beam navigator and (b) right hemidiaphragm (RHD) projection navigator. An extra delay (100 ms) between the projection navigator and imaging data is used to guarantee sufficient signal from the RHD allowing elimination of the navigator restore pulse.
Analogous to the pencil-beam navigator, free-breathing PV LGE images from the RHD projection navigator were acquired with the exception of the navigator restore pulse and with the additional short delay of 100 ms between the projection navigator and start of data acquisition in each heart beat. This short delay was determined in a pilot study in five healthy subjects for investigating the signal recovery of the RHD experiencing an inversion pulse. An inversion recovery prepared T1-weighted segmented 2D echo-planar imaging (Look-Locker) sequence (14) with variable inversion time was used to determine sufficient RHD signal after experiencing an inversion. This additional delay guaranteed the signal recovery of the RHD with the added penalty of potential respiratory motion artifacts on the PV images.
Imaging Protocol
Eight healthy adults (6 females, age 25 ± 9 years) were enrolled in the study. In addition, ten patients (8 males, age 58 ± 14 years), who underwent RF ablation for treatment of AF, were recruited. Exclusion criteria included renal impairment (eGFR < 60 ml/min/1.73m2) or contraindication to MRI. All subjects were in sinus rhythm.
Three-orthogonal stacks of multiple 2D bright blood images were acquired for PV localization and navigator positioning using a steady-state free precession (SSFP) sequence with 3.1 × 3.1 mm2 in-plane resolution and 10 mm slice thickness. LGE images covering the PV and LA were acquired at 15 to 20 min after a bolus infusion of 0.2 mmol/kg of Gd-BOPTA (MultiHance®, Bracco, Rome, Italy) or 0.2 mmol/kg of Gd-DTPA (Magnevist, Bayer Schering Pharma AG, Germany). A Look-Locker sequence was used to determine the appropriate inversion time for LGE. The 3D PV LGE images were then acquired using the RHD projection and pencil-beam navigators, with random ordering, to cancel out differences in acquisition time after contrast infusion.
The 3D PV LGE was acquired using an inversion recovery gradient echo imaging sequence with the following parameters: ECG triggered at end diastole, TE/TR/α = 2.5 ms/5.2 ms/25°, field-of-view (FOV) of 320×320 mm2, 40 slices, spatial resolution of 1.41×1.41×4 mm3 reconstructed to a resolution of 1.25×1.25×2 mm3 using zero-padding, and 25 shots in each cardiac cycle. A navigator acceptance window of 7 mm with a tracking factor of 0.6 was used for gating and tracking respiratory motion. Fold-over suppression was used to reduce the aliasing artifact. The total acquisition time was ~3 minutes assuming a heart rate of 60 bpm and 100% navigator acceptance rate. The inversion time for the second LGE scan was updated by the second Look-Locker sequence prior to imaging. The imaging parameters for the both LGE scans were identical except for the chosen respiratory navigator method.
Image Analysis and Statistics
All images were reconstructed online using the MR console. Data were transferred to the ViewForum (Philips Healthcare, Best, Netherland) for visualization and analysis. The PV LGE was evaluated by two readers, blinded to the acquisition technique and each with > 10 years of cardiac MR experience. Only raw axial images, without any reformatting, were used for scoring. Readers scored the artifacts using a 4 point scale (1 = severe, 2 = moderate, 3 = mild, 4 = none). Separate scores were given, in consensus by both readers, for motion and blood artifacts, and the scores were analyzed separately. SNR and contrast-to-noise ratio (CNR) were not measured due to the dependence of signal level on the imaging time after contrast infusion. A paired two-sided Wilcoxon test was performed on the visual grading where a p value of < 0.05 considered statistically significant. All measurements were represented as mean ± one standard deviation, and all statistical analyses were performed using SAS software (V4.2, SAS Institute Inc., Cary NC).
RESULTS
Figure 4 shows a sample slice from the 3D PV LGE acquired in two healthy subjects using the 2D pencil-beam and RHD projection navigators. Subject 1 showed severe artifacts in the right superior PV (yellow arrow). These artifacts were not seen in the LGE images acquired with the RHD projection navigator. Subject 2 showed moderate artifacts with the pencil-beam navigator that was not visible using the RHD projection navigator. A zoomed section of the region where the blood artifact was located was also shown for both slices. Figure 5 shows PV LGE images of an AF patient imaged after an RF ablation. The images acquired using the pencil-beam navigator, contain signal enhancement from both the ablation (dotted yellow arrow) and navigator inflow artifact (solid white arrow). The navigator inflow artifact was obviated by using the RHD projection navigator. The image contrast between the two acquisitions in this example is fundamentally different for the pencil-beam and RHD projection navigators, presumably due to the differences in sequence timing and/or contrast washout.
