Abstract
Whole-heart coronary MRA is a promising method for detecting coronary artery disease. However, the imaging time is relatively long (typically 10-15 minutes). The goal of this study was to implement a radial echo planar imaging (radial EPI) sequence for contrast-enhanced whole-heart coronary MRA, with the aim of combining the scan efficiency of EPI with the motion insensitivity of radial k-space sampling. A self-calibrating phase correction technique was used to correct for off-resonance effects, trajectory measurement was used to correct for k-space trajectory errors and variable density sampling was used in the partition direction to reduce streaking artifacts. 7 healthy volunteers and 2 patients were scanned with the proposed radial EPI sequence, and the images were compared with a traditional GRE and x-ray angiography techniques respectively. Whole-heart images with the radial EPI technique were acquired with a resolution of 1.0 × 1.0 × 2.0 mm3 in a scan time of 5 minutes. In healthy volunteers the average image quality scores and visualized vessel lengths of the RCA and LAD were similar for the radial EPI and GRE techniques (p value > 0.05 for all). Anecdotal patient studies showed excellent agreement of the radial EPI technique with x-ray angiography.
Keywords: magnetic resonance angiography, coronary arteries, radial sampling, EPI, contrast agent
INTRODUCTION
Whole-heart coronary MRA (1) acquires a thick axial slab covering the entire heart and allows the coronary arteries to be imaged in a single volume. Whole-heart coronary MRA has previously been applied at both 1.5T (2) and 3T (3) and has shown promising results for detecting coronary artery disease. The main advantage of this technique is that it eliminates the time-consuming process of localization of the coronary arteries and substantially improves the workflow of coronary MRA. Furthermore, it is possible to retrospectively reconstruct arbitrary views for optimal visualization of each vessel. However, the major drawback of this technique is the relatively long data acquisition time on the order of 10–15 minutes despite an acceleration factor of 2 with the use of parallel imaging methods (1–7). Further significant increase in the acceleration factor is limited by the available SNR and coil array geometry. Drifts in diaphragm position, patient motion and heart rate variations during these long imaging times may compromise the robustness of whole-heart coronary MRA and result in imaging artifacts (8).
Gradient echo interleaved echo planar imaging with Cartesian k-space sampling (referred to as Cartesian EPI in this paper) (9) is one of the techniques which can be used to reduce the scan time of whole-heart coronary MRA and it is unique since it combines high scan efficiency with increased signal (due to its longer repetition time (TR)). Cartesian EPI has previously been used to acquire contrast-enhanced whole-heart coronary MRA (10) in a scan time of approximately 5 minutes. The major drawback of Cartesian EPI is its sensitivity to motion and flow artifacts (11,12), which reduces the robustness of this technique. Radial EPI is a technique which acquires multiple radial projections in each TR. This technique has previously been used with gradient echo (GRE) readout for real-time imaging (13), and steady state free precession (SSFP) readout for cardiac cine imaging (14). Radial EPI provides a hybrid approach which combines the advantages of both Cartesian EPI (high scan efficiency combined with higher signal) and radial k-space sampling (motion insensitivity compared with Cartesian sampling (15)).
The goal of this work was to implement a radial EPI acquisition scheme for contrast-enhanced whole-heart coronary MRA at 3T field strength. Healthy volunteers were scanned with both the proposed radial EPI sequence and a 3D gradient echo (GRE) sequence for comparison purposes. A pilot study with 2 patients was performed with the proposed radial EPI sequence. Slow infusion of an extravascular, paramagnetic contrast agent, gadopentetate dimeglumine (Gd-DTPA, Magnevist, Schering AG, Berlin, Germany) was used to enhance the SNR of the wholeheart radial EPI acquisition.
MATERIALS AND METHODS
7 healthy volunteers (5 male and 2 female, average age 29 ± 5 years) and 2 patients with suspected coronary artery disease (1 male age 39 years and 1 female age 74 years) were scanned on a clinical 3T scanner (MAGNETOM Trio, Siemens AG Healthcare Sector, Erlangen, Germany). Gd-DTPA as used as the contrast agent. Written consent was obtained from the volunteers in compliance with the Institutional Review Board of our institution.
