Abstract
Background
A recent clinical trial revealed that folic acid supplementation is associated with an increased incidence of prostate cancer (1). As tumor cells in culture proliferate directly in response to available folic acid, the goal of our study was to determine if there is a similar relationship between patient folate status, and the proliferative capacity of tumors in men with prostate cancer.
Methods
Serum folate and/or prostate tissue folate was determined in 87 randomly selected patients undergoing surgery for prostate cancer, and compared to tumor proliferation in a subset.
Results
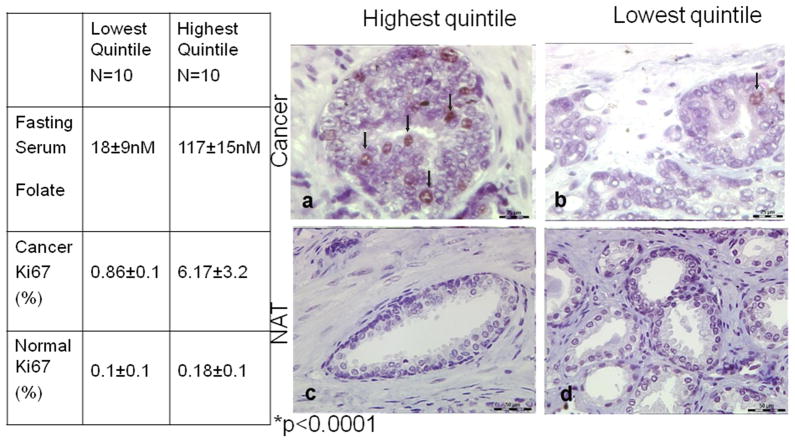
Fasting serum folate levels were positively correlated with prostate tumor tissue folate content (n= 15; r= 0.577, p<0.03). Mean serum folate was 62.6 nM [7.5–145.2 nM], 39.5% of patients used supplements containing folic acid (n=86). The top quartile of patients had serum folates above 82 nM, six times the level considered adequate. Of these, 48% reported no supplement use. Among 50 patients with Gleason 7 disease, the mean proliferation index as determined by Ki67 staining was 6.17 ± 3.2% and 0.86 ± 0.92% in the tumors from patients in the highest (117 ± 15 nM) and lowest (18 ± 9 nM) quintiles for serum folate, respectively (p<0.0001).
Conclusions
Increased cancer cell proliferation in men with higher serum folate concentrations is consistent with an increase in prostate cancer incidence observed with folate supplementation. Unexpectedly, more than 25% of patients had serum folate levels greater than 6-fold adequate. Nearly half of these men reported no supplement use, suggesting either altered folate metabolism and/or sustained consumption of folic acid from fortified foods.
Keywords: folic acid, PSMA, fortification, B-vitamins
Introduction
Folate, a water soluble B vitamin, is an essential factor in the one-carbon metabolism pathway which is responsible for nucleotide synthesis, and for biological methylation reactions, including DNA, RNA and histone methylation (reviewed in Mason, 2009(2)). Low circulating levels of folate have been associated with higher risk of breast, lung, pancreatic and colorectal cancer, while a generally high folate status has been associated with a modest risk reduction for cancer development in some prospective studies (2). Conversely, higher plasma folate levels in Swedish women have been associated with an increased incidence of estrogen receptor-beta negative breast cancer (3). Further, in a recent randomized controlled trial investigating the potential anti-neoplastic effect of supplementary folic acid (a synthetic folate not found in nature) in subjects with a history of colorectal adenoma, a 67% increased risk of advanced lesions was reported with supplementation when compared to placebo (4). Taken together, these findings suggest folate may play a dual role in carcinogenesis (5), and the timing of folate administration may modify disease progression; folate supplementation prior to the existence of pre-neoplastic lesions may prevent tumor development by enhancing genomic DNA stability, whereas folate supplementation appears to increase established cancer growth (reviewed in Ulrich and Potter, 2007(6)). This increased cellular proliferation may reflect the necessary role of folate in the de novo synthesis of thymidine, considered a rate-limiting step in DNA synthesis. Thus, folate availability may be particularly critical for more rapidly proliferating dysplastic or cancerous cells with excessive folate intake providing abundant quantities of thymidine.