Figure 4.
Axial slices for the pulmonary vein (PV) late gadolinium enhancement datasets from two healthy adult subjects acquired with the pencil-beam and right hemidiaphragm (RHD) projection navigators, placed at the dome of the RHD. The arrows display the navigator inflow artifact in the right PVs and the left atrium (LA) which is associated with the pencil-beam navigator.
Figure 5.
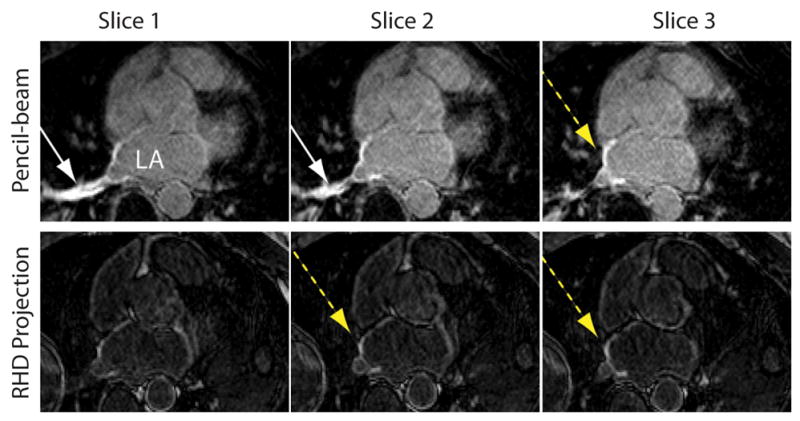
Three slices from a pulmonary vein late gadolinium enhanced dataset acquired using the pencil-beam (top-row) and right hemidiaphragm (RHD) projection navigator (bottom-row) in a post-radio frequency ablation patient. Both navigator inflow artifact (solid white arrow) and late enhancement from ablation procedure (dashed yellow arrow) are seen with the pencil-beam navigator. The elimination of the inflow enhancement is seen with the RHD projection navigator.
Overall, the inflow artifact suppression score was superior for the RHD projection navigator (3.8 ± 0.4) compared to the pencil-beam navigator (2.1 ± 0.7) with p < 0.001. The navigator inflow artifacts were present in most of the LGE images acquired using the pencil-beam navigator (3 severe, 10 moderate, and 5 mild cases) while with the RHD projection navigator only 3 mild cases were observed. The subjective score for the motion artifacts were near identical (3.0 ± 0.5 for the projection vs. 3.1 ±0.5 for the pencil-beam, p = NS). This finding demonstrates that the additional delay between the acquisition navigator signal and data acquisition in the cardiac cycle does not result in a noticeable respiratory motion artifact in PV LGE.
DISCUSSION
In this study, we evaluated a prospective RHD projection navigator and demonstrated that it allows for reduction of the navigator inflow artifacts in the free-breathing PV LGE. We found that the respiratory signal can be derived from the projection profile of the RHD in real-time and used for motion gating and tracking. The LGE images acquired using the pencil-beam navigator showed severe to mild artifacts in the right PVs and LA that were never or mildly present on the images acquired using the RHD projection navigator. There were minimal respiratory motion artifacts from either of the two navigator techniques.
We have used a projection of RHD to monitor the respiratory motion of the PVs. An alternative approach would entail monitoring the PV motion directly. Our initial experience using this alternative approach suggests that the projection signal is heavily dominated by blood flow in the PVs instead of by the PV motion itself. Therefore, we chose to use the RHD motion as a surrogate for the PVs’ motion. Based on data from coronary MRI studies (15), a tracking factor of 0.6 was used for PV LGE.
To reduce the motion artifact from the chest wall, a phase encoding direction of right-left was used for all PV LGE. This resulted in wrapping of the arm into the imaging field-of-view. As shown in Figure 3. b, due to the additional delay between the RHD projection navigator and imaging sequences, the spatial saturation of the arms become less effective and therefore, the warping of the arms becomes more apparent in the images reconstructed by the RHD projection navigator. Additional spatial saturation pulses performed immediately prior to imaging or an increase in the field of view in the right-left direction (with the penalty of increased acquisition time) can be used to suppress this artifact. A spectrally-selective fat saturation pulse can also be employed during this delay to suppress the fat signal for LGE.
In the present implementation, two spatially selective saturation slabs were employed to prevent the chest wall, back and thoracic signals from interfering with the projection profile of the navigator signal. An alternative approach would involve adapting the double projection method (16–17) to exclude additional signals superimposed on the RHD’s projection profile. The body coil was used to measure the projection navigator signal. Use of a single element of the phased-array coil adjacent to the RHD, or a combination of multiple phased-array coils, could potentially improve the navigator signal and reduce the signal from the stationary spins.