Sequence Design
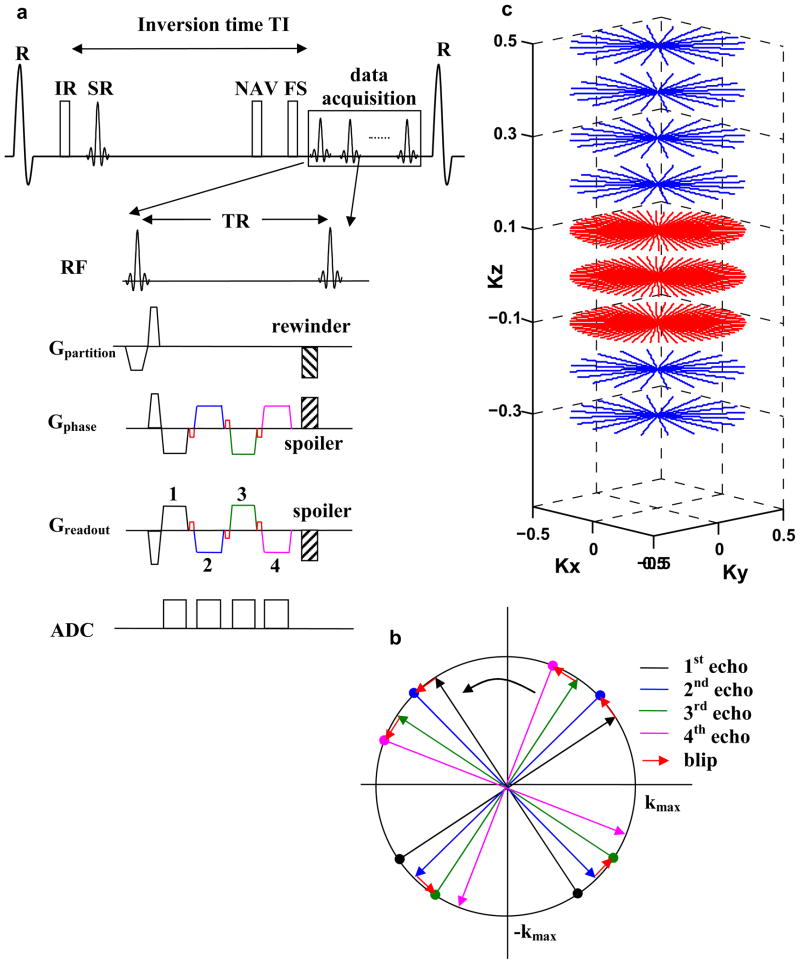
A stack-of-stars radial trajectory (16,17) was used for whole-heart coronary MRA. Figure 1a shows a schematic of the radial EPI pulse sequence. During contrast-enhanced imaging blood myocardium contrast was achieved with inversion recovery preparation. Fat saturation was applied prior to data acquisition. The sequence was ECG-triggered and respiratory-gated using the navigator approach. To restore the magnetization for the navigator echo, a selective reinversion (SR) pulse was applied after the nonselective inversion pulse. Gradient blips (shown in red in Fig. 1a and 1b) were applied on both the readout and phase encoding axis and data sampling was not performed during the blips or gradient ramps. 4 adjacent projections (shown by the black, blue, green and pink lines in Fig. 1a and 1b) were sampled after each RF pulse. This resulted in minimal blip duration and TR and it also facilitated the self-calibrating phase correction procedure for off-resonance correction (described in the following section). The full range of projections (0 to π) was sampled in each heart-beat in an interleaved fashion. Gridding was used to reconstruct the radially sampled data (18).
Figure 1.
1a: Inversion Recovery (IR) prepared, fat saturated (FS), ECG triggered and navigator (NAV) gated stack-of-stars radial EPI sequence used for contrast-enhanced whole-heart coronary MRA. SR stands for the selective reinversion pulse which is used to restore the magnetization for the navigator echo. 1b: Reordering scheme showing the 4 adjacent projections sampled after each RF pulse. 1c: Variable density sampling used in the kz direction.
K-space trajectory
Traditional stack-of-stars PR trajectories sample the same number of projections in each partition encoding (kz) step. They are referred to as trajectories with constant density sampling in this section. These constant density stack-of-stars trajectories utilize the undersampling capabilities of PR acquisition only in the in-plane (kx-ky) direction. In this work we used a trajectory which has variable density sampling in the kz direction. An optimal version of this trajectory was proposed in Ref (19) where the number of projections was varied sinusoidally as a function of partition number. In this work we used a simplified version of this trajectory in which the central 10 kz partitions acquired 288 projections (shown in red in Fig. 1c), and the outer kz partitions acquired 144 projections (shown in blue in Fig. 1c). In PR imaging undersampling produces aliasing which is manifested as streaking artifacts outside the region of support called the reduced FOV (FOVr). The FOVr depends on the number of projections acquired and is given as (17):
| [1] |
where: Np is the number of projections and Nr is the number of readout points which is 256 in this case. The proposed variable density trajectory has Np = 288 in the central kz partitions, leading to FOVr = 0.72 FOVfull. In comparison, the constant density trajectory with Np = 144 has FOVr = 0.36 FOVfull. This increase by a factor of 2 in the reduced FOV for the variable density stack-of-stars trajectory results in lesser streaking artifacts in the images. Another advantage of the variable density trajectory is that it leads to better spatial resolution in the off-resonance phase map (described in the Self-calibrating phase correction section). The disadvantage of this variable density trajectory is that it requires additional scan time to oversample the central k-space partitions (compared with the constant density trajectory with Np = 144). To offset this increase asymmetric k-space sampling was used in the kz direction and partial Fourier reconstruction was used to synthesize the unacquired k-space region (20).
