Abstract
Rationale
Conditioned cues can elicit relapse to drug- and food-seeking behavior over prolonged periods of abstinence. If seeking behavior depends on mesolimbic dopamine D1 receptors, blocking these receptors should reduce seeking behavior.
Objectives
We examined the effects of either systemic or intra-nucleus accumbens administration of the D1 antagonist SCH 23390 on extinction responding (sucrose seeking) by rats either 1 or 30 days into forced abstinence.
Materials and methods
Rats self-administered 10% sucrose paired with a tone+light cue for 10 days. After either 1 or 30 days of forced abstinence, rats received systemic (0, 1, 5, or 25 µg/kg IP) or bilateral nucleus accumbens core or shell (0.3 or 0.6 µg/site) injections of SCH 23390 prior to extinction testing.
Results
Saline-treated rats responded more during extinction following 30 vs. 1 day of forced abstinence (“incubation of craving”). Systemic SCH 23390 reduced sucrose seeking after 1 day of forced abstinence, significantly reducing responding following pretreatment with 1, 5, and 25 µg/kg SCH 23390, but only 25 µg/kg significantly reduced sucrose seeking after 30 days of forced abstinence. SCH 23390 (0.3 or 0.6 µg/site) in the core or shell of the nucleus accumbens reduced sucrose seeking in all groups.
Conclusion
Nucleus accumbens D1 receptors are involved in sucrose seeking, but it is not clear if they are involved in the incubation of craving. The fact that D1 antagonism reduced sucrose seeking across an extended period of abstinence may be of use for development of treatment strategies for relapse.
Keywords: Addiction, Craving, Eating disorders, Incubation, Motivation, Relapse
Introduction
Drug addiction and disordered eating share neurobehavioral features (Wang et al. 2004; Volkow and Wise 2005; Nair et al. 2009). For example, craving and relapse characterize cocaine addicts (O'Brien et al. 1988; Mendelson and Mello 1996) and obese or overweight patients attempting to lose weight (Brownell and Wadden 1992; Wadden 1993; Grodstein et al. 1996). Craving can be triggered by drug or food cues (Carter and Tiffany 1999; Childress et al. 1999; Jansen et al. 2003; Sobik et al. 2005; Epstein et al. 2009). While the exact neural substrates that mediate drug versus food reinforcement differ, identification of general neurobehavioral mechanisms underlying craving behaviors is needed to develop effective treatments against relapse.
Craving has been operationally defined as rats responding for (seeking) a cue associated with reinforcement (Markou et al. 1993; Grimm et al. 2000). Robust seeking behavior is observed when rats respond for this cue as a conditioned reinforcer (Cador et al. 1989; Grimm et al. 2000) and during extinction testing (Lu et al. 2005). The dopamine system has been implicated in conditioned responding for both foods and drugs (Wise 2004). Medium spiny neurons in the nucleus accumbens (NAcc) that fire in response to self-administration of natural versus drug reward and also to reward-predictive cues receive input from midbrain dopamine projections (Wheeler and Carelli 2009). Thus, dopamine input may serve a modulatory role over goal-directed behavior (Wheeler and Carelli 2009).
Systemic and intracranial administration of D1 antagonist has been shown to be effective at reducing responding for, or in the presence of, drug-paired cues (Weissenborn et al. 1996; Ciccocioppo et al. 2001; See et al. 2001,Weiss et al. 2001; Alleweireldt et al. 2002, 2006; Crombag et al. 2002; Liu and Weiss 2002; Bossert et al. 2007; Hamlin et al. 2007; Bossert et al. 2009; Chaudhri et al. 2009; Mashhoon et al. 2009; See 2009; Liu et al. 2010). Systemic antagonism of D1 receptors was found to reduce contextual-mediated renewal of sucrose seeking (Hamlin et al. 2006) and intra-NAcc delivery of SCH 23390 reduced measures of food seeking (Wakabayashi et al. 2004; Yun et al. 2004; Lex and Hauber 2010) assessed in discriminative stimulus and Pavlovian–instrumental transfer paradigms.
The present study examined the effects of the D1 antagonist SCH 23390 on sucrose-seeking behavior in rats using an extinction procedure adapted from a well-established model of drug craving (Markou et al. 1993; Shalev et al. 2002; Epstein et al. 2006; Reichel and Bevins 2009). On the sucrose-seeking test day, rats were pretreated with either systemic or intra-NAcc (core or shell) SCH 23390. D1 receptors in the NAcc were targeted due to the above-mentioned role of accumbal dopamine in goal-directed behavior and also because a previous study identified a role for accumbal core and shell in discrete (core) or contextual (shell) D1 receptor-mediated reinstatement of extinguished heroin-seeking rats (Bossert et al. 2007). In addition, as drug or sucrose seeking incubates over forced abstinence (Grimm et al. 2001; Grimm et al. 2005), rats were tested after either 1 or 30 days of forced abstinence.
Materials and methods
Subjects
Two hundred and forty-eight male Long–Evans rats [3 months old; 396.2±3.8 g (mean±standard error of the mean) (SEM) at start of study; Simonsen-derived, Gilroy, CA, USA] bred in the Western Washington University vivarium were housed individually on a 12-h reverse day/night cycle (lights off at 0700) with Purina Mills Inc. Mazuri Rodent Pellets (Gray Summit, MO, USA) and water available ad libitum. All training and testing took place between 0900 and 1300 with cohorts of rats always trained and tested at the same time daily. Rats were weighed each Monday, Wednesday, and Friday for the duration of the experiment. Immediately prior to the training phase, the animals were deprived of water for 17 h to encourage sucrose self-administration on the first day of training. All procedures followed the guidelines outlined in the “Principles of Laboratory Animal Care” (NIH publication no. 86-23) and were approved by the Western Washington University Institutional Animal Care and Use Committee.
Guide cannulae placement
Rats were anaesthetized with a ketamine/xylazine combination (100 mg+10 mg/kg, IP) followed by an injection of the analgesic buprenorphine (0.01 mg/kg, SC). Using a stereotaxic device (Stoelting, Wood Dale, IL, USA), bilateral stainless steel cannulae (22-gauge; Plastics One, Roanoke, VA, USA) were lowered at a 10° angle from the skull surface into the NAcc core or shell: core anteroposterior (AP), +1.2 mm; mediolateral (ML), ±3.2 mm; dorsoventral (DV), −6.3 mm; shell AP, +1.2 mm; ML, ±2.7 mm; DV, −7.0 mm relative to bregma as identified in a stereotaxic atlas of the rat brain (Paxinos and Watson 2007). To identify whether any behavioral effects of drug microinjection might be due to movement of the drug up the sides of the guide cannulae to regions above the desired injection sites, two other groups of rats were implanted with bilateral cannulae to a site in between core and shell guide cannulae tracks and approximately 3 mm dorsal and equidistant to the average DV level of core and shell microinjection sites (dorsal injection comparison groups—DV, +1.2 mm; ML, ±3.0 mm; DV, −4.2 mm). The cannulae assembly was held in place with dental cement bound to four jeweler’s screws mounted to the skull. Rats were provided 5–7 days to recover from surgery prior to initiating sucrose self-administration training.
Systemic and intracranial injections
The D1 antagonist SCH 23390 (Sigma, St. Louis, MO, USA) was dissolved in sterile saline. For the systemic study, the drug was injected 15 min prior to a test session (0, 1, 5, or 25 µg/kg IP). All rats in the systemic study received two acclimatization saline injections the two afternoons prior to their test day. For the microinjection studies, microinjection needles (28-gauge) that projected 1 mm beyond the end of the guide cannulae were lowered into the guide cannulae immediately prior to a test session and saline or antagonist (0.3 or 0.6 µg/site) was infused over 1 min. Microinjection cannulae were left in place for 2 min. Rats were then placed in the operant conditioning chambers and testing began. All rats in the microinjection studies received one acclimatization saline microinjection in the afternoon 2 days prior to their test day. Only one test was conducted for each rat. The doses of antagonist were based on previous studies examining the effect of D1 antagonism on drug-seeking behavior (Crombag et al. 2002; Bossert et al. 2007). A systemic dose just above the range chosen was shown to produce elevated brain levels of drug by the first 15 min post-injection, remaining elevated for at least 1 h (Kilts et al. 1985). As indicated by Bossert et al. (2007), the intracranial doses were lower than in some previous reports as SCH 23390 moves rapidly from a microinjection site (Caine et al. 1995). Rats that had intracranial microinjections were euthanized after testing and their brains were removed and placed in 10% formalin. Then 70-µm coronal sections were later taken on a vibratome, placed on glass slides, and subsequently stained with cresyl violet. Sections were examined for microinjection tip placements under the 4× objective of an Olympus BX41 light microscope.