The RHD projection navigator used in this study has a selective excitation with the size of 50 mm in right-left and 150 mm in anterior-posterior directions. This is analogous to the 2D pencil-beam, which has a typical diameter of 30 mm. The larger excitation volume results in more signals for the RHD projection navigator. The diameter of the pencil-beam navigator can also be increased for more SNR but potentially with the penalty of increasing susceptibility to B0 inhomogeneity. Therefore, further investigation is needed to evaluate if the pencil-beam navigator with the additional delay can be used similarly to eliminate the need for navigator restore.
The additional 100 ms delay can have an adverse impact on the accuracy of motion compensation which can results in decreasing PV and LA sharpness. The effect of such delay has been previously investigated for coronary MRI (18–19). Although at high spatial resolution, such delay reduces sharpness in coronary MRI; but, at lower resolution (1.4×1.4×4 mm3), the reduced sharpness is negligible (19). In our study, we did not measure vessel sharpness due to presence of artifacts in LA and PVs. The impact of this delay in PV may be more forgiving due to the several factors including lower spatial resolution of PV LGE, smaller respiratory-induced PV motion, and domination of PV diameter change during data acquisition (20). Despite earlier technique in which PVs were the major target of AF ablation for isolation of electrical activity, current approaches mainly include ablation of LA to eliminate the PV stenosis risk associated with direct PV ablation. The contrast agent uptake may differ among patients. A delay that coincides with nulling the liver signal will result in failure of navigator due to the poor signal quality. In this study, based on our pilot experiment, we chose a fixed delay of 100 ms to simplify the acquisition. An additional Look-Locker scan, covering the liver, immediately prior to the LGE may help to determine the patient-specific delay.
Our study has several limitations. Our imaging population was small. Further studies using a larger AF cohort are needed to confirm the performance of our proposed method. We have combined both pre- and post-RF ablation patients to permit proper statistical analysis of the data. In a larger patient series, these two patient populations could be studied separately to investigate the utility of LGE for the detection of pre-existent scar/fibrosis in the pre-ablation patients.
CONCLUSIONS
The proposed RHD projection navigator reduces the navigator inflow artifact associated with the 2D pencil-beam navigator in free-breathing PV LGE.
Acknowledgments
The project described was supported by NIH R01EB008743-01A2, AHA SDG-0730339N and NIH UL1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resources. Mehdi H. Moghari acknowledges the fellowship support from NSERC (Natural Sciences and Engineering Research Council of Canada).
Appendix
To evaluate the impact of the additional 100 ms delay between the RHD projection navigator and start of imaging, we have performed another study on three healthy volunteers (3 males, 20±1) and sixteen patients (9 males, years old 53±16). For each subject, two scans were performed to calculate the maximum respiratory-induced PV motion displacement which can occur during this delay. Free-breathing, 2D ECG triggered, single shot coronal images were acquired covering the PVs and RHD using the following imaging parameters: The parameters for the real-time 2D coronal images were as follows: 270×337 mm2 field of view (FOV); 1.5×3 mm2 in-plane spatial resolution; TE/TR = 1.49/3; flip angle 60°; SENSE with acceleration factor 3; navigator gating window 100 mm for accepting all the images in different respiratory cycles. Each real-time 2D image acquisition took approximately 90 ms. Subsequently, for each subject, the 2D pencil-beam navigator was used to continuously monitor the respiratory motion of the RHD. Both data were exported to Matlab for further analysis. The tracking factor was calculated by manually drawing two regions of interest around the RHD and PVs to extract their signals over time. A cross-correlation was used to calculate their displacements along SI direction, and the tracking factor. The estimated respiratory tracking factor between RHD and PV was measured as 0.45 ± 0.1. Assuming a sinusoidal model for the RHD respiratory motion between the end-expiration and beginning of inspiration, the maximum PV motion was estimated to be 1.3 ± 0.7 mm, and 2.5 ± 1.4 mm for delays of 100ms and 200 ms, respectively. The central k-space is acquired at about 100ms delay while the outer region are acquired later in the acquisition window. This result demonstrates the potential impact of the additional delay and degradation of motion compensation in PV LGE.