The effect of folate availability on prostate cancer incidence remains unclear. In the Aspirin/Folate Polyp Prevention Study, a placebo-controlled randomized trial of aspirin and folic acid supplementation for the chemoprevention of colorectal adenomas, the supplementation group was analyzed for the diagnosis of prostate cancer, and an age-adjusted hazard ratio of 2.63 (95% CI 1.23 to 5.65, p< .01) over a 10 year follow-up period was observed (1,4). However while one Swedish study found an increased risk of prostate cancer with increased plasma folate (7), two other European prospective studies did not reveal an association between plasma folate levels and prostate cancer risk (8,9). As a group, European men have lower serum folate levels compared to U.S. men, in part because mandatory folic acid fortification of cereal grains occurred in the U.S. in the late 1990s, but not in Europe. In a recent study (8), 90% of Swedish subjects had serum folate levels less than 11.1 nM, while in the Finnish study (9) 75% of cases had serum folate levels less than 10.8 nM. By comparison, only 2.5% of U.S. men have serum folate levels less than 10.4 nM (10). This large difference in serum folate levels in different countries may explain, in part, the inconsistent findings regarding a potential relationship between circulating folate levels and prostate cancer incidence. This idea is supported by the results of two recently published double-blind, placebo-controlled trials carried out in Norway, that found that treatment with moderate amounts of folic acid and its co-factor vitamin B (12) increased both cancer incidence and cancer mortality (11). Finally, in a recent UK study examining serum folate levels in patients with localized prostate cancer, Collin et al., (12) found that increased folate levels correlated with increased PSA velocity, indicating more rapid disease progression and suggesting that serum folate levels may drive cancer cell proliferation in vivo.
The present study was designed to test the hypotheses that 1) there is a positive relationship between basal circulating folate levels and prostate tumor and/or normal prostate tissue folate levels, and 2)if there was a positive relationship between tissue folate and circulating folate levels, then patients with higher serum folate levels would have increased proliferation in the primary tumor relative to patients with lower folate. Finally we asked whether higher folate levels correlated with expression of PSMA (also known as Folate Hydrolase 1), which is significantly up-regulated in prostate cancer and independently associated with disease progression (13). Our findings suggest that there is a positive relationship between folate status and the proliferation of prostate tumors, however expression of PSMA was not changed with regard to folate levels.
Materials and Methods
Patients and blood sample collection
All serum and tissues were obtained from the Department of Pathology and the University of Pittsburgh Health Sciences Tissue Bank after University of Pittsburgh Institutional Review Board approval for this study (Protocol #PRO10010060). Written consent to have tissue banked was obtained from patients (frozen tissues and serum). Use of clinical specimens from patients (paraffin embedded) was approved by the University of Pittsburgh Institutional Review Board as exempt from Human Subjects Research Policy under the U.S. Federal Code of Regulations 45CFR46.101(b)(4). All patient information was de-identified by an honest broker and research was carried out in a manner consistent with the Helsinki Declaration. Fasting blood serum samples were obtained on the day of surgery in 87 patients undergoing prostatectomy, and at the time of organ donation in 25 brain dead organ donors. Causes of death among organ donors included head trauma, gunshot wounds, and motor vehicle crashes, and organs were harvested shortly after traumatic injury. Details of tissue pathology and patient characteristics are listed in Table I.
Table 1.
Clinical characteristics of patients with primary prostate carcinoma.
| Clinical parameter | All men studied |
|---|---|
| Age range (years) | Frequency (% total) |
| 40–49 | 23 (26.4%) |
| 50–59 | 18 (20.7%) |
| 60–69 | 43 (49.5%) |
| ≥70 | 3 (3.4%) |
| Preoperative serum PSA (ng/mL) | |
| Mean ± SD | 6.20 ± 4.6 |
| Median (range) | 5.29 (0–32.1) |
| Pathologic stage | |
| pT1-pT2xN0M0 | 56 (64.4%) |
| pT3aN0M0 & pT3bN0M0 | 25 (28.7%) |
| pTanyN1M0 | 6 (6.9%) |
| Gleason Score | |
| 3+3=6 | 8 (9.2%) |
| 3+4=7 | 54 (62.1%) |
| 4+3=7 | 14 (16.1%) |
| 4+4=8 | 4 (4.6%) |
| 4+5=9 | 7 (8.0%) |
| Use of folic acid containing supplements, including multi-vitamins | 34 (39.1%) |
To determine tissue folate levels, bulk frozen tissues from 19 surgically resected primary prostate cancer tissues or normal tissue from the same prostate (age range 41–71 years) and 25 cancer-free controls (age range <40–71 years) were used. The absence of cancer was verified histologically by a genitourinary pathologist.