Apparatus
Operant training and testing took place in operant conditioning chambers (30×20×24 cm; Med Associates, St. Albans, VT, USA) containing two levers (one stationary and one retractable), a tone generator, a white stimulus light above the retractable lever, and a red house light on the opposite wall. An infusion pump delivered sucrose into a reward receptacle to the right of the active lever. Operant conditioning chambers were enclosed in sound-attenuating cabinets with ventilation fans.
Sucrose self-administration training
Rats spent 2 h/day for 10 consecutive days in operant conditioning chambers where they were allowed to press the retractable (active) lever for a 0.2 ml delivery of 10% sucrose solution into the receptacle to the right of the lever. This response also activated a compound stimulus consisting of a tone (2 kHz, 15 dB over ambient noise) and the white light. The compound stimulus lasted for 5 s and was followed by a 40-s time out, during which presses on the active lever were recorded but had no programmed consequence. A response on the inactive (stationary) lever had no programmed consequence, but presses were recorded. Four infrared photobeams crisscrossed the chamber. The total number of beam breaks was recorded during cue-reactivity testing. At the end of each training session, rats were returned to home cages.
Forced abstinence
The forced-abstinence phase began the day immediately following the 10th day of the training phase (“Day 1”).
Cue reactivity testing
On Day 1 or Day 30, rats were tested in the operant conditioning chambers for sucrose cue reactivity (sucrose seeking). This session was identical to the 2-h training procedure, but sucrose was not delivered following a lever response.
Experiment 1a: effect of systemic SCH 23390 injection on sucrose seeking
Rats were administered acclimatizing injections of saline outside of the operant test room the two afternoons immediately prior to their sucrose cue-reactivity test. Different rats were then injected with the various doses of SCH 23390 15 min prior to cue reactivity testing after either 1 or 30 days of forced abstinence.
Experiment 1b: effect of systemic SCH 23390 injection on sucrose self-administration
A separate group of rats was tested for effects of the low and high doses (1 and 25 µg/kg) of SCH 23390 on sucrose self-administration using a counterbalanced within-subjects design. The purpose of examining the effects of SCH 23390 on sucrose self-administration was to identify whether the drug affects the relatively high rate of responding engendered by sucrose. If an effect of the drug on this responding were to be found, it might be indicative of a motor impairment. These rats first received core or shell guide cannulae placements for later microinjection studies (see Experiment 2b). As was done in Experiment 1a, rats were administered acclimatizing injections of saline prior to their first drug challenge. The first systemic drug injections were given after the rats had completed 10 self-administration training sessions. The rats never had a forced-abstinence period and two self-administration sessions separated each test session. Test sessions were the same as self-administration training sessions with the addition of the drug injection preceding the test.
Experiment 2a: effect of NAcc core or shell SCH 23390 injection on sucrose seeking
Rats were administered an acclimatizing injection of saline outside of the operant test room in the afternoon 2 days prior to testing with drug. Different rats were then injected with SCH 23390 (0.3 or 0.6 µg/site) immediately prior to cue reactivity testing after either 1 or 30 days of forced abstinence.
Experiment 2b: effect of NAcc core or shell SCH 23390 injection on sucrose self-administration
As in Experiment 1b, a study was conducted to identify possible motor impairing effects of SCH 23390 on operant responding. A separate group of rats (same rats as in Experiment 1b) was tested for effects of both doses of intracranial SCH 23390 on sucrose self-administration using a counterbalanced within-subjects design. These rats were tested after approximately 1 week of self-administration sessions following the last systemic injection for Experiment 1b. As with Experiment 2a, rats received one acclimatization injection of saline 2 days prior to their first drug challenge. Two self-administration sessions separated each test session and test sessions were the same as self-administration training sessions with the addition of the drug microinjection preceding the test.
Experiment 3: dorsal injection site
Different rats were injected with either saline or the high dose (0.6 µg/site) of SCH 23390 after 30 days of forced abstinence. A dorsal injection comparison was included to verify whether SCH 23390-induced changes in cue reactivity were localized to the target regions (NAcc core or shell) or whether they might have been caused by movement of drug up the sides of the guide cannulae. The location chosen was approximately the dorsomedial striatum, a region where injection of SCH 23390 was recently shown to not affect drug seeking in rats (Bossert et al. 2009). This region of the striatum is directly in the trajectory of guide cannulae for both core and shell microinjections. As with the sucrose-seeking tests in Experiments 1 and 2, sucrose was not delivered during the test session.
Statistical analyses
Data were analyzed separately for each experiment. In addition, data in Experiment 2 were analyzed separately for the two brain regions examined. Active lever responding during sucrose self-administration training was analyzed using multi-factorial repeated measures (RM) ANOVA of the 10 days of training (TIME) using between-group factors of DAY (1 or 30), DOSE (dose of SCH 23390), and for Experiment 2, REGION (core or shell). This analysis was used to verify that all treatment groups received equal training. Acquisition of sucrose self-administration was defined as an average of 20 or more daily sucrose deliveries over the final 4 days of self-administration training, and an increase in responding for sucrose over the 10 days of training. For the 2-h cue-reactivity tests, the effects of SCH 23390 on the dependent measures (active lever responses, inactive lever responses, photobeam breaks) were evaluated using ANOVA. Active lever responses and photobeam breaks in the first 2 min of these tests were also evaluated using ANOVA. These early test session evaluations were made to determine whether drug injections had an effect on the initiation of responding, potentially indicative of a motor impairment effect of the drug (Alleweireldt et al. 2002), and to identify whether locomotor activity was correlated with this initial active lever responding. A decrease in both measures simultaneously could be indicative of a non-selective motor impairment caused by the drug. For the sucrose self-administration comparison groups in Experiments 1 and 2, data were analyzed using one-way RM ANOVA. For all ANOVAs, post hoc comparisons were made with either LSD tests for between-group comparisons or paired t tests for within-group comparisons. ANOVAs were calculated using SPSS version 18.0. The t tests were calculated using Microsoft Excel 2007. Group data are presented as means±SEMs in the text and figures. For statistical comparisons, p <0.05 was the criterion for statistical significance. In general, only the statistics for significant effects and interactions are indicated in the text.
Results
Of 248 rats that were trained for sucrose self-administration, 10 were removed from the study because they either did not meet a minimum response criterion for acquisition of an average of 20 sucrose deliveries/day over the last 4 days of training or there was an improper microinjection tip placement. One rat was dropped from the sucrose self-administration core microinjection study due to a blocked guide cannula. Final group size ranges are indicated in the figure captions.