Trajectory Measurement
To correct for k-space trajectory errors in the radial EPI acquisition, trajectory measurement was used with the measured k-space trajectory used during data gridding. The trajectory measurement procedure proposed in Refs (21,22) was used in this work. In our experience the two techniques give slightly variable results depending on factors like slice position and which coil is used to estimate the trajectory. As a result we used both these methods and averaged the results. Based on our observation, the trajectories measured in volunteers had low SNR and resulted in poor image quality. As a result, we measured the trajectory once on a spherical water phantom and used this for all the subsequent volunteer studies. Scan parameters for the trajectory measurement were: flip angle = 5°, slice thickness = 1mm, slice locations measured: −6 mm, −3 mm, 0 mm and 3 mm, 20 averages per slice location. All of this data was averaged to give the final trajectory. The trajectory was independently measured in the x and y directions and their linear combinations were used to generate trajectories of the different radial projection angles (23). Total scan time for the trajectory measurement was approximately 900 msec.
Self-calibrating phase correction
Off-resonance phase has three effects on the radial EPI trajectory (13). The first is the off-resonance phase accumulation during each echo. This leads to the well known blurring effect in radial k-space acquisitions. In the current study a high readout bandwidth of 977 Hz/pixel was used, making this effect negligible. The second is the amplitude difference between echoes due to T2* signal decay. This leads to increased streaking artifacts in the images which are partially mitigated by the variable density k-space sampling used in the kz direction. The third and most significant effect for this work is the off-resonance phase accumulation between echoes. This leads to phase cancellation when the projections acquired at different echo times (TE) are combined during gridding, resulting in signal voids in the image (14) (bottom right in Fig. 2). We used a self-calibrating phase correction technique similar to Refs (24,25) to compensate for this effect. As shown in Fig. 2, the entire k-space data is split into n undersampled sections, each acquired at a particular TE (in this case n = 4). A low resolution fully sampled image is reconstructed at each TE from the central k-space region (shown as circles in Fig. 2) and the off-resonance phase φn(x, y) is estimated from these images. This low resolution phase information is used to correct the corresponding undersampled high resolution data, and the multiple high resolution undersampled images (one for each TE) are combined by complex summation to give the final image for a particular coil. The final image I(x, y) (bottom left in Fig. 2) is obtained by standard sum of squares combination of all the coils. The image reconstruction operation can be represented as:
| [2] |
where C is the number of coils, N is the echo train length (in this case 4), Pcn(x, y) is the complex image reconstructed after gridding for coil number c and TE = TEn, φcn(x, y) is the low resolution off-resonance phase for coil number c and TE = TEn.
Figure 2.
Self-calibrating phase correction technique used to correct for off-resonance effects in the radial EPI sequence. The image on the bottom right is without the phase correction and on the bottom left is with the phase correction. k-space is split into 4 undersampled sections, each acquired at a particular TE, and a low resolution fully sampled image is reconstructed from the central k-space region (shown as circles). The off-resonance phase φn(x, y)is estimated from these low resolution images and is used to correct the corresponding undersampled high resolution data.
Volunteer Studies
An inversion recovery prepared radial EPI sequence was used to acquire contrast-enhanced whole-heart coronary MRA during free breathing. Scan parameters were: TR = 8.2 ms, 4 echoes (projections) acquired after each RF pulse, flip angle = 30o, readout bandwidth = 977 Hz/pixel, number of radial views = 288 for the central 10 kz partitions and 144 for all the other outer kz partitions, partial Fourier factor of 5/6 in the kz direction (which results in an undersampling factor of ~ 2.8 compared with Nyquist), 48 to 72 projections in each heart-beat in a data acquisition window of 98 to 147 msec, non selective inversion pulse with TI = 200 ms, matrix: 260 × 260 × 60, voxel size: 1 × 1 × 2 mm3, interpolated to 0.5 × 0.5 × 1 mm3. The estimated imaging time for the radial EPI whole-heart scan would be 2 minutes, assuming a heart-rate of 60 bpm and navigator gating efficiency of 100%. 0.2 mmol/kg body weight of Gd-DTPA was injected at a rate of 0.3 ml/sec followed by a flush of saline using the same amount and rate.
