Abstract
We sought to analyze the outcome of hemodynamically significant acute graft rejection in pediatric heart transplant recipients from a single-center experience. Acute graft rejection remains a major cause of morbidity and mortality for patients who undergo orthotopic heart transplantation and has been associated with the severity of the rejection episode. A retrospective review of all children experiencing a hemodynamically significant rejection episode after orthotopic heart transplantation was performed. Fifty-three patients with 54 grafts had 70 rejection episodes requiring intravenous inotropic support. Forty-one percent of these patients required high-dose inotropic support, with the remaining 59% of patients requiring less inotropic support. Overall graft survival to hospital discharge was 41% for patients in the high-dose group compared to 94% in the low-dose group. Six-month graft survival in patients who required high-dose inotropes remained at 41% compared to 44% in the low-dose group. Hemodynamically significant acute graft rejection in pediatric heart transplant recipients is a devastating problem with poor short- and long-term outcomes. Survival to hospital discharge is dismal in patients who require high-dose inotropic support. In contrast, survival to discharge is quite good in patients who require only low-dose inotropic support; however, six-month graft survival in this group is low secondary to a high incidence of graft failure related to worsening or aggressive transplant coronary artery disease.
Keywords: Acute graft rejection, Pediatric heart transplant
Background
Heart transplantation has been a surgical option for infants and children with heart disease since Dr. Adrian Kantrowitz and his group at Maimonides Medical Center in Brooklyn, New York performed the first pediatric orthotopic heart transplant in a 3-week-old child with Ebstein’s anomaly and pulmonary atresia on December 3, 1967 [6]. At this time, success was measured in hours and it was not until 1984 that Dr. Denton Cooley performed the first clinically successful infant heart transplant in an 8-month-old infant with subendocardial fibroelastosis [4]. In the intervening period, orthotopic heart transplantation has become an important component of the therapeutic armamentarium of the treatment of infants and children with congenital and acquired heart disease.
As surgical techniques have improved and advances have been made in immunosuppression, there has been a continual improvement in overall pediatric heart transplantation survival [1, 7]. Despite improved survival, acute graft rejection remains a major cause of morbidity and mortality for patients who have undergone orthotopic heart transplantation [13]. Survival after the first year posttransplant has not changed in the past 20 years despite the advent of new drug therapies and treatment strategies [7, 10]. Acute graft rejection is associated with repeated and sometimes prolonged hospitalizations, interventional procedures, and complex medical therapies, all of which have related morbidity risks and certainly negatively impact on a child’s quality of life. Furthermore, in the pediatric population, acute graft rejection is the leading cause of mortality from 30 days to 3 years posttransplantation [7]. The risk of an adverse outcome associated with an episode of rejection is related to the timing and severity of the event. The development of late rejection has been linked to transplant coronary artery disease (TCAD) and poor outcome [11, 14, 16]. In the pediatric population, studies have demonstrated that heart transplant recipients with a history of previous rejection episodes are at higher risk for symptomatic or fatal rejection [5]. Additionally, in pediatric patients experiencing late rejection following a previous episode of rejection, severe hemodynamic compromise has been identified as a risk factor for death [3, 11]. Finally, in studies evaluating the outcome of acute graft rejection, hemodynamically significant episodes requiring inotropic support resulted in a 1-year mortality of 30–50% [8, 12].
In this article we describe our single-center experience of the short- and long-term outcome of hemodynamically significant acute graft rejection in pediatric heart transplant recipients. Specifically, we hypothesize that the severity of hemodynamic compromise associated with an episode of acute rejection is directly related to graft loss and mortality.
Methods
Patient Population
A retrospective review of the pediatric heart transplant database at our institution was conducted with institutional review board (IRB) approval. All patients who required inotropic or mechanical support during an episode of acute graft rejection from June 1996 until February 2007 were identified and included for analysis. Demographic data obtained included the following: age at transplant, age at time of rejection episode, gender, both the number of prior rejections and the number of hemodynamically significant rejection episodes, the time from last rejection, the presence of TCAD, and graft function at baseline prior to the rejection episode.