Experiment 1a: effect of systemic SCH 23390 injection on sucrose seeking
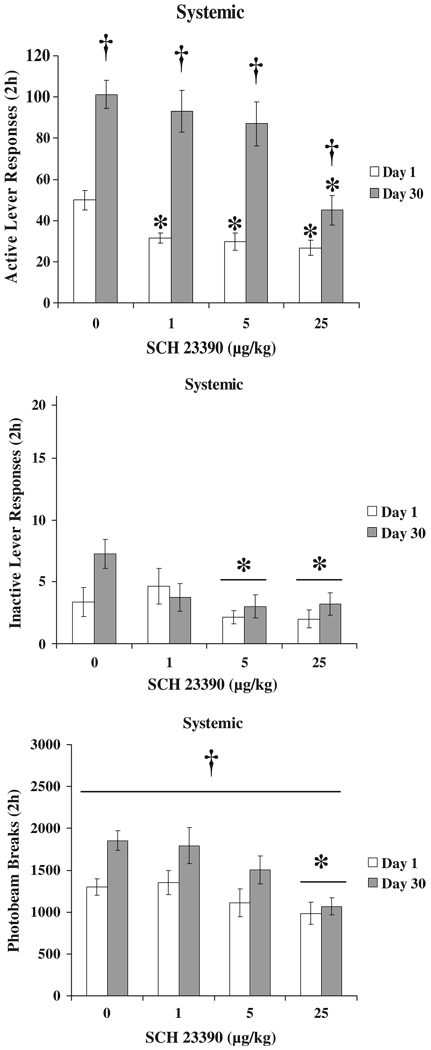
All rats acquired sucrose self-administration [TIME F (9,621)=10.1, p<0.001]. There were no significant differences between groups of animals. The average rate of active lever responding for the final 4 days of self-administration training was 109.3±5.2 per 2-h session. Active lever responding during the 2-h test session was generally higher after 30 days of forced abstinence indicating an incubation of sucrose seeking, DAY F(1,69)=80.0, p<0.001. SCH 23390 pretreatment had an abstinence-dependent effect of reducing active lever responding on the sucrose-seeking test day, DOSE F(3,69)=11.4, p<0.001 and DAY×DOSE F(3,69)=3.3, p<0.05. Inactive lever responding was reduced by SCH 23390, DOSE F(3,69)=3.0, p<0.05 as was locomotor activity, DOSE F(3,69)=6.7, p<0.001. Locomotor activity was increased by forced abstinence period DAY F(1,69)=13.1, p<0.01. Overall SCH 23390 was most effective at reducing sucrose seeking after 1 vs. 30 days of forced abstinence. The highest dose (25 µg/kg) reduced both active lever responding and locomotor activity after either 1 or 30 days of forced abstinence. Active and inactive lever responses and photobeam breaks are indicated with results of appropriate post hoc comparisons in Fig. 1.
Fig. 1.
Active and inactive lever responding and locomotion following systemic injection with SCH 23390. Each bar represents a separate group of animals (n=8–12 per group). Means±SEMs are indicated on the figure. Asterisk indicates significant difference from 0 dose and dagger indicates significant difference from Day 1 group at that dose of SCH 23390, p<0.05
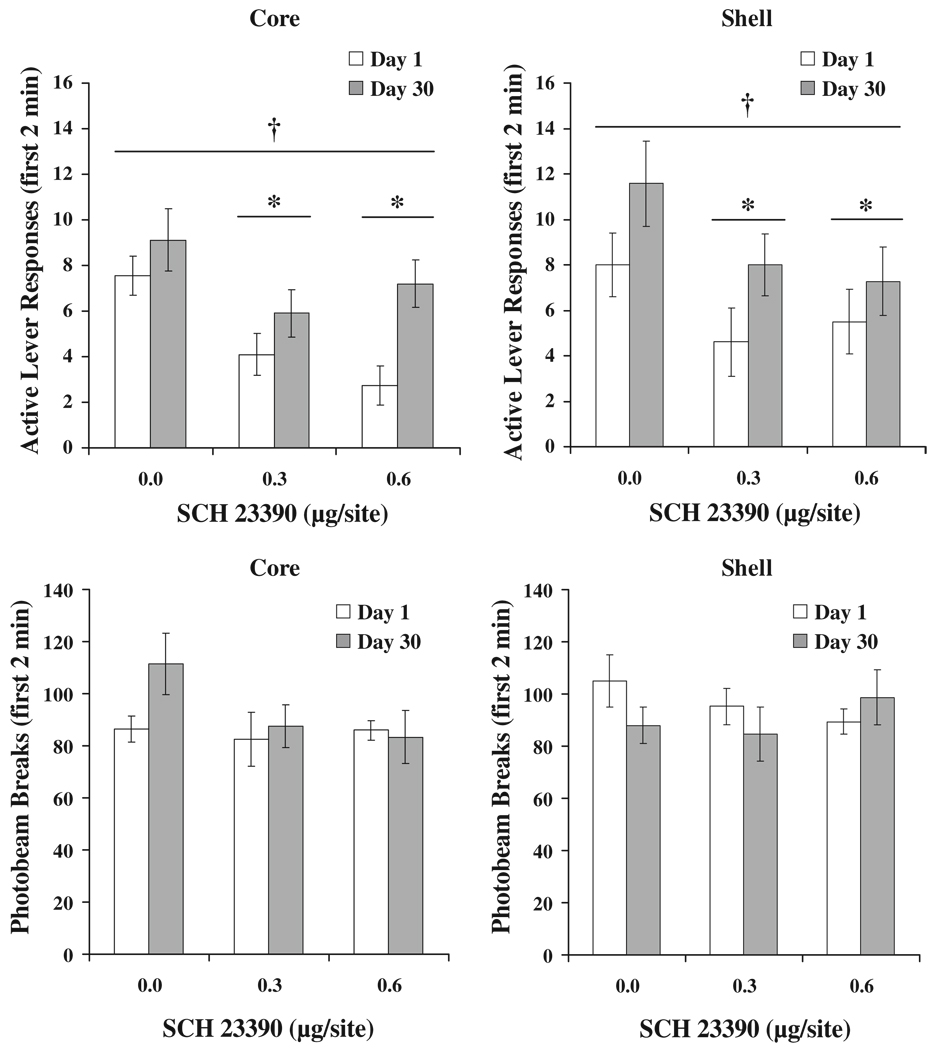
Analyses of active lever responses and locomotor activity in the first 2 min of the test indicated that active lever responding was greater after 30 days of forced abstinence, DAY F(1,69)=23.2, p<0.001, but there was no significant effect of drug DOSE, nor a significant DAY×DOSE interaction. There were no significant effects of DAY or DOSE, nor an interaction of these variables on locomotor activity. These data are indicated in Fig. 2.
Fig. 2.
First 2 min active lever responding and locomotion following systemic injection with SCH 23390. Each bar represents a separate group of animals (n=8–12 per group). Means±SEMs are indicated on the figure. Dagger indicates significant overall difference from Day 1 groups, p<0.001
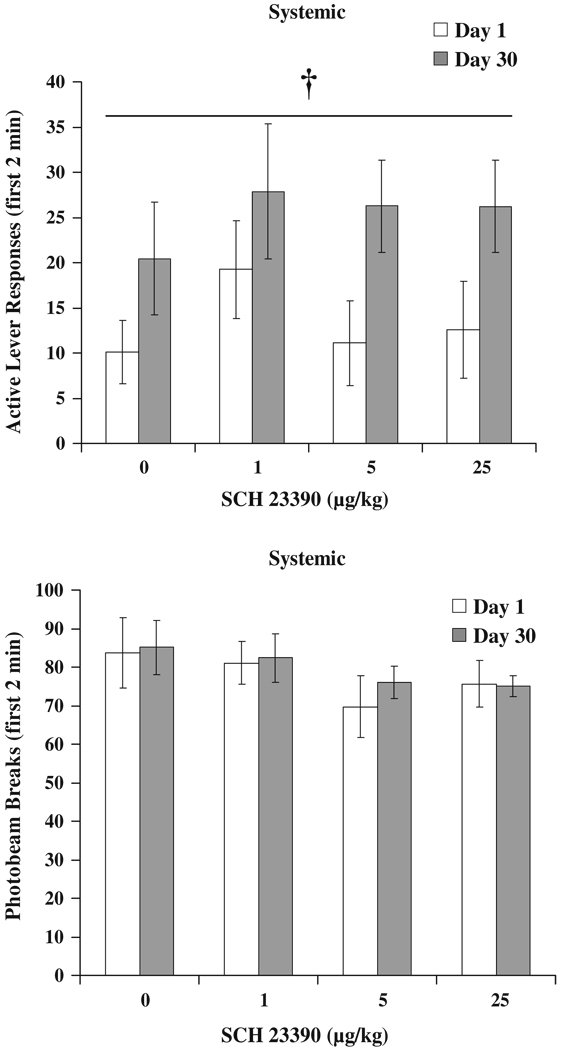
Experiment 1b: effect of systemic SCH 23390 injection on sucrose self-administration
All rats acquired sucrose self-administration [TIME F (9,153)=4.5, p<0.001]. The average rate of active lever responding for the final 4 days of self-administration training was 164.5±15.1 per 2-h session. The low and high doses of systemic SCH 23390 had no significant effects on responding for sucrose itself, nor did they affect inactive lever responding. The high (25 µg/kg) dose reduced locomotor activity by 29.5% compared to saline treatment DOSE F(2,34)=17.1, p<0.001 and significant post hoc comparison (data not shown). Active lever responding is depicted in Fig. 3. Response rates of animals returned to Saline-pretreatment levels the day following drug injections (RM ANOVAs comparing days following SCH 23390 injections to Saline n.s., data not shown).