Folate assay
Folate levels were determined by the microbiological (Lactobacillus casei, ATCC 7469) assay in a 96-well microtitre plate (14,15). Serum samples were analyzed directly. To extract folate from tissues, 25–75 mg was homogenized in 600 μl of extraction buffer containing 2% sodium ascorbate. An aliquot was removed for protein quantitation, and the remainder incubated at 99°C for 5 minutes. The cooled supernatant was treated with rat serum hydrolase to deglutamate intracellular folates and stored at −80°C until analysis.
Samples were run in replicates of 6. Plates were incubated in the dark for 16 hours at 37°C, and then read at 600 nm using a Molecular Devices Spectramax M5 plate reader, and the folate concentration was determined against a standard curve generated from folic acid stock solution that was verified for concentration spectrophotometrically, as previously described (16).
Immunohistochemistry
Immunohistochemical staining of the nuclear antigen Ki67 using anti-human Ki67 (BD Biosciences Pharmingen, San Jose, California; 1:50 dilution) and FOLH1 (PM2J004.5, a gift from HybritechInc., San Diego, CA; 1:800 dilution) was carried out as previously described (17). Determination of the Ki67 staining index (S.I.) was performed in a blinded fashion as described (18)on at least 500 cancer cells per specimen, and expressed as percent positively stained nuclei over the total cancer cell nuclei considered. To account for the heterogeneous distribution of positively stained cells, only tissue areas harboring the highest number of stained cells were chosen to be investigated.
Semi-quantitative assessment of FOLH1 staining was scored as: 0 = none; 1+ = mild; 2+ = moderate; and 3+ = marked. Three cancer containing areas of the gland were reviewed per case. The score was multiplied by the percent of positively stained cancer glands for each triplicate, and the sum of the three scores resulted in an overall score for each patient.
Statistics
Values are reported as mean ± s.d. unless otherwise indicated. Statistical analysis was carried out using SigmaStat version 3.5 (Systat Software, Inc.).
Results
The clinical characteristics of patients with primary prostate carcinoma are summarized in Table 1. Serum was obtained from 12, 5, and 3 controls ranging in age from 41–49, 50–59, and ≥60 years of age, respectively. Mean serum folate in controls and patients are shown in Figure 1 and Table 2. Mean serum folate concentrations were significantly higher at all age ranges in men with prostate cancer versus cancer-free controls (utilizing the Mann-Whitney Rank Sum test; age 40–49 P<0.00001, 50–59 P<0.007, and ≥60 years P<0.002; Table 2). As shown in Table 3, fasting serum folate was significantly higher in users of supplements containing folic acid (P<0.05), when the group as a whole was compared to non-users. However when the group was divided into quartiles there were no significant differences in the serum folate levels between users and non-users of supplements. Of interest, the patients in the top two quartiles have serum folate levels ranging from more than 4-fold to more than 10-fold the level considered adequate, yet only 55% of these used folate-containing supplements.
Figure 1.
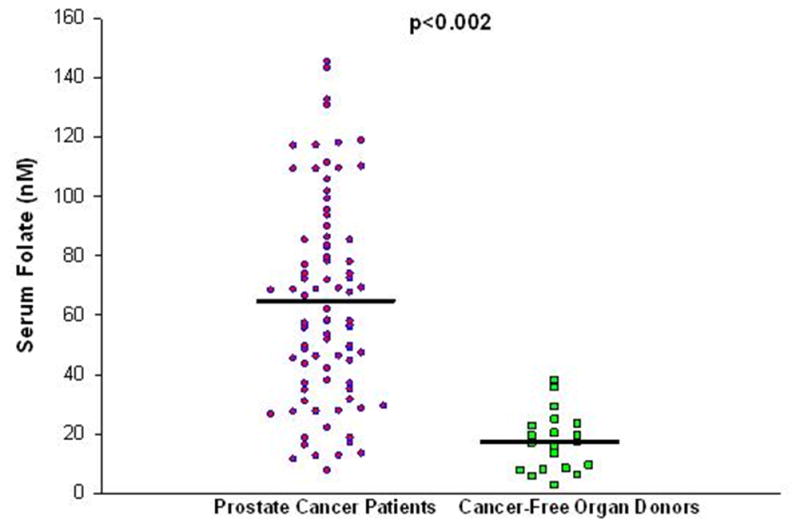
Fasting serum folate concentrations (nM) in patients with prostate cancer versus cancer-free controls.