For comparison purposes, all the volunteers were scanned again in a separate scan session using a traditional GRE sequence (26) with the same contrast agent dose and injection rate. The spatial resolution was the same as the radial EPI acquisition. The scan parameters for the GRE sequence were: TR = 3.1 ms, TE = 1.5 ms, flip angle = 20°, non selective inversion pulse with TI = 200 ms, 31 to 47 lines per heartbeat in a data acquisition window of 98 to 147 msec, readout bandwidth = 700 Hz/pixel, GRAPPA acceleration factor of 2 in the phase encoding direction. The estimated imaging time for the GRE whole-heart scan would be 3 minutes, assuming a heart-rate of 60 bpm and navigator gating efficiency of 100%.
In addition, for a qualitative comparison, 2 of the volunteers were rescanned in separate scan sessions with the Cartesian EPI technique (10,27) with the same contrast agent dose and injection rate. The spatial resolution was the same as the radial EPI and GRE acquisitions. The scan parameters for the Cartesian EPI sequence were: TR = 10.6 ms, TE = 3.5 ms, 6 echoes after each RF pulse, flip angle = 25o, readout bandwidth = 1221 Hz/pixel, non selective inversion pulse with TI = 300 ms, The estimated imaging time for the Cartesian EPI whole-heart scan would be 2 minutes, assuming a heart-rate of 60 bpm and navigator gating efficiency of 100%.
For all the scans, a four chamber cine scan was used to determine the quiescent period (in mid-diastole) for coronary artery imaging.
Patient Studies
The radial EPI coronary MRA sequence used for the patient studies was exactly the same as that used in the healthy volunteer studies (described above). X-ray coronary angiography was also performed in the two patients and evaluated by two cardiologists in consensus who were blinded to the coronary MRA results. Lesions with a diameter reduction of at least 50% at X-ray coronary angiography were considered to be significant.
Image Reformatting and Data Analysis
Whole-heart coronary MRA images were reformatted using the CoronaViz software (Siemens Corporate Research, Inc., Princeton, NJ, USA) to project multiple vessel branches onto a single image (28). The contrast-enhanced radial EPI images were compared with the contrast-enhanced GRE images in terms of relative SNR (rSNR), relative CNR (rCNR), image quality scores and visualized RCA and LAD lengths as described in Ref. (27).
RESULTS
Phantom Studies
Fig. 3 shows phantom images acquired with the radial EPI sequence and reconstructed with the different correction techniques. TC stands for trajectory correction, PC for phase correction, VD for variable density sampling and PF for partial Fourier acquisition. From 3a-d it is clear that both the trajectory and phase correction steps are critical for producing acceptable image quality with the radial EPI technique. The reduction of streaking artifacts with variable density sampling is apparent from Figs. 3d and e. The image with 5/6 partial Fourier acquisition in kz (3f) is identical to the one without partial Fourier acquisition (3e).
Figure 3.

Phantom images acquired with the radial EPI sequence and reconstructed with the different correction techniques. TC stands for trajectory correction, PC for phase correction, VD for variable density sampling and PF for partial Fourier acquisition. From 3a-d it is clear that both the trajectory and phase corrections steps are critical for producing acceptable image quality with the radial EPI technique. The reduction of streaking artifacts with variable density sampling is apparent from Figs. 3d and e. The image with 5/6 partial Fourier acquisition in kz (3f) is similar to the one without partial Fourier acquisition (3e).
Volunteer and Patient Studies
All the volunteer studies were successfully completed. Quantitative comparison between the contrast-enhanced radial EPI and GRE acquisitions is shown in Table 1. The average scan time for the radial EPI sequence was 5.3 ± 1.7 minutes with an average navigator efficiency of 43.4 ± 12.4%. In comparison, the average scan time for the GRE technique was 7.0 ± 2.5 minutes with an average navigator efficiency of 49.7 ± 13.8%. The GRE technique had a significantly higher navigator efficiency (p value < 0.05) compared with the radial EPI technique, however, assuming similar navigator efficiencies, radial EPI reduces the scan time by 33% compared with the GRE technique. Compared to the GRE technique the radial EPI technique had: i) rSNR decreased by 25% (32.0 ± 10.0 to 24.2 ± 4.6, the difference between them was statistically significant p value < 0.05), and, ii) rCNR decreased by 45% (26.9±11.9 to 14.1 ± 2.1, the difference between them was statistically significant p value < 0.05). The average image quality score for the contrast-enhanced radial EPI acquisition was 3.3 ± 0.2, compared to 3.4 ± 0.5 for the contrast-enhanced GRE acquisition. The difference between them was not statistically significant (p value > 0.05). The average visualized vessel lengths of the RCA and LAD were similar for both techniques (p value > 0.05 for both).
Table 1.