Fig. 3.
Active lever responses for sucrose following systemic injection with SCH 23390. Means±SEMs are indicated on the figure, n=18 per dose
Experiment 2a: effect of NAcc core or shell SCH 23390 injection on sucrose seeking
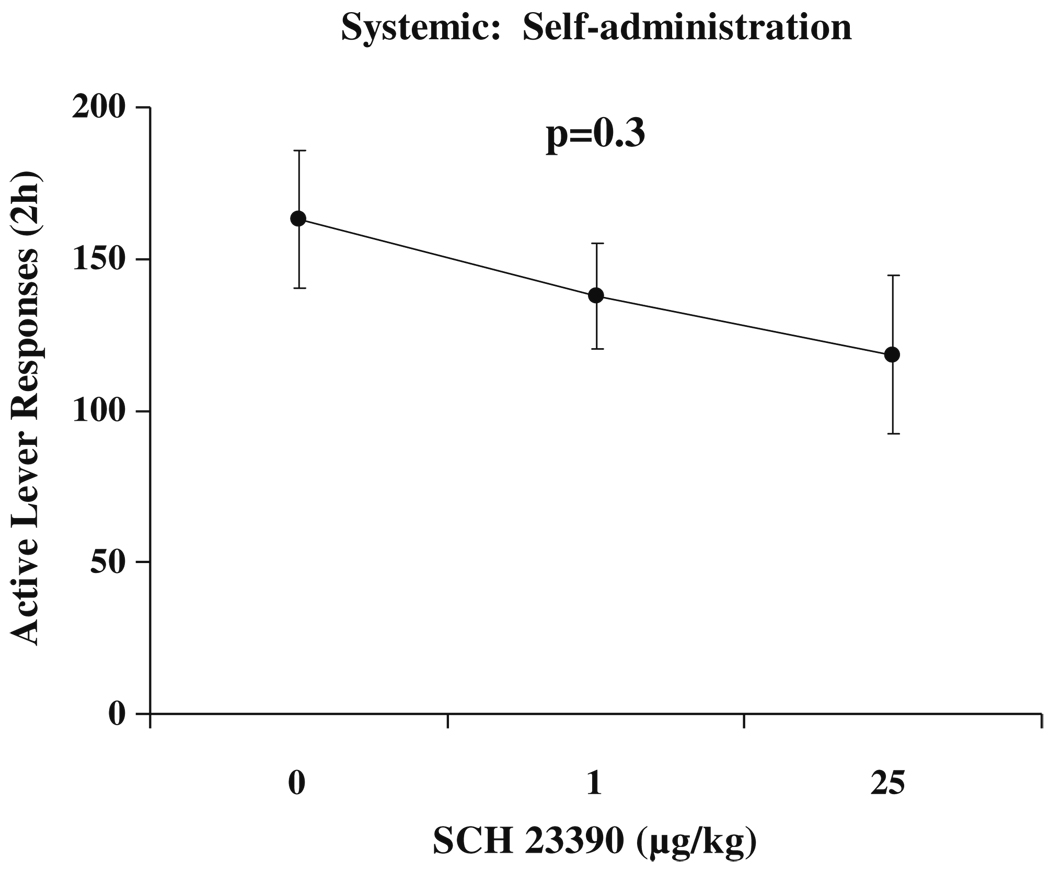
As in Experiment 1, all rats acquired sucrose self-administration [TIME F(9,981)=22.4, p<0.001]. There were no significant differences between groups of animals. The average rate of active lever responding for the final 4 days of self-administration training was 157.1±8.8 responses per 2-h session.
Core
Active lever responding over the 2-h test session was higher after 30 days of forced abstinence (incubation of sucrose seeking), DAY F(1,55)=8.7, p<0.01. Active lever responding was reduced by SCH 23390, DOSE F(2,55)=10.9, p<0.001. There was no significant interaction between DAY and DOSE. Inactive lever responding was greater after 30 days of forced abstinence, DAY F(1,55)=10.5, p<0.01. Both inactive lever responding and locomotor activity were reduced by SCH 23390, F(2,55)=7.1, p<0.01 and F(2,55)=10.0, p<0.001, respectively. Overall, both doses of SCH 23390 reduced all three behavioral measures. Active and inactive lever responses and photobeam breaks are indicated with results of appropriate post hoc comparisons in Fig. 4.
Fig. 4.
Active and inactive lever responding and locomotion following microinjection with SCH 23390. Each bar represents a separate group of animals (n=9–11 per group). Means±SEMs are indicated on the figure. Asterisk indicates significant difference from 0 dose and dagger indicates significant difference from Day 1 group at that dose of SCH 23390, p’s<0.01
Analyses of active lever responses and locomotor activity in the first 2 min of the NAcc core test indicated that active lever responding was greater after 30 days of forced abstinence, DAY F(1,55)=10.0, p<0.01, and that there was a significant effect of drug DOSE, F(2,55)=7.2, p<0.01. There was no significant DAY×DOSE interaction. Post hoc tests revealed that both doses of SCH 23390 reduced active lever responding. There were no significant effects of DAY or DOSE, nor an interaction of these variables on locomotor activity. These data are indicated in Fig. 5.
Fig. 5.
First 2 min active lever responding and locomotion following microinjection with SCH 23390. Each bar represents a separate group of animals (n=9–11 per group). Means±SEMs are indicated on the figure. Asterisk indicates significant difference from 0 dose and dagger indicates overall significant difference from Day 1 groups, p<0.05
Shell
Active lever responding over the 2-h test session was higher after 30 days of forced abstinence (incubation of sucrose seeking), DAY F(1,54)=8.0, p<0.01. Active lever responding was reduced by SCH 23390, DOSE F(2,54)=9.7, p<0.001. There was no significant interaction between DAY and DOSE. Inactive lever responding was not affected by length of forced abstinence or by SCH 23390. Locomotor activity was decreased by SCH 23390, DOSE F(2,54)=8.1, p<0.01. Both doses of SCH 23390 reduced active lever responding and locomotor activity. Active and inactive lever responses and photobeam breaks are indicated with results of appropriate post hoc comparisons in Fig. 4. Analyses of active lever responses and locomotor activity in the first 2 min of the NAcc shell test indicated that active lever responding was greater after 30 days of forced abstinence, DAY F(1,54)=5.6, p<0.05, and that there was a significant effect of drug DOSE, F(2,54)=3.3, p<0.05. There was no significant DAY×DOSE interaction. Post hoc tests revealed that both doses of SCH 23390 reduced active lever responding. There were no significant effects of DAY or DOSE, nor an interaction of these variables on locomotor activity. These data are indicated in Fig. 5.