Diagnosis of Rejection
Diagnosis of acute graft rejection was based on one or more of the following: clinical presentation, echocardiographic changes, hemodynamics at time of catheterization, and/or endomyocardial biopsy. Echocardiographic data routinely collected in the rejection surveillance protocol has been previously described and involves the computerized generation of a rejection score based on an m-mode-derived determination of left ventricle size, mass, function and systolic and diastolic wall motion [2, 15]. Valve regurgitation as well as the presence of pericardial or pleural effusions are also determined. Cardiac catheterization data include the mean right atrial pressure (RAP), the pulmonary capillary wedge pressure (PCWP), and endomyocaridal biopsy results at the time of rejection.
Definition of Hemodynamically Significant Rejection
Hemodynamically significant rejection was defined as an episode of acute graft rejection for which the patient required intravenous inotropic support while undergoing rejection treatment. These patients were then categorized based on level of inotropic support: (1) Low-dose support is defined as ≤2 inotropes [milrinone (≤0.5 mcg/kg/min) and/or dopamine or dobutamine at <10 mcg/kg/min] and (2) high-dose support is defined as epinephrine or vasopressin infusion, more than 2 inotropes simultaneously and/or dopamine/dobutamine infusion ≥10 mcg/kg/min.
Treatment of Rejection
Our standardized institutional protocol for all patients experiencing rejection regardless of hemodynamic significance includes anti-T-cell antibodies [antithymocyte globulin (ATG) or OKT3] for 7–10 days in combination with intravenous steroids (four doses) and intravenous immune globulin administration (IVIG, 0.5 g/kg). T-Cell antibody choice is based primarily on heterologous antibody levels and past rejection treatment history. Plasmaphoresis (five times volume exchange over 4 days) is utilized if the treating physician is concerned about a component of humoral rejection. Alemtuzumab (anti-CD52 antibody) along with photophoresis was utilized in select patients with resistant rejection. Patients treated with alemtuzumab received an initial dose of 12 mg/m2 on day 1 and 20 mg/m2 on days 3 and 5 for a total dose of 52 mg/m2. Photophoresis was performed twice weekly for a month following alemtuzumab treatment.
Outcome Variables
The outcome variables we examined for all episodes of hemodynamically significant rejection included the following: (1) graft survival to hospital discharge, (2) graft survival at 6 months and 1 year after episode, (3) graft function at follow-up, and (4) the diagnosis of new TCAD within 1 year of a hemodynamically significant rejection episode.
Statistics
Categorical variables were compared with chi-squared and Fisher’s exact tests, as appropriate. Continuous variables were compared using t-tests. A Wilcoxon rank sum test was used to compare the number of rejection episodes. Time to next rejection episode and time from first rejection to graft failure were compared with a log rank test. All analyses were performed using SAS Version 9.1.
Results
Patients
Of the 208 patients transplanted at our institution between 1996 and February 2007, 53 patients (25%) with 54 grafts had 70 rejection episodes requiring intravenous inotropic support (Table 1). There were 26 episodes (37%) in 22 patients/grafts (41%) that required high-dose inotropic support and 44 episodes in 32 patients/grafts that required low-dose support. There were a total of 25 female and 28 male patients who experienced hemodynamically significant graft dysfunction during a rejection episode. The mean graft age at the time of the rejection episode was 4.05 years (SD = ±3.23 years). There was no significant difference in time from transplantation in those who required high-dose (3.39 ± 0.63 years) or low-dose (4.44 ± 0.48 years) therapy (p = 0.19). Of the 53 patients, 43 had experienced at least one prior episode of rejection (the number of prior rejection episodes ranged from 0 to 16), with 12 patients (22.6%) experiencing more than one episode of hemodynamically significant rejection. The time between episodes of rejection for all patients ranged from 9 days to 11.1 years (mean = 1.5 years). For each individual episode of rejection, the time from the previous rejection episode (1.65 ± 0.71 years and 1.45 ± 0.3 years in the high- and low-dose inotrope groups, respectively, p = 0.76) and the total number of prior rejection episodes were not significantly different between the low-dose and high-dose groups.
Table 1.