Table 2.
Fasting serum folate concentrations (nM) in patients with prostate cancer versus cancer-free controls, by age.
| Prostate Cancer | Controls | P-value | |
|---|---|---|---|
| Age range (PC, OD) | mean (median) [n] | mean (median) [n] | |
| 40–49 | 54.7 ± 31.4 (49.6) [23] | 19.8 ±10.3 (18.5) [12] | <0.00001 |
| 50–59 | 62.2 ± 33.8 (67.5) [18] | 14.4 ± 9.1 (15.8) [5] | <0.007 |
| 60 | 67.1 ± 35.5 (68.4) [46] | 12.6 ± 9.6 (8.2) [3] | <0.002 |
| Overall | 64.9 ± 35.2 [87] | 17.4 ± 9.9 [20] | <0.001 |
Table 3.
Fasting Serum Folate Levels in Prostate Cancer Patients by Use of Folic Acid Containing Supplements.
| All Patients (n=86) | First Quartile (n=22) | Second Quartile (n=21) | Third Quartile (n=22) | Fourth Quartile (n=21) | |
|---|---|---|---|---|---|
| Mean serum folate (nM) | 62.6 | 22.0 | 48.0 | 71.3 | 110.8 |
| Median (nM) | 57.3 | 21.9 | 48.4 | 71.8 | 109.4 |
| Supplement users (%) | 34 (39.5) | 7 (31.8) | 3 (14.3) | 13 (59.1) | 11 (52.4) |
| Mean serum folate in supplement users (nM) | 73.2* | 21.1 | 47.3 | 73.5 | 113.2 |
| Mean serum folate in non-users of supplements (nM) | 55.7* | 22.4 | 48.1 | 68.2 | 108.1 |
P=0.02
Median tissue folate concentrations were significantly higher in prostate cancer tissues (n=17) compared to cancer-free prostate tissues from similar age (>50 years) controls (n=9), 0.075 pg/μg and 0.022 pg/μg, respectively (P<0.05; Kruskal-Wallis test using Dunn’s method for multiple comparisons). The median folate level in the histologically normal prostate tissue adjacent to the cancer was 0.037 pg/μg (n=17) and was not significantly different to either the corresponding cancer samples or to the cancer free prostates. In addition, fasting serum folate concentration had a significant positive correlation with prostate tissue folate content in the cancer patients (n= 15; Spearman correlation for cancer; r=0.577, p<0.03; for normal tissues adjacent to the cancer n=15, r=0.546, P<0.04, Figure 2). In the control group, there was no significant correlation between prostate tissue and serum folate content, although the trend was positive (n=19, r=0.36, P=0.12). When prostate tissue folates amongst cancer free controls were compared by age, there was no significant difference (age <40, n=8, median 0.053 pg/μg, age 40–49, n=8, 0.049 pg/μg and age 50+, n=9, median 0.022 pg/μg; Kruskal-Wallis test, P=0.07).
Figure 2.
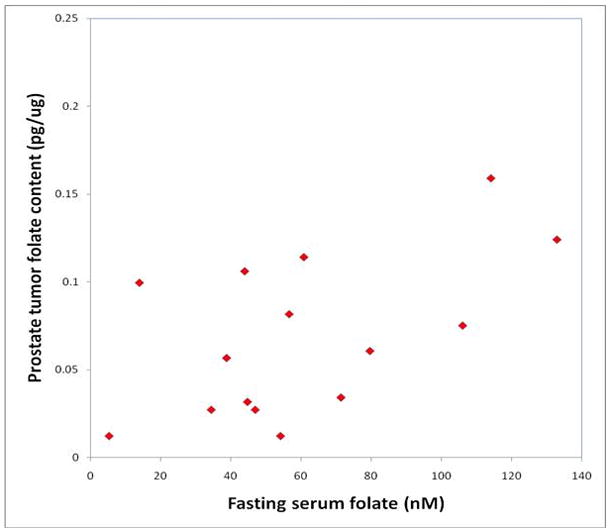
Fasting serum folate has a significant positive correlation with prostate cancer folate content (correlation coefficient using the Spearman rank correlation test is 0.577, p=0.023).