Experiment 2b: effect of NAcc core or shell SCH 23390 injection on sucrose self-administration
Core
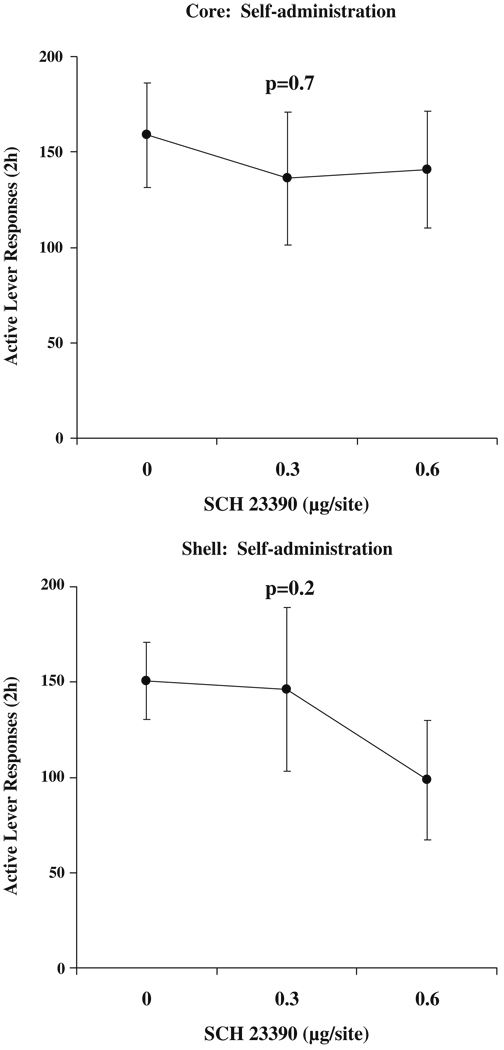
Neither dose of SCH 23390 affected sucrose self-administration, nor did they affect inactive lever responding or locomotor activity. Active lever responses are indicated in Fig. 6.
Fig. 6.
Active lever responses for sucrose following microinjection with SCH 23390. Means±SEMs are indicated on the figure, n=8 per dose Core, and n=9 per dose Shell
Shell
Neither dose of SCH 23390 affected sucrose self-administration, nor did they affect inactive lever responding. The high dose reduced locomotor activity, DOSE F(2,16)=4.4, p<0.05 and significant post hoc comparison (data not shown). Active lever responses are indicated in Fig. 6. Response rates of animals in either core or shell groups returned to Saline-pretreatment levels the day following drug microinjections (RM ANOVAs comparing days following SCH 23390 injections to Saline n.s., data not shown).
Experiment 3: dorsal injection site
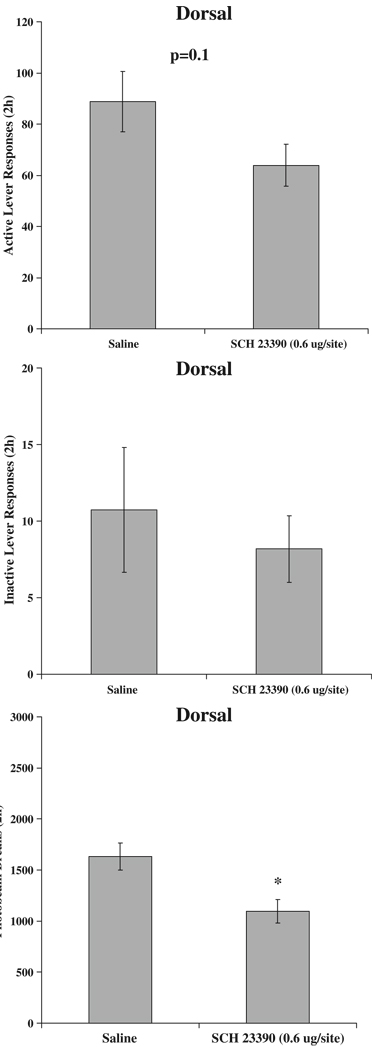
As in Experiments 1 and 2, all rats acquired sucrose self-administration [TIME F(9,180)=4.3, p<0.001]. There were no significant differences between the two groups of animals. The average rate of active lever responding for the final 4 days of self-administration training was 145.5±24.2 responses per 2-h session. Microinjection of the high (0.6 µg/site) dose of SCH 23390 did not have a significant effect on active lever responding for the sucrose-paired cue (p=0.1), nor did it affect inactive lever responding. The drug did produce a significant reduction in locomotor activity [DOSE F(1,20)=9.3, p<0.01]. Active and inactive lever responses and photobeam breaks are indicated in Fig. 7.
Fig. 7.
Active and inactive lever responding and locomotion following microinjection with SCH 23390. Each bar represents a separate group of animals (n=11 per group). Means±SEMs are indicated on the figure. Asterisk indicates significant difference from Saline, p<0.05
Histology
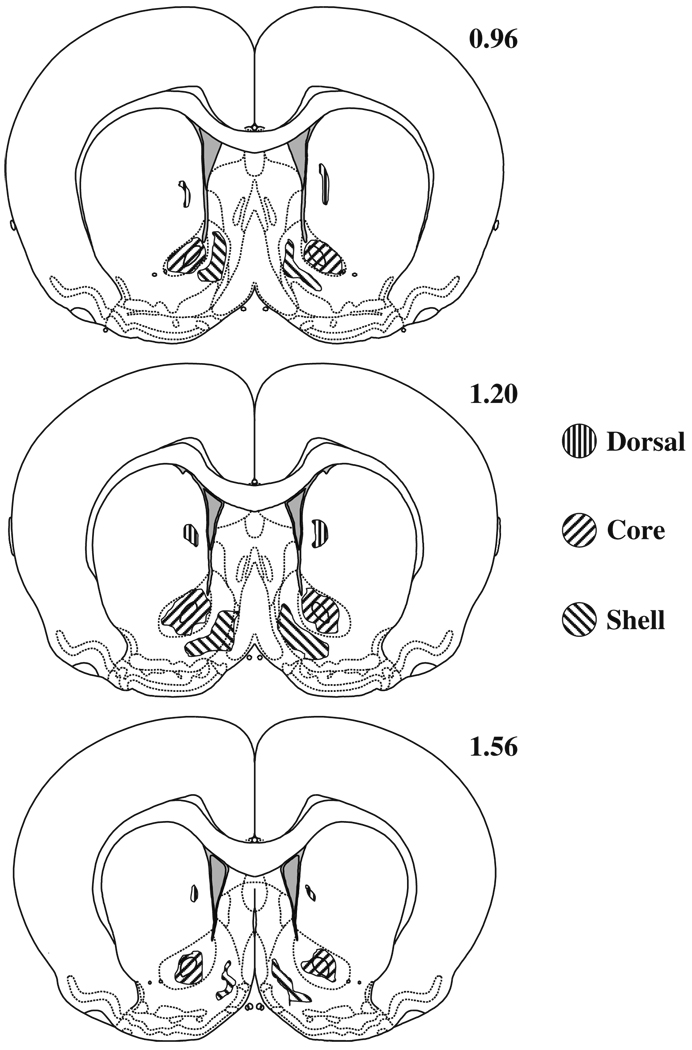
The ranges of all microinjection tip placements (core and shell of NAcc and dorsal injection placements) are depicted in Fig. 8. Representative micrographs are presented in Fig. 9. The distribution of microinjection tip placements in the sucrose self-administration study was indistinguishable from the core and shell placements in Experiments 1 and 2.
Fig. 8.
Ranges of microinjection tip placements into the dorsal site, NAcc core, or NAcc shell. Anatomical plates were adapted from Paxinos and Watson (2007) and values indicate distance from bregma
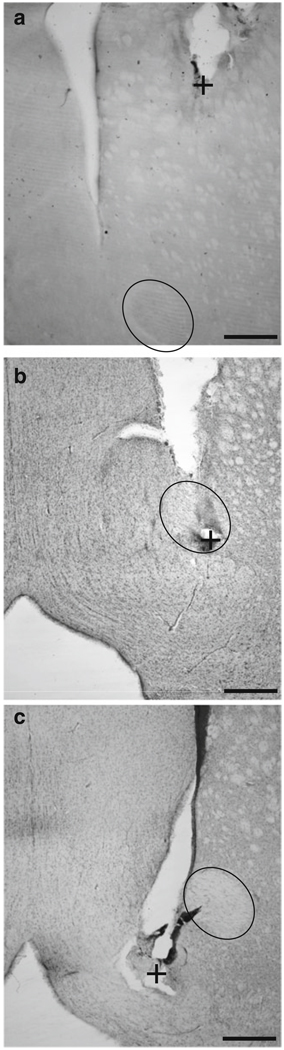
Fig. 9.