Quantitative comparison between the contrast-enhanced radial EPI and GRE techniques.
| Imaging sequence (n = 7) | Imaging time (minutes) | Navigator efficiency (%) | rSNR | rCNR | Image quality score | Vessel length (cm) | |
|---|---|---|---|---|---|---|---|
| LM + LAD | RCA | ||||||
| contrast-enhanced radial EPI | 5.3 ± 1.7 | 43.4 ±12.4 | 24.2 ± 4.6 | 14.1 ± 2.1 | 3.3 ± 0.2 | 10.9 ± 2.4 | 10.9 ± 2.3 |
| contrast-enhanced GRE | 7.0 ± 2.5* | 49.7 ± 13.8* | 32.0 ± 10.0* | 26.9±11.9* | 3.4 ± 0.5 | 10.7 ± 1.6 | 11.0 ± 2.0 |
indicates statistically significant
Fig. 4 shows volunteer images acquired with the radial EPI sequence and reconstructed with the different correction techniques. TC stands for trajectory correction and PC for phase correction. From 4a-d it is clear that both trajectory and phase correction are critical for producing acceptable image quality with radial EPI.
Figure 4.
Volunteer images acquired with the radial EPI sequence and reconstructed with the different correction techniques. TC stands for trajectory correction and PC for phase correction. From 4a-d it is clear that both trajectory and phase correction are critical for producing acceptable image quality with radial EPI.
Figure 5 shows reformatted coronary artery images from 2 healthy volunteers using the contrast-enhanced radial EPI (5a, d), GRE (5b, e), and Cartesian EPI (5c, f) acquisitions acquired in separate scan sessions. The volunteer in the first row in Fig. 5 had a stable heart-rate of approximately 60 beats per minute and all the three techniques resulted in similar coronary artery delineation. The volunteer in the second row in Fig. 5 had a highly variable heart rate, changing between 60 and 80 beats per minute. In this case the image quality of Cartesian EPI deteriorated due to motion artifacts, however the radial EPI and GRE techniques still resulted in good coronary artery delineation. This qualitative comparison demonstrates the improvement in radial EPI compared with Cartesian EPI in the presence of motion.
Figure 5.

Reformatted coronary artery images from 2 healthy volunteers using the contrast-enhanced radial EPI (5a, d), GRE (5b, e), and Cartesian EPI (5c, f) acquisitions acquired in separate scan sessions. The volunteer in the first row had a stable heart-rate of approximately 60 beats per minute and the three techniques resulted in similar coronary artery delineation. The volunteer in the second row had a highly variable heart rate, changing between 60 and 80 beats per minute. In this case the image quality of Cartesian EPI deteriorated due to motion artifacts, however the radial EPI and GRE techniques still resulted in good coronary artery delineation.
Figure 6 shows reformatted coronary artery images from 2 healthy volunteers using the contrast-enhanced radial EPI acquisition (6a, c) and contrast-enhanced GRE acquisition (6b, d), acquired in separate scan sessions. Both sequences show excellent delineation of the major coronary arteries, however, compared with the GRE sequence, the radial EPI sequence has scan time reduced by approximately 30%.
Figure 6.

Reformatted coronary artery images from 2 healthy volunteers using the contrast-enhanced radial EPI (6a, c) and GRE techniques (6b, d) acquired in separate scan sessions. Both sequences show excellent delineation of the major coronary arteries.
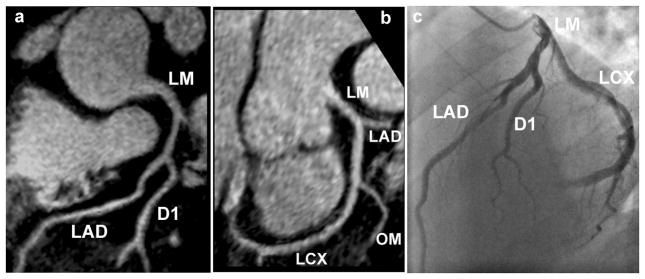
Figures 7a and 7b show reformatted coronary artery images from a 39 year old male patient acquired with the radial EPI technique. Long segments of the LM, LAD, and LCX are well depicted. No significant stenosis was found in this case and the coronary MRA images correlate well with the x-ray angiography image (Fig. 7c). Figure 8 shows coronary artery images from a 74 year old female patient illustrating the detection of significant stenoses in the distal LCX (shown as white arrow) and total occlusion of the LAD. Once again there is excellent correlation between the radial EPI coronary MRA technique (8a) and x-ray angiography (8b).
Figure 7.
Reformatted coronary artery images (7a and 7b) from a 39 year old male patient acquired with the radial EPI technique. No significant stenosis was found in this case and the coronary MRA images correlate well with the x-ray angiography image (7c).
Figure 8.