References
- 1.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace. 2007;9(6):335–379. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 2.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N Engl J Med. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 3.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000;102(21):2619–2628. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 4.Hamdan A, Charalampos K, Roettgen R, Wellnhofer E, Gebker R, Paetsch I, Jahnke C, Schnackenburg B, Tang M, Gerds-Li H, Fleck E. Magnetic resonance imaging versus computed tomography for characterization of pulmonary vein morphology before radiofrequency catheter ablation of atrial fibrillation. Am J Cardiol. 2009;104(11):1540–1546. doi: 10.1016/j.amjcard.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Yang M, Akbari H, Reddy GP, Higgins CB. Identification of pulmonary vein stenosis after radiofrequency ablation for atrial fibrillation using MRI. J Comput Assist Tomogr. 2001;25(1):34–35. doi: 10.1097/00004728-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, Josephson ME, Manning WJ. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243(3):690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 7.Peters DC, Wylie JV, Hauser TH, Nezafat R, Han Y, Woo JJ, Taclas J, Kissinger KV, Goddu B, Josephson ME, Manning WJ. Recurrence of atrial fibrillation correlates with the extent of post-procedural late gadolinium enhancement: a pilot study. JACC Cardiovasc Imaging. 2009;2(3):308–316. doi: 10.1016/j.jcmg.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJE, Rao SN, DiBella EVR, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and Quantification of Left Atrial Structural Remodeling With Delayed-Enhancement Magnetic Resonance Imaging in Patients With Atrial Fibrillation. Circulation. 2009;119(13):1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGann CJ, Kholmovski EG, Oakes RS, Blauer JJ, Daccarett M, Segerson N, Airey KJ, Akoum N, Fish E, Badger TJ, DiBella EV, Parker D, MacLeod RS, Marrouche NF. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52(15):1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 10.Reddy VY, Schmidt EJ, Holmvang G, Fung M. Arrhythmia recurrence after atrial fibrillation ablation: can magnetic resonance imaging identify gaps in atrial ablation lines? J Cardiovasc Electrophysiol. 2008;19(4):434–437. doi: 10.1111/j.1540-8167.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 11.Malchano ZJ, Neuzil P, Cury RC, Holmvang G, Weichet J, Schmidt EJ, Ruskin JN, Reddy VY. Integration of Cardiac CT/MR Imaging with Three-Dimensional Electroanatomical Mapping to Guide Catheter Manipulation in the Left Atrium: Implications for Catheter Ablation of Atrial Fibrillation. Journal Of Cardiovascular Electrophysiology. 2006;17(11):1221–1229. doi: 10.1111/j.1540-8167.2006.00616.x. [DOI] [PubMed] [Google Scholar]
- 12.Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology. 1989;173(1):255–263. doi: 10.1148/radiology.173.1.2781017. [DOI] [PubMed] [Google Scholar]
- 13.Spuentrup E, Buecker A, Karassimos E, Gunther RW, Stuber M. Navigator-gated and real-time motion corrected free-breathing MR Imaging of myocardial late enhancement. Rofo. 2002;174(5):562–567. doi: 10.1055/s-2002-28271. [DOI] [PubMed] [Google Scholar]
- 14.Look DC, Locker DR. Time Saving in Measurement of NMR and EPR Relaxation Times. Review of Scientific Instruments. 1970;41(2):250. [Google Scholar]
- 15.Wang Y, Riederer S, Ehman R. Respiratory motion of the heart: kinematics and the implications for the spatial resolution in coronary imaging. Magnetic Resonance in Medicine. 1995;33(5):713–719. doi: 10.1002/mrm.1910330517. [DOI] [PubMed] [Google Scholar]
- 16.Lai P, Bi X, Jerecic R, Li D. A respiratory self-gating technique with 3D-translation compensation for free-breathing whole-heart coronary MRA. Magn Reson Med. 2009;62(3):731–738. doi: 10.1002/mrm.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai P, Larson AC, Bi X, Jerecic R, Li D. A dual-projection respiratory self-gating technique for whole-heart coronary MRA. J Magn Reson Imaging. 2008;28(3):612–620. doi: 10.1002/jmri.21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spuentrup E, Stuber M, Botnar RM, Manning WJ. The impact of navigator timing parameters and navigator spatial resolution on 3D coronary magnetic resonance angiography. J Magn Reson Imaging. 2001;14(3):311–318. doi: 10.1002/jmri.1188. [DOI] [PubMed] [Google Scholar]
- 19.Spuentrup E, Manning WJ, Botnar RM, Kissinger KV, Stuber M. Impact of navigator timing on free-breathing submillimeter 3D coronary magnetic resonance angiography. Magn Reson Med. 2002;47(1):196–201. doi: 10.1002/mrm.10026. [DOI] [PubMed] [Google Scholar]
- 20.Hauser TH, Yeon SB, Kissinger KV, Josephson ME, Manning WJ. Variation in pulmonary vein size during the cardiac cycle: implications for non-electrocardiogram-gated imaging. Am Heart J. 2006;152(5):974, e971–976. doi: 10.1016/j.ahj.2006.05.018. [DOI] [PubMed] [Google Scholar]