Representative left hemisphere microinjection cannulae tracts and injector tip placements in the dorsal site (a), NAcc core (b), or NAcc shell (c); all placements in these examples were approximately +1.2 mm from bregma as identified in an atlas of the rat brain (Paxinos and Watson 2007). Landmarks identified are the anterior commissure (encircled) and the approximate location of the microinjection needle tip (+) for each micrograph. Scale bars represent 500 µm
Discussion
In Experiments 1 and 2 where length of forced abstinence was manipulated, rats responded much greater after 30 days of forced abstinence. This “incubation of craving” effect was similar to our previously published studies (e.g., Grimm et al. 2008; Harkness et al. 2009). When administered systemically, 1 and 5 µg/kg doses of the D1 receptor antagonist SCH 23390 only reduced cue reactivity in rats after 1 vs. 30 days into forced abstinence from sucrose self-administration. The highest dose administered (25 µg/kg) reduced cue reactivity at both forced abstinence time points. Microinjection of the drug into either the core or the shell of the NAcc reduced cue reactivity to a similar extent at both time points. Locomotor activity was reduced following the 25 µg dose of SCH 23390 in the systemic study and following both doses of SCH 23390 in the microinjection studies. However, analyses of active lever responding and locomotor activity in the first 2 min of test sessions revealed that the ability of SCH 23390 to reduce sucrose seeking was not necessarily tied to a general motor impairment effect of the drug. Furthermore, comparison studies wherein rats were challenged with SCH 23390 either systemically or intra-NAcc core or shell immediately prior to a sucrose self-administration session revealed that the drug had rather minimal effects on ongoing operant responding, supporting a selective effect of the drug on conditioned responding in the cue reactivity studies.
Effect of systemic SCH 23390 on sucrose seeking
Systemic D1 receptor antagonism has been demonstrated to be effective at reducing drug cue- or drug context-directed responding in rats in several studies examining self-administration of cocaine, heroin, a cocaine+heroin combination, nicotine, and alcohol (Weissenborn et al. 1996; Ciccocioppo et al. 2001; Weiss et al. 2001; Alleweireldt et al. 2002; Crombag et al. 2002; Liu and Weiss 2002; Bossert et al. 2007; Hamlin et al. 2007; Liu et al. 2010). D1 antagonism was also effective at attenuating food deprivation-induced heroin seeking (Tobin et al. 2009). If D1 receptors are involved in reward seeking in a general sense (e.g., drugs or food), it would be expected that systemic challenge with SCH 23390 would affect food-seeking behavior in a manner similar to the aforementioned drug seeking findings. This is what we observed (present study) with rats responding in extinction. At this time, the only other published study of systemic D1 receptor antagonism of sucrose seeking has been with context renewal of sucrose seeking, and in that study SCH 23390 (10 µg/kg) markedly attenuated sucrose seeking (Hamlin et al. 2006). The time dependence of the systemic effect in the present study qualifies the effectiveness of SCH 23390 on sucrose seeking, however, to being most effective in early forced abstinence.
One explanation for the enhanced efficacy of the drug on the lower rate of responding on Day 1 could be that this lower rate of responding is more susceptible to disruption. Studies on rate dependency support the generalization that lower rates of responding should actually be more susceptible to disruption (Gonzalez and Goldberg 1977; Phillips et al. 1991). Indeed, all three doses (1, 5, 25 µg/kg) of systemically administered SCH 23390 reduced active lever responding for the sucrose-paired cue on the first day of forced abstinence but only the 25 µg/kg dose was effective after 30 days of forced abstinence. However, we feel that there is good evidence to support the interpretation that the time-dependent effect of SCH 23390 was not simply due to rate dependency. For example, we have reported that naloxone is most effective at reducing the higher rate of sucrose seeking found after 30 vs. 1 day of forced abstinence (Grimm et al. 2007). In addition, in studies where SCH 23390 has been effective at reducing food self-administration, the self-administration reducing effect of the drug was on high rates of responding engendered by use of higher ratio schedules of reinforcement as compared to the FR1 schedule used in the present study (e.g., FR10 Sanger 1987; FR15 Haile and Kosten 2001). These findings would suggest that if the effect of SCH 23390 on operant responding is subject to rate-dependent effects, it is higher rates of responding that are most sensitive. Therefore, we conclude that the abstinence-dependent effect of systemically delivered SCH 23390 on sucrose seeking is not due to a simple rate-dependent effect of the drug on operant responding. The abstinence-dependent effect would therefore likely relate to abstinence-dependent changes in D1 signaling and/or abstinence-dependent changes in other signaling pathways that affect sucrose seeking.
Effect of intracranial SCH 23390 on sucrose seeking
As with findings regarding the effects of systemic administration of D1 antagonists on reward seeking, most studies of intracranial-mediated effects have been in drug reinstatement studies. For example, antagonism of D1 receptors in the basolateral amygdala reduced cue-induced cocaine seeking (See et al. 2001; Alleweireldt et al. 2006; Mashhoon et al. 2009). Similar injections into the prelimbic cortex also were effective at reducing responding for a heroin-paired cue (See 2009). Bossert et al. (2009) also found that SCH 23390 microinjected into the dorsolateral, but not dorsomedial, striatum decreased contextual renewal of heroin seeking. At this time, only two studies have examined the effects of D1 antagonism within the NAcc on drug seeking. Bossert et al. (2007) found that SCH 23390 delivered to the NAcc core attenuated heroin seeking mediated by a discrete heroin-paired cue, while injections into the NAcc shell did not affect this behavior. Shell, but not core, microinjections decreased heroin context-mediated renewal of extinguished heroin seeking (Bossert et al. 2007). In contrast, Chaudhri et al. (2009) found that SCH 23390 microinjected into either the core or shell of the NAcc reduced alcohol context-mediated renewal of extinguished alcohol seeking. The results of the present study with sucrose appear to fit with both of these previous reports in that our test procedure was an extinction session where all sucrose-paired cues (lever, tone+light, operant box, etc.) were presented at once. As Bossert et al. (2007) found, the NAcc shell and core have dissociable roles in contextual vs. discrete cue heroin seeking, respectively, perhaps due to the fact that the shell receives inputs from the more spatially oriented hippocampus (Floresco et al. 2001; Ito et al. 2006) while the core receives inputs from the more discrete-cue oriented amygdala (Groenewegen et al. 1999; Tye et al. 2008). It would make sense, therefore, that blocking activity in either structure would affect responding in our procedure. That being stated, this logic does not explain why core microinjections by Chaudhri et al. were effective at reducing context-mediated renewal. As stated in Chaudhri et al., this discrepancy calls for further evaluation of core vs. shell contributions to context-mediated drug seeking.
Regardless, our findings support the hypothesis that NAcc dopamine serves as a signal of incentive motivation relegated to the reward-paired cue (Di Ciano et al. 2008). How the dopamine is released in the accumbens in response to these cues is still a matter of debate. One hypothesis is that cue exposure excites glutamate release from hippocampus and/or amygdala into the NAcc resulting in a presynaptic release of dopamine that results in excitation of accumbal medium spiny neurons via D1 receptor activation (Chaudhri et al. 2009). These are the neurons that have been found to respond to reward-paired cues (Wheeler and Carelli 2009). Blocking accumbens dopamine D1 receptors may therefore reduce the incentive motivation the rat attributes to a cue and thereby reduces responding directed toward that cue.
Effects of SCH 23390 on motivational vs. motor output
Distinguishing motivational versus motor impairments following experimental manipulations is complicated as motivated behavior is often assessed as overt motor responding. In fact, it might be argued that it would be impossible to completely separate the two phenomena, as the mesolimbic dopamine system appears to be responsible for the forward locomotor behavior necessary for exploration and approach to incentive stimuli (Wise 2004). Nonetheless, many studies examining the effects of dopamine antagonists on motivated behavior have presented convincing demonstrations of dissociations between diminished motivated behavior and overt motor impairment by showing one or more of the following: (1) that initiation of operant behavior is similar to controls early in a session (e.g., Wise et al. 1978; Alleweireldt et al. 2002), or under the first exposure to an antagonist (e.g., Wise et al. 1978); (2) that inactive lever responding is not affected by exposure to an antagonist (e.g., Grimm et al. 2007); (3) that the antagonist does not produce a general depression in locomotor activity alongside a decrease in operant responding (e.g., Grimm et al. 2007); and (4) that the antagonist does not reduce a relatively high rate of responding engendered by food, such as sucrose (e.g., Bossert et al. 2007). Many published studies address one or more of these criteria, but rarely all of them, so it is difficult to evaluate several of these previous studies in terms of motivational versus motor effects of experimental manipulations.