Mean Ki67 staining index in the cancer tissue from patients with Gleason 7 disease (and regardless of age) in the highest quintile for serum folate (n=10; 117 ± 15 nM) was 6.17 ± 3.2% versus 0.86 ± 0.92% for those in the lowest quintile of serum folate (n=10; 18 ± 9 nM) concentration (p< 0.0001) (Figure 3). In normal prostate glands from the same patients, we found no significant difference in mean Ki67 staining index between patients in the highest (0.18 ± 0.1%) and lowest (0.10 ± 0.1%) quintiles for serum folate (p>0.17). We found no significant difference in mean FOLH1 staining scores in patients with high (343 ± 292) and low (497 ± 279) serum folate concentrations (p>0.23).
Figure 3.
Mean Ki67 staining index in grade-matched cancers from patients in the highest (n=10; mean = 117±15 nM) and lowest quintiles (n=10; mean= 18 ± 9 nM) with respect to fasting serum folate concentrations (p<0.0001). Representative light photomicrograph (63×) of Ki67 immunohistochemistry reveals significantly greater staining (arrows) in prostate cancer patients with high (a) versus low (b) serum folate concentrations. Normal prostate glands from the same patients revealed no difference in Ki67 staining in patients with high (c) versus low (d) serum folate concentrations.
Discussion
To our knowledge, this is the first study to examine prostate tissue folate levels in either prostate cancer patients or cancer-free controls. Previous studies have compared prostate cancer incidence to serum or red blood cell folate, or, in many cases, dietary recall assessments of folate intake, in either a prospective or retrospective manner. However, there are great differences between tissues in regard to their sensitivity to changes in folate intake (19). Fasting serum folate levels at time of radical prostatectomy were therefore measured to determine systemic folate status, and both cancer and histologically normal tissue folate concentrations were measured to determine folate status of the prostate.
Both cancer and normal prostate tissue from prostate cancer patients were positively correlated with the patient’s baseline serum folate, which contrasted with a lack of significant correlation among the control subjects. The potential for folate to serve as a growth factor for neoplastic cells is amplified by their tendency to up-regulate the membrane transporters that mediate their uptake of folate (20): this upregulation might better enable tumor-bearing prostates to extract folate from the serum and to thereby maintain a closer concordance with circulating levels of the vitamin. Moreover, cancer folate was significantly higher than normal prostate tissue folate levels from cancer free controls. The mean serum folate level among the control group in our cohort (17.4 nM) approximates the mean folate level in the placebo group (22.7 nM) of the colon cancer chemoprevention study (4). In addition, the mean level of serum folate observed among prostate cancer patients (62.6 nM) approaches the post-folate supplementation serum levels (72 nM) from that study (4), suggesting that this cohort is representative of the U.S. population consuming folic acid supplements or large amounts of folic acid fortified food. Although folate is considered a water-soluble vitamin and therefore expected to be excreted when in excess, this may not be true in practice as a recent study indicated that 40% of older adults in the United States have unmetabolized serum folic acid that persists after fasting, the presence of which is not easily explained by folic acid intake alone (21).
The wide range of prostate tissue folate concentrations observed is of interest. Prostate tissue folate concentrations varied by greater than 40-fold (range: 0.006 pg/ug to 0.223 pg/ug). Within the confines of either the cancer group or the cancer-free group, the range of tissue folate concentrations varied by 19 and 13-fold, respectively. Thus, considerable variability in prostate tissue folate concentrations exists within this population. If prostate tissue folate levels are related to the risk of prostate carcinogenesis or progression, such a large variation between individuals may contribute to the variable risk seen in the population.