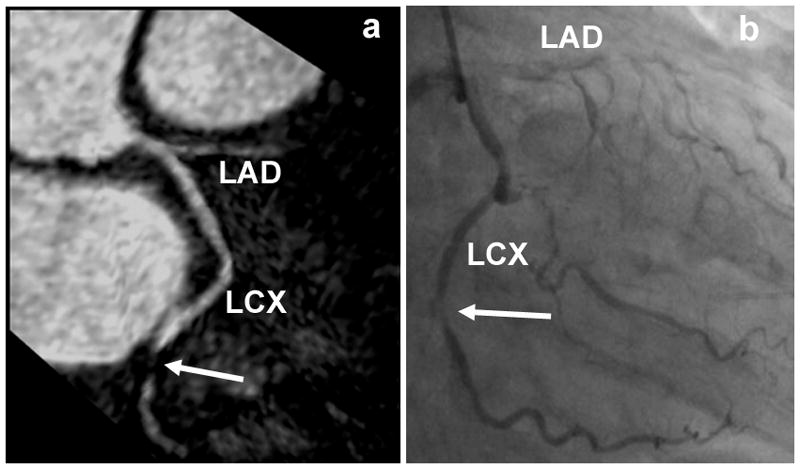
Coronary artery images from a 74 year old female patient illustrating the detection of significant stenoses in the distal LCX (shown as white arrow) and total occlusion of the LAD. There is excellent correlation between the radial EPI coronary MRA (8a) and x-ray angiography (8b) images.
DISCUSSION
In this study, contrast-enhanced whole-heart coronary artery images were acquired in 5 minutes using a radial EPI sequence. The radial EPI sequence was compared with a traditional GRE technique for contrast-enhanced whole-heart coronary MRA at 3T. Similar to previous studies with radial k-space sampling (29,30) the relative SNR and CNR were reduced for the radial EPI sequence; however there was no statistically significant difference between the image quality scores and lengths of visualized coronary arteries between the GRE and radial EPI sequences. The radial EPI technique had scan time reduced by approximately 33% compared with the tested GRE technique. In addition, 2 anecdotal patient studies were performed with radial EPI and the results were well correlated with x-ray coronary angiography.
The main motivation to use EPI for whole-heart coronary MRA is its unique combination of high scan and signal efficiency (9). However, the Cartesian EPI technique is susceptible to motion and flow artifacts (11). Radial EPI provides a hybrid approach which combines the high scan and signal efficiency of Cartesian EPI with the relative motion insensitivity of stack-of-stars radial k-space sampling compared with Cartesian acquisitions. The Cartesian EPI technique is also very sensitive to k-space amplitude modulations which restricts the flip angle and TI that can be used for contrast-enhanced whole-heart coronary MRA (10). This problem is alleviated in radial EPI where the amplitude modulations in k-space lead to streaking artifacts as opposed to the more obstructive ghosting artifacts in Cartesian EPI.
Several steps were taken in this work to facilitate the radial EPI sequence. First, trajectory measurement (21,22) was used to correct for the k-space trajectory errors caused by gradient delays. As demonstrated by the phantom and volunteer images, this was a critical step in getting acceptable image quality with radial EPI. In this study the trajectory was measured once on a phantom and used this for all the subsequent volunteer studies. This has the disadvantage of not being able to correct for either the eddy current related errors or changes in gradient delays with scanner/gradient coil utilization. Also, the trajectory measurement was performed independently for each gradient axis ignoring the cross talk between axes which can cause additional errors. Inspite of these drawbacks the trajectory measurement technique resulted in excellent image quality in all the volunteer studies. Secondly, variable density k-space sampling was used in the kz direction and as shown in the resolution phantom images it resulted in reduced streaking artifacts in the images. Thirdly, a self-calibrating phase correction technique was implemented for correction of the off-resonance phase accumulation between projections acquired at different TE’s. This effect leads to phase cancellation between the projections and results in severe signal voids in the heart at 3T. The self-calibrating phase correction technique estimated the off-resonance phase from the radial EPI data itself and did not require any additional calculations like phase unwrapping, making it very straightforward to apply to the magnitude reconstructions performed in this work. For any phase sensitive application like flow imaging this self-calibrating phase correction method will not work directly and a traditional field map approach will be needed. However, as the data already has multiple low resolution images acquired at different TE’s, no extra data acquisition will be required, only more processing will be needed to estimate the field map from the multiple TE images.
Several previous studies (15,29) have used the stack-of-stars radial k-space trajectory for acquiring 3D coronary MRA. Compared to these approaches the major advantage of the current radial EPI technique is its higher scan efficiency as demonstrated by the effective echo spacing of 8.2/4 = 2.05 msec. The traditional single echo radial acquisitions do not require any additional correction steps. In comparison the current radial EPI approach requires trajectory measurement, phase correction and variable density sampling in kz to produce acceptable and diagnostic image quality.
In this work, parallel imaging was not used along with the radial EPI technique. Several methods have been proposed for combining radial k-space sampling with parallel imaging (31,32) and the use of these techniques combined with radial EPI should be investigated for further reducing the scan time of whole-heart coronary MRA.