Regarding the effect of SCH 23390 on motivated vs. motor responding, we have presented data from cue-reactivity sessions and from sucrose self-administration sessions that, according to these four measures, generally supports a motivational decreasing effect of D1 antagonism, versus a simple motor impairment effect. First, the more ambiguous findings were that over a 2-h test session, the 5 and 25 µg/kg doses of systemically administered SCH 23390 slightly decreased inactive lever responding and the 25 µg/kg dose decreased locomotor activity (Fig. 1). The effect of SCH 23390 microinjections was rather similar: a slight reduction in already low levels of inactive lever responding and some decrease in locomotor activity (Fig. 4). In contrast, evaluation of responding at the beginning of the session (first 2 min) revealed no effect of systemically administered drug on initiating responding or locomotor activity (Fig. 2), and a dissociation between an initial decrease in responding and locomotor activity when drug was administered directly to the core or shell of the NAcc. That is, SCH 23390 directly into the NAcc immediately decreased sucrose seeking, but did not change locomotor activity (Fig. 5). The results of the sucrose self-administration studies (Figs. 3 and 6) indicated that SCH 23390 (systemic or intracranial) did not produce a significant decrease in responding for sucrose itself. Therefore, related to the four general criteria above, we found that our results with systemic administration of SCH 23390 satisfy all of the items with the exception of a decrease in locomotor activity following the 25 µg/kg dose when examining responding in a 2-h session. The results of the NAcc microinjection studies satisfy the majority of the criteria, with the exceptions of a slight reduction in inactive lever responding and a reduction in locomotor activity across 2-h test sessions, a reduction in initiation in responding, and a reduction in locomotor activity during sucrose self-administration in the high dose Shell group. Yet overall there was no consistent relationship between operant responding and locomotor activity following either systemic or intracranial SCH 23390.
As noted in the “Materials and methods” section, we used low doses of SCH 23390 that are not typically associated with motor impairment and included several measures that may assess motor compromising effects of drugs. Our general conclusion, as outlined above, was that SCH 23390 reduces sucrose seeking dissociable from motor impairment. This is a largely consistent finding in the literature regarding the effects of either systemic or intra-accumbens SCH 23390 (Baldo et al. 2002; Bachtell et al. 2005; Bossert et al. 2007) and the few studies that did report an effect of SCH 23390 on rate of responding for food were evaluating rats responding at higher rates (e.g., FR10 Sanger 1987) or used much higher doses (e.g., 100 µg/kg Rusk and Cooper 1994; 1.25 µg/site Bari and Peirce 2005). It is also possible that the effects of D1 antagonism may be to reduce seeking behavior by reducing “effort” (e.g., Nunes et al. 2010) or “response likelihood” (e.g., Choi et al. 2009). Regardless, we feel that in the present study, especially in the context of findings from other laboratories identified above, that our results provide compelling evidence for a selective effect of our D1 antagonist manipulations on motivated behavior vs. motor output. However, given the caveats listed in the section above, it is clear that further evaluation of potential motor impairing effects of D1 antagonism are warranted.
Systemic vs. accumbens effects of SCH 23390 on cue reactivity
As noted in sections above, systemic administration of SCH 23390 had an abstinence-dependent effect on sucrose seeking, whereas it decreased sucrose seeking to a similar extent after a brief or long period of abstinence when directly administered to the nucleus accumbens. There are two general hypotheses to account for this dissociation. First, the microinjected doses of the drug could have been too high to allow detection of abstinence-dependent effects. As noted above, however, we did use relatively low doses of drug for the microinjection studies. In fact, the low dose (0.3 µg/site) was particularly low compared to other studies of accumbal D1-mediated effects in reward and reinforcement paradigms (Bossert et al. 2007). That being said, further studies would be required using a lower dose range of SCH 23390 to accurately determine if the threshold for attenuating sucrose seeking differs by length of forced abstinence. The second general explanation could be that blocking accumbal D1 receptors is sufficient to attenuate sucrose seeking, but that with systemic administration of SCH 23390 the drug is most effective early in forced abstinence due to an abstinence-dependent interaction of the drug with D1 receptors outside of the nucleus accumbens and/or an abstinence-dependent modulation of the efficacy of the drug due to another neurotransmitter system that may or may not interact with the nucleus accumbens, but ultimately affects seeking behavior.
Future studies will be required to determine which hypothesis is correct and will be designed to identify brain structures and signaling systems that respond to sucrose cues and dopamine antagonists in an abstinent-dependent manner. Regions of interest beyond the nucleus accumbens are those already identified to be involved in the incubation of craving for drugs of abuse including the central amygdala (Li et al. 2008) and ventral medial prefrontal cortex (Koya et al. 2009). We are also interested in glutamate signaling as systemic and intra-central amygdala microinjections of a glutamate antagonist attenuate the incubation of sucrose seeking (Uejima et al. 2007) and glutamate/dopamine interactions are critical for certain aspects of incentive learning (Novak et al. 2010). One other area of interest is the dorsolateral striatum, as this region has recently been demonstrated to be involved in responding for drug-paired cues (See et al. 2007; Di Ciano et al. 2008; Bossert et al. 2009). The dorsolateral striatum has emerged within addiction theory as a region where appetitive responding as a habit becomes encoded (Belin et al. 2009). This type of responding is less flexible in many ways, including that it is less susceptible to revaluation of the primary reinforcement contingency (Holland et al. 2008). This effect may be related to the incubation of sucrose seeking as we recently found that revaluation of a sucrose-paired cue was abstinence dependent (Harkness et al. 2009).
The lack of effect of SCH 23390 microinjected into the dorsal site (essentially the dorsomedial striatum) on cue reactivity indicates that the cue-reactivity reducing effects of the drug when microinjected into either the core or shell of the NAcc were mediated locally. These results complement those of Bossert et al. (2009) who reported a lack of effect of microinjection of SCH 23390 into the dorsomedial striatum on context-mediated renewal of responding for heroin-paired cues. The results of this comparison study support a conclusion that reductions in active lever responding for the sucrose-paired cue following microinjection of SCH 23390 into the NAcc were not due to action of the drug at a site directly above the target sites (the dorsomedial striatum). However, as the core of the NAcc is penetrated by most shell guide cannulae placements, this comparison study does not clearly indicate whether shell microinjection effects were mediated partly by movement of drug into the core. That being noted, studies using similar microinjection coordinates to the present study were able to discriminate behavioral effects of GABA agonist inactivation or dopamine receptor antagonism between the core and shell (Bari and Pierce 2005; Bossert et al. 2007; Di Ciano et al. 2008).
Summary and conclusions
We found a time-dependent effect of the dopamine D1 antagonist on sucrose seeking wherein responding for a sucrose-paired cue was most effectively attenuated by systemically administered SCH 23390 after 1 vs. 30 days of forced abstinence from sucrose self-administration. However, microinjection of the antagonist directly into either the core or the shell of the NAcc reduced sucrose seeking to a similar extent in early and late forced abstinence. Our findings complement findings from several other laboratories indicating that dopamine D1 receptors in the NAcc are central to drug- and food-seeking behaviors. Further research will determine whether pharmacological targeting of D1 receptors will have beneficial effects at reducing drug and food relapse behaviors in the clinical population. So far, D1 antagonist pharmacotherapy for clinical relapse has met with limited success (e.g., Nann-Vernotica et al. 2001 examining cocaine craving). Given the abstinence-dependent effect of systemic SCH 23390 in the present study, it may be beneficial to consider that the clinical efficacy of D1 antagonist pharmacotherapy might also be abstinence dependent.