A significantly greater mean Ki67 staining index in grade matched (Gleason score 7) prostate cancers from men with high versus low serum folate concentrations was observed. Ki67, a proliferation marker, is strongly associated with the biological behavior of prostate cancer (22). Many studies have demonstrated the independent prognostic value of Ki67 staining both in clinically localized disease treated by radical prostatectomy (23)or radiation (18,24,25), and in patients with advanced disease (25,26). Most recently, Gunia et al. (26) reported that Ki67 SI >5% is an independent predictor of biochemical recurrence following radical prostatectomy. In a rat model of colorectal carcinogenesis, moderate folic acid supplementation positively correlated with tumor multiplicity and rectal epithelial cell proliferation (27). Kim’s “dual effect” hypothesis (28) proposes that prior to neoplastic transformation folate is protective, however once the tumor is initiated, folate drives cell division. Thus our results would support the second part of Kim’s hypothesis, implying that at least in prostate cancer patients, very high serum folate concentrations may potentiate tumor proliferation (29). In the presence of limiting levels of folate in vitro (≤50 nM), PSMA expression increases cell folate uptake and proliferation (30). We therefore examined if expression of PSMA in tumors varied according to serum folate level; however our non-significant findings suggest PSMA expression is not regulated by folate levels and therefore it is unlikely that PSMA directly contributes in to the increased proliferation seen in the patients with high serum folate.
As suggested by a recent study (31), the high serum folate levels observed in this cohort may indicate an underlying perturbation of one-carbon metabolism that supports prostate carcinogenesis in some individuals. The finding that 25% of the study cohort had more than 6-fold “adequate” serum folate levels (>81.72 nM) was unexpected. Such high levels are not likely to be achieved without long term folic acid supplementation, whether from consuming fortified foods or vitamin preparations. As half of the patients in this quartile reported that they do not take vitamin supplements, it is likely that their folate levels are due to sustained consumption of fortified food.
Although these observations are novel and may have important implications regarding folate supplementation in populations at risk for developing prostate cancer, the study has limitations. As a cross-sectional analysis, causality can only be implied, and not proven, by the correlations observed between folate concentrations and the presence and characteristics of prostate cancer. Unknown variables may be responsible for the observed differences in serum and prostate tissue folate concentrations rather than variances created by the effects of differences in the use of vitamin supplements or by tumor biology, as postulated. The number of subjects studied is limited, and it will be useful to confirm our observations in a larger cohort.
Given widespread dietary folate supplementation the relationship between folate intake and prostate cancer should be better defined.
Acknowledgments
We would like to thank Michelle Bisceglia and Brandy Greenawalt of the University of Pittsburgh Health Sciences tissue bank for their assistance in obtaining tissues.
Financial Support: DODPCRP W81XWH-07-1-0146 (DJB). NIH R21 CA124892 and American Institute for Cancer Research (DSOK), NIHR01CA138444 (DSOK and DJB) and NIH 5P20CA103730 (DSOK, DJB and RD). This work also supported by the U.S. Department of Agriculture, under agreement No. 581950-9-001 (JBM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure statement: Nothing to disclose
References
- 1.Figueiredo JC, Grau MV, Haile RW, Sandler RS, Summers RW, Bresalier RS, Burke CA, McKeown-Eyssen GE, Baron JA. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101(6):432–435. doi: 10.1093/jnci/djp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mason JB. Folate, cancer risk, and the Greek god, Proteus: a tale of two chameleons. Nutr Rev. 2009;67(4):206–212. doi: 10.1111/j.1753-4887.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ericson U, Borgquist S, Ivarsson MI, Sonestedt E, Gullberg B, Carlson J, Olsson H, Jirstrom K, Wirfalt E. Plasma Folate Concentrations Are Positively Associated with Risk of Estrogen Receptor {beta} Negative Breast Cancer in a Swedish Nested Case Control Study. J Nutr. doi: 10.3945/jn.110.124313. [DOI] [PubMed] [Google Scholar]