In conclusion, a radial EPI sequence was implemented for contrast-enhanced whole-heart coronary MRA at 3 Tesla. In healthy volunteers, all the major coronary arteries were clearly depicted in a scan time of approximately 5 minutes. Preliminary patient studies showed excellent agreement of the radial EPI technique with x-ray angiography and further testing of this technique in patients is currently underway.
Acknowledgments
National Institute of Health grants nos. NIBIB EB002623 and NHLBI HL38698; Siemens Medical Solutions USA, Inc., Malvern, PA; National Natural Science Foundation of China grant nos. 30828009 and 30900355; Captain and Mrs. Roberts Fellowship.
References
- 1.Weber OM, Martin AJ, Higgins CB. Whole-heart steady-state free precession coronary artery magnetic resonance angiography. Magn Reson Med. 2003;50(6):1223–1228. doi: 10.1002/mrm.10653. [DOI] [PubMed] [Google Scholar]
- 2.Sakuma H, Ichikawa Y, Chino S, Hirano T, Makino K, Takeda K. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. J Am Coll Cardiol. 2006;48(10):1946–1950. doi: 10.1016/j.jacc.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Yang Q, Li K, Liu X, Bi X, Liu Z, An J, Zhang A, Jerecic R, Li D. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3.0-T a comparative study with x-ray angiography in a single center. J Am Coll Cardiol. 2009;54(1):69–76. doi: 10.1016/j.jacc.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gharib AM, Ho VB, Rosing DR, Herzka DA, Stuber M, Arai AE, Pettigrew RI. Coronary artery anomalies and variants: technical feasibility of assessment with coronary MR angiography at 3 T. Radiology. 2008;247(1):220–227. doi: 10.1148/radiol.2471070274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuber M, Weiss RG. Coronary magnetic resonance angiography. J Magn Reson Imaging. 2007;26(2):219–234. doi: 10.1002/jmri.20949. [DOI] [PubMed] [Google Scholar]
- 6.Stehning C, Boernert P, Nehrke K. Advances in coronary MRA from vessel wall to whole heart imaging. Magn Reson Med Sci. 2007;6(3):157–170. doi: 10.2463/mrms.6.157. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Bi X, Huang J, Jerecic R, Carr J, Li D. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3.0 T: comparison with steady-state free precession technique at 1.5 T. Invest Radiol. 2008;43(9):663–668. doi: 10.1097/RLI.0b013e31817ed1ff. [DOI] [PubMed] [Google Scholar]
- 8.Chang S, Cham MD, Hu S, Wang Y. 3-T navigator parallel-imaging coronary MR angiography: targeted-volume versus whole-heart acquisition. AJR Am J Roentgenol. 2008;191(1):38–42. doi: 10.2214/AJR.07.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKinnon GC. Ultrafast interleaved gradient-echo-planar imaging on a standard scanner. Magn Reson Med. 1993;30(5):609–616. doi: 10.1002/mrm.1910300512. [DOI] [PubMed] [Google Scholar]
- 10.Bhat H, Zuehlsdorff S, Bi X, Li D. Whole-heart contrast-enhanced coronary magnetic resonance angiography using gradient echo interleaved EPI. Magn Reson Med. 2009;61(6):1388–1395. doi: 10.1002/mrm.21963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duerk JL, Simonetti OP. Theoretical aspects of motion sensitivity and compensation in echo-planar imaging. J Magn Reson Imaging. 1991;1(6):643–650. doi: 10.1002/jmri.1880010605. [DOI] [PubMed] [Google Scholar]
- 12.Luk Pat GT, Meyer CH, Pauly JM, Nishimura DG. Reducing flow artifacts in echo-planar imaging. Magn Reson Med. 1997;37(3):436–447. doi: 10.1002/mrm.1910370323. [DOI] [PubMed] [Google Scholar]
- 13.Rasche V, Holz D, Proksa R. MR fluoroscopy using projection reconstruction multi-gradient-echo (prMGE) MRI. Magn Reson Med. 1999;42(2):324–334. doi: 10.1002/(sici)1522-2594(199908)42:2<324::aid-mrm15>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Larson AC, Simonetti OP. Real-time cardiac cine imaging with SPIDER: steady-state projection imaging with dynamic echo-train readout. Magn Reson Med. 2001;46(6):1059–1066. doi: 10.1002/mrm.1299. [DOI] [PubMed] [Google Scholar]
- 15.Larson AC, Simonetti OP, Li D. Coronary MRA with 3D undersampled projection reconstruction TrueFISP. Magn Reson Med. 2002;48(4):594–601. doi: 10.1002/mrm.10262. [DOI] [PubMed] [Google Scholar]