Acknowledgments
This study was supported by National Institute on Drug Abuse/National Institutes of Health grant R15 DA016285-02 and the Western Washington University Biomedical Research Activities in Neuroscience Initiative. The authors wish to thank Addison Tice, David Goodman, and Jon Koerber for helping with data collection.
Footnotes
Conflict of interest The authors have no conflicts of interest or financial disclosures to report.
References
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31:363–374. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2002;159:284–293. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell KD, Wadden TA. Etiology and treatment of obesity: understanding a serious, prevalent, and refractory disorder. J Consult Clin Psychol. 1992;60:505–517. doi: 10.1037//0022-006x.60.4.505. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus–reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology (Berl) 2009;207:303–314. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WY, Morvan C, Balsam PD, Horvitz JC. Dopamine D1 and D2 antagonist effects on response likelihood and duration. Behav Neurosci. 2009;123:1279–1287. doi: 10.1037/a0017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D (1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez FA, Goldberg SR. Effects of cocaine and d-amphetamine on behavior maintained under various schedules of food presentation in squirrel monkeys. J Pharmacol Exp Ther. 1977;201:33–43. [PubMed] [Google Scholar]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Kruzich PJ, See RE. Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology. 2000;28:383–386. [Google Scholar]
- Grimm JW, Manaois M, Osincup D, Wells B, Buse C. Naloxone attenuates incubated sucrose craving in rats. Psychopharmacology (Berl) 2007;194:537–544. doi: 10.1007/s00213-007-0868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Osincup D, Wells B, Manaois M, Fyall A, Buse C, Harkness JH. Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol. 2008;19:777–785. doi: 10.1097/FBP.0b013e32831c3b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodstein F, Levine R, Troy L, Spencer T, Colditz GA, Stampfer MJ. Three-year follow-up of participants in a commercial weight loss program. Can you keep it off? Arch Intern Med. 1996;156:1302–1306. [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann NY Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Haile CN, Kosten TA. Differential effects of D1- and D2-like compounds on cocaine self-administration in Lewis and Fischer 344 inbred rats. J Pharmacol Exp Ther. 2001;299:509–518. [PubMed] [Google Scholar]
- Hamlin AS, Blatchford KE, McNally GP. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143:25–38. doi: 10.1016/j.neuroscience.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2007;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Harkness JH, Webb S, Grimm JW. Abstinence-dependent transfer of lithium chloride-induced sucrose aversion to a sucrose-paired cue in rats. Psychopharmacology (Berl) 2009;208:521–530. doi: 10.1007/s00213-009-1755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Lasseter H, Agarwal I. Amount of training and cue-evoked taste-reactivity responding in reinforcer devaluation. J Exp Psychol Anim Behav Process. 2008;34:119–132. doi: 10.1037/0097-7403.34.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, McNaughton BL, Everitt BJ. Selective excitotoxic lesions of the hippocampus and basolateral amygdala have dissociable effects on appetitive cue and place conditioning based on path integration in a novel Y-maze procedure. Eur J Neurosci. 2006;23:3071–3080. doi: 10.1111/j.1460-9568.2006.04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Theunissen N, Slechten K, Nederkoorn C, Boon B, Mulkens S, Roefs A. Overweight children overeat after exposure to food cues. Eat Behav. 2003;4:197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Dew KL, Ely TD, Mailman RB. Quantification of R-(+)-7-chloro-8-hydroxy-1-phenyl-2, 3, 4, 5-tetrahydro-1H-3-methyl-3-benzazepine in brain and blood by use of reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1985;342:452–457. doi: 10.1016/s0378-4347(00)84543-0. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56:177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex B, Hauber W. The role of nucleus accumbens dopamine in outcome encoding in instrumental and Pavlovian conditioning. Neurobiol Learn Mem. 2010;93:283–290. doi: 10.1016/j.nlm.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, Shaham Y, Lu L. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci. 2008;28:13248–13257. doi: 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jernigen C, Gharib M, Booth S, Caggiula AR, Sved AF. Effects of dopamine antagonists on drug cue-induced reinstatement of nicotine-seeking behavior in rats. Behav Pharmacol. 2010;21:153–160. doi: 10.1097/FBP.0b013e328337be95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J Pharmacol Exp Ther. 2002;300:882–889. doi: 10.1124/jpet.300.3.882. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine B, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Mashhoon Y, Tsikitas LA, Kantak KM. Dissociable effects of cocaine-seeking behavior following D1 receptor activation and blockade within the caudal and rostral basolateral amygdala in rats. Eur J Neurosci. 2009;29:1641–1653. doi: 10.1111/j.1460-9568.2009.06705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. N Engl J Med. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nann-Vernotica E, Donny EC, Bigelow GE, Walsh SL. Repeated administration of the D1/5 antagonist ecopipam fails to attenuate the subjective effects of cocaine. Psychopharmacology (Berl) 2001;155:338–347. doi: 10.1007/s002130100724. [DOI] [PubMed] [Google Scholar]
- Novak M, Halbout B, O'Connor EC, Rodriguez Parkitna J, Su T, Chai M, Crombag HS, Bilbao A, Spanagel R, Stephens DN, Schutz G, Engblom D. Incentive learning underlying cocaine-seeking requires mGluR5 receptors located on dopamine D1 receptor-expressing neurons. J Neurosci. 2010;30:11973–11982. doi: 10.1523/JNEUROSCI.2550-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Santerre JL, Given AB, Sager TN, Correa M, Salamone JD. Differential effects of selective adenosine antagonists on the effort-related impairments induced by dopamine D1 and D2 antagonism. Neuroscience. 2010;170:268–280. doi: 10.1016/j.neuroscience.2010.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Arndt IO, McLellan AT, Woody GE, Maany I. Pharmacological and behavioral treatments of cocaine dependence: controlled studies. J Clin Psychiatry. 1988;49:17–22. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th edn. San Diego: Elsevier Academic; 2007. [Google Scholar]
- Phillips G, Willner P, Sampson D, Nunn J, Muscat R. Time-, schedule-, and reinforcer-dependent effects of pimozide and amphetamine. Psychopharmacology (Berl) 1991;104:125–131. doi: 10.1007/BF02244566. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusk IN, Cooper SJ. Parametric studies of selective D1 or D2 antagonists: effects on appetitive and feeding behaviour. Behav Pharmacol. 1994;5:615–622. doi: 10.1097/00008877-199410000-00007. [DOI] [PubMed] [Google Scholar]
- Sanger DJ. The actions of SCH 23390, a D1 receptor antagonist, on operant and avoidance behavior in rats. Pharmacol Biochem Behav. 1987;26:509–513. doi: 10.1016/0091-3057(87)90157-2. [DOI] [PubMed] [Google Scholar]
- See RE. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2009;12:431–436. doi: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology. 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–261. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Tobin S, Newman AH, Quinn T, Shalev U. A role for dopamine D1-like receptors in acute food deprivation-induced reinstatement of heroin seeking in rats. Int J Neuropsychopharmacol. 2009;12:217–226. doi: 10.1017/S1461145708008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Stuber GD, De Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uejima JL, Bossert JM, Poles GC, Lu L. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res. 2007;181:292–296. doi: 10.1016/j.bbr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med. 1993;119:688–693. doi: 10.7326/0003-4819-119-7_part_2-199310011-00012. [DOI] [PubMed] [Google Scholar]
- Wakabayashi KT, Fields HL, Nicola SM. Dissociation of the role of nucleus accumbens dopamine in responding to reward-predictive cues and waiting for reward. Behav Brain Res. 2004;154:19–30. doi: 10.1016/j.bbr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25:361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Deroche V, Koob GF, Weiss F. Effects of dopamine agonists and antagonists on cocaine-induced operant responding for a cocaine-associated stimulus. Psychopharmacology. 1996;126:311–322. doi: 10.1007/BF02247382. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009;56:149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced "anhedonia" in rats: pimozide blocks reward quality of food. Science. 1978;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci. 2004;20:249–263. doi: 10.1111/j.1460-9568.2004.03476.x. [DOI] [PubMed] [Google Scholar]