- 4.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, Snover DC, Church TR, Allen JI, Robertson DJ, Beck GJ, Bond JH, Byers T, Mandel JS, Mott LA, Pearson LH, Barry EL, Rees JR, Marcon N, Saibil F, Ueland PM, Greenberg ER. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297(21):2351–2359. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 5.Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention? Gut. 2006;55(10):1387–1389. doi: 10.1136/gut.2006.095463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulrich CM, Potter JD. Folate and cancer--timing is everything. JAMA. 2007;297(21):2408–2409. doi: 10.1001/jama.297.21.2408. [DOI] [PubMed] [Google Scholar]
- 7.Hultdin J, Van Guelpen B, Bergh A, Hallmans G, Stattin P. Plasma folate, vitamin B12, and homocysteine and prostate cancer risk: a prospective study. Int J Cancer. 2005;113(5):819–824. doi: 10.1002/ijc.20646. [DOI] [PubMed] [Google Scholar]
- 8.Johansson M, Appleby PN, Allen NE, Travis RC, Roddam AW, Egevad L, Jenab M, Rinaldi S, Kiemeney LA, Bueno-de-Mesquita HB, Vollset SE, Ueland PM, Sanchez MJ, Quiros JR, Gonzalez CA, Larranaga N, Chirlaque MD, Ardanaz E, Sieri S, Palli D, Vineis P, Tumino R, Linseisen J, Kaaks R, Boeing H, Pischon T, Psaltopoulou T, Trichopoulou A, Trichopoulos D, Khaw KT, Bingham S, Hallmans G, Riboli E, Stattin P, Key TJ. Circulating concentrations of folate and vitamin B12 in relation to prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition study. Cancer Epidemiol Biomarkers Prev. 2008;17(2):279–285. doi: 10.1158/1055-9965.EPI-07-0657. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein SJ, Hartman TJ, Stolzenberg-Solomon R, Pietinen P, Barrett MJ, Taylor PR, Virtamo J, Albanes D. Null association between prostate cancer and serum folate, vitamin B(6), vitamin B(12), and homocysteine. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1271–1272. [PubMed] [Google Scholar]
- 10.Pfeiffer CM, Johnson CL, Jain RB, Yetley EA, Picciano MF, Rader JI, Fisher KD, Mulinare J, Osterloh JD. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988–2004. Am J Clin Nutr. 2007;86(3):718–727. doi: 10.1093/ajcn/86.3.718. [DOI] [PubMed] [Google Scholar]
- 11.Ebbing M, Bonaa KH, Nygard O, Arnesen E, Ueland PM, Nordrehaug JE, Rasmussen K, Njolstad I, Refsum H, Nilsen DW, Tverdal A, Meyer K, Vollset SE. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302(19):2119–2126. doi: 10.1001/jama.2009.1622. [DOI] [PubMed] [Google Scholar]
- 12.Collin SM, Metcalfe C, Refsum H, Lewis SJ, Davey Smith G, Cox A, Davis M, Marsden G, Johnston C, Lane JA, Donovan JL, Neal DE, Hamdy FC, Smith AD, Martin RM. Associations of folate, vitamin B12, homocysteine, and folate-pathway polymorphisms with prostate-specific antigen velocity in men with localized prostate cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2833–2838. doi: 10.1158/1055-9965.EPI-10-0582. [DOI] [PubMed] [Google Scholar]
- 13.Ross JS, Sheehan CE, Fisher HA, Kaufman RP, Jr, Kaur P, Gray K, Webb I, Gray GS, Mosher R, Kallakury BV. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9(17):6357–6362. [PubMed] [Google Scholar]
- 14.Horne DW, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin Chem. 1988;34(11):2357–2359. [PubMed] [Google Scholar]
- 15.Tamura T, Freeberg LE, Cornwell PE. Inhibition of EDTA of growth of Lactobacillus casei in the folate microbiological assay and its reversal by added manganese or iron. Clin Chem. 1990;36(11):1993. [PubMed] [Google Scholar]
- 16.McCullough JL, Chabner BA, Bertino JR. Purification and properties of carboxypeptidase G 1. J Biol Chem. 1971;246(23):7207–7213. [PubMed] [Google Scholar]
- 17.Yao V, Parwani A, Maier C, Heston WD, Bacich DJ. Moderate expression of prostate-specific membrane antigen, a tissue differentiation antigen and folate hydrolase, facilitates prostate carcinogenesis. Cancer Res. 2008;68(21):9070–9077. doi: 10.1158/0008-5472.CAN-08-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowen D, Troncoso P, Khoo VS, Zagars GK, von Eschenbach AC, Meistrich ML, Pollack A. Ki-67 staining is an independent correlate of biochemical failure in prostate cancer treated with radiotherapy. Clin Cancer Res. 2002;8(5):1148–1154. [PubMed] [Google Scholar]