- 16.Peters DC, Korosec FR, Grist TM, Block WF, Holden JE, Vigen KK, Mistretta CA. Undersampled projection reconstruction applied to MR angiography. Magn Reson Med. 2000;43(1):91–101. doi: 10.1002/(sici)1522-2594(200001)43:1<91::aid-mrm11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Vigen KK, Peters DC, Grist TM, Block WF, Mistretta CA. Undersampled projection-reconstruction imaging for time-resolved contrast-enhanced imaging. Magn Reson Med. 2000;43(2):170–176. doi: 10.1002/(sici)1522-2594(200002)43:2<170::aid-mrm2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 18.Jackson JI, Meyer CH, Nishimura DG, Macovski A. Selection of a convolution function for Fourier inversion using gridding [computerised tomography application] IEEE Trans Med Imaging. 1991;10(3):473–478. doi: 10.1109/42.97598. [DOI] [PubMed] [Google Scholar]
- 19.Peters DC, Nezafat R, Manning WJ. Radial Undersampling that is Variable in kz. 2007. Proceedings of the 16th Annual Meeting of ISMRM; Berlin, Germany. 2007. p. 304. [Google Scholar]
- 20.Noll DC, Nishimura DG, Macovski A. Homodyne detection in magnetic resonance imaging. IEEE Trans Med Imaging. 1991;10(2):154–163. doi: 10.1109/42.79473. [DOI] [PubMed] [Google Scholar]
- 21.Duyn JH, Yang Y, Frank JA, van der Veen JW. Simple correction method for k-space trajectory deviations in MRI. J Magn Reson. 1998;132(1):150–153. doi: 10.1006/jmre.1998.1396. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Hetherington HP, Stokely EM, Mason GF, Twieg DB. A novel k-space trajectory measurement technique. Magn Reson Med. 1998;39(6):999–1004. doi: 10.1002/mrm.1910390618. [DOI] [PubMed] [Google Scholar]
- 23.Lu A, Brodsky E, Grist TM, Block WF. Rapid fat-suppressed isotropic steady-state free precession imaging using true 3D multiple-half-echo projection reconstruction. Magn Reson Med. 2005;53(3):692–699. doi: 10.1002/mrm.20389. [DOI] [PubMed] [Google Scholar]
- 24.Nayak KS, Nishimura DG. Automatic field map generation and off-resonance correction for projection reconstruction imaging. Magn Reson Med. 2000;43(1):151–154. doi: 10.1002/(sici)1522-2594(200001)43:1<151::aid-mrm19>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 25.Nayak KS, Tsai CM, Meyer CH, Nishimura DG. Efficient off-resonance correction for spiral imaging. Magn Reson Med. 2001;45(3):521–524. doi: 10.1002/1522-2594(200103)45:3<521::aid-mrm1069>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Bi X, Carr JC, Li D. Whole-heart coronary magnetic resonance angiography at 3 Tesla in 5 minutes with slow infusion of Gd-BOPTA, a high-relaxivity clinical contrast agent. Magn Reson Med. 2007;58(1):1–7. doi: 10.1002/mrm.21224. [DOI] [PubMed] [Google Scholar]
- 27.Bhat H, Yang Q, Zuehlsdorff S, Li K, Li D. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3 T using interleaved echo planar imaging. Invest Radiol. 45(8):458–464. doi: 10.1097/RLI.0b013e3181d8df32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aharon S, Oksuz O, Lorenz C. Simultaneous projection of multibranched vessels with their surroundings on a single image from coronary MRA. Proceedings of the 14th Annual Meeting of ISMRM; Seattle, WA, USA. 2006. p. 365. [Google Scholar]
- 29.Spuentrup E, Katoh M, Buecker A, Manning WJ, Schaeffter T, Nguyen TH, Kuhl HP, Stuber M, Botnar RM, Gunther RW. Free-breathing 3D steady-state free precession coronary MR angiography with radial k-space sampling: comparison with cartesian k-space sampling and cartesian gradient-echo coronary MR angiography--pilot study. Radiology. 2004;231(2):581–586. doi: 10.1148/radiol.2312030451. [DOI] [PubMed] [Google Scholar]
- 30.Weber OM, Pujadas S, Martin AJ, Higgins CB. Free-breathing, three-dimensional coronary artery magnetic resonance angiography: comparison of sequences. J Magn Reson Imaging. 2004;20(3):395–402. doi: 10.1002/jmri.20141. [DOI] [PubMed] [Google Scholar]
- 31.Pruessmann KP, Weiger M, Bornert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magn Reson Med. 2001;46(4):638–651. doi: 10.1002/mrm.1241. [DOI] [PubMed] [Google Scholar]
- 32.Seiberlich N, Breuer FA, Blaimer M, Barkauskas K, Jakob PM, Griswold MA. Non-Cartesian data reconstruction using GRAPPA operator gridding (GROG) Magn Reson Med. 2007;58(6):1257–1265. doi: 10.1002/mrm.21435. [DOI] [PubMed] [Google Scholar]