- 19.Varela-Moreiras G, Selhub J. Long-term folate deficiency alters folate content and distribution differentially in rat tissues. J Nutr. 1992;122(4):986–991. doi: 10.1093/jn/122.4.986. [DOI] [PubMed] [Google Scholar]
- 20.Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer. 2006;119(2):243–250. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- 21.Bailey RL, Mills JL, Yetley EA, Gahche JJ, Pfeiffer CM, Dwyer JT, Dodd KW, Sempos CT, Betz JM, Picciano MF. Unmetabolized serum folic acid and its relation to folic acid intake from diet and supplements in a nationally representative sample of adults aged >=60 y in the United States. Am J Clin Nutr. doi: 10.3945/ajcn.2010.29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zellweger T, Gunther S, Zlobec I, Savic S, Sauter G, Moch H, Mattarelli G, Eichenberger T, Curschellas E, Rufenacht H, Bachmann A, Gasser TC, Mihatsch MJ, Bubendorf L. Tumour growth fraction measured by immunohistochemical staining of Ki67 is an independent prognostic factor in preoperative prostate biopsies with small-volume or low-grade prostate cancer. Int J Cancer. 2009;124(9):2116–2123. doi: 10.1002/ijc.24174. [DOI] [PubMed] [Google Scholar]
- 23.Rubio J, Ramos D, Lopez-Guerrero JA, Iborra I, Collado A, Solsona E, Almenar S, Llombart-Bosch A. Immunohistochemical expression of Ki-67 antigen, cox-2 and Bax/Bcl-2 in prostate cancer; prognostic value in biopsies and radical prostatectomy specimens. Eur Urol. 2005;48(5):745–751. doi: 10.1016/j.eururo.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Khoo VS, Pollack A, Cowen D, Joon DL, Patel N, Terry NH, Zagars GK, von Eschenbach AC, Meistrich ML, Troncoso P. Relationship of Ki-67 labeling index to DNA-ploidy, S-phase fraction, and outcome in prostate cancer treated with radiotherapy. Prostate. 1999;41(3):166–172. doi: 10.1002/(sici)1097-0045(19991101)41:3<166::aid-pros3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Pollack A, DeSilvio M, Khor LY, Li R, Al-Saleem TI, Hammond ME, Venkatesan V, Lawton CA, Roach M, 3rd, Shipley WU, Hanks GE, Sandler HM. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy plus androgen deprivation: Radiation Therapy Oncology Group Trial 92–02. J Clin Oncol. 2004;22(11):2133–2140. doi: 10.1200/JCO.2004.09.150. [DOI] [PubMed] [Google Scholar]
- 26.Gunia S, Albrecht K, Koch S, Herrmann T, Ecke T, Loy V, Linke J, Siegsmund M, May M. Ki67 staining index and neuroendocrine differentiation aggravate adverse prognostic parameters in prostate cancer and are characterized by negligible inter-observer variability. World J Urol. 2008;26(3):243–250. doi: 10.1007/s00345-008-0257-0. [DOI] [PubMed] [Google Scholar]
- 27.Lindzon GM, Medline A, Sohn KJ, Depeint F, Croxford R, Kim YI. Effect of folic acid supplementation on the progression of colorectal aberrant crypt foci. Carcinogenesis. 2009;30(9):1536–1543. doi: 10.1093/carcin/bgp152. [DOI] [PubMed] [Google Scholar]
- 28.Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention? Gut. 2006;55(10):1387–1389. doi: 10.1136/gut.2006.095463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aaltomaa S, Karja V, Lipponen P, Isotalo T, Kankkunen JP, Talja M, Mokka R. Expression of Ki-67, cyclin D1 and apoptosis markers correlated with survival in prostate cancer patients treated by radical prostatectomy. Anticancer Res. 2006;26(6C):4873–4878. [PubMed] [Google Scholar]
- 30.Yao V, Berkman CE, Choi JK, O’Keefe DS, Bacich DJ. Expression of prostate-specific membrane antigen (PSMA), increases cell folate uptake and proliferation and suggests a novel role for PSMA in the uptake of the non-polyglutamated folate, folic acid. Prostate. 2010;70(3):305–316. doi: 10.1002/pros.21065. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein SJ, Mackrain K, Stolzenberg-Solomon RZ, Selhub J, Virtamo J, Albanes D. Serum Creatinine and Prostate Cancer Risk in a Prospective Study. Cancer Epidemiol Biomarkers Prev. 2009 doi: 10.1158/1055-9965.EPI-09-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]