Patients and variables
| Variable | High-dose group | Low-dose group | p-Value |
|---|---|---|---|
| Grafts | 22/54 (41%) | 32/54 (59%) | |
| Episodes | 26/70 (37%) | 44/70 (63%) | |
| Age at transplantation (years) | 6.8 ± 1.2 | 7.9 ± 0.95 | 0.43 |
| Range: 0.17–15.6 | Range: 0.08–18.8 | ||
| Graft age (years) | 3.39 ± 0.63 | 4.44 ± 0.48 | 0.19 |
| No. of grafts with prior rejection episodes | 12/26 | 39/44 | 0.13 |
| Time from previous rejection episode (days) | 604 ± 260 | 530 ± 110 | 0.76 |
Rejection Episode Characteristics
None of the echo parameters measured was significantly different between the high- and low-dose groups (Table 2). Of the 70 episodes of hemodynamically significant rejection, 29 episodes of rejection were evaluated by cardiac catheterization for hemodynamic assessment; endomyocardial tissue adequate for biopsy was obtained in 27 of these 29 episodes. Biopsy data alone are available for two additional patients. There was no difference in severity of rejection based on biopsy results and survival of the rejection episode. Biopsy results were not statistically worse in the high-dose group when compared with the low-dose group (Table 3; p = 0.08). Additionally, there was no difference in RAP or PCWP between the high- and low-dose groups. Baseline immunosuppresion and rejection treatment therapy were similar between the high- and low-dose groups (Table 4). The only difference between groups was the use of more IVIG in the low-dose group.
Table 2.
Rejection characteristics
| Variable | High-dose group | Low-dose group | p-Value |
|---|---|---|---|
| Prerejection FS | 33.78 ± 2.391 | 33.52 ± 1.716 | 0.93 |
| FS at time of diagnosis | 22.688 ± 13.638 | 23.944 ± 9.6469 | 0.94 |
| LVEDD | 44.168 ± 6.5936 | 43.31 ± 9.2057 | 0.69 |
| TR at time of diagnosis | 12 (3 moderate and 1 severe) | 37 (12 moderate and 1 severe) | 0.63 |
| MR at time of diagnosis | 9 (1 moderate and 1 severe) | 29 (4 moderate and no severe) | 0.23 |
| Mitral inflow abnormalities at time of diagnosis | 9 | 19 | 0.37 |
| RAP pressure (mmHg) | 14.333 ± 6.4395 | 17.15 ± 6.9757 | 0.38 |
| PCWP (mmHg) | 20.833 ± 4.9967 | 20.238 ± 6.6099 | 0.81 |
| Pericardial or pleural effusions at the time of diagnosis | 12 | 7 | 0.23 |
| Presence of TCD prior to rejection episode | 2 | 9 | 0.16 |
FS = fractional shortening; LVEDD = left ventricular end diastolic dimension; TR = tricuspid valve regurgitation; MR = mitral valve regurgitation; RAP = right atrial pressure; PCWP = pulmonary capillary wedge pressure; TCD = transplant coronary disease
Table 3.
Endomyocardial biopsy
| Biopsy grade | High-dose group (No. of patients) |
Low-dose group (No. of patients) |
|---|---|---|
| Zero | 1 | 7 |
| 1A | 3 | |
| 1B | 1 | 2 |
| 2A | 1 | |
| 2B | ||
| 3A | 1 | 7 |
| 3B | 1 | |
| 4 | 4 | 1 |
Table 4.
Medical therapy
| Treatment | Low dose | High dose | p-Value |
|---|---|---|---|
| Cyclosporine | 27 | 21 | 0.0936 |
| Mycophenolate mofetil | 25 | 16 | 0.7035 |
| Azathioprine | 7 | 4 | 0.9544 |
| Tacrolimus | 18 | 5 | 0.0635 |
| Sirolimus | 9 | 3 | 0.3461 |
| Steroids | 42 | 22 | 0.1209 |
| Antithymocyte globulin | 17 | 13 | 0.3605 |
| Muromonab-CD3 | 21 | 12 | 0.9004 |
| IVIG | 20 | 3 | 0.0031 |
| Alemtuzumab | 1 | 0 | ns |
| Plasmaphoresis | 19 | 11 | 0.9441 |
| Photophoresis | 1 | 1 | 0.7076 |
| TLI | 1 | 1 | 0.7076 |
Italic values are statistically significant
Outcome
High-Dose Inotrope Group
Of the 70 episodes of rejection, 22 patients (26 episodes, 39%) required high-dose inotrope support. The overall mortality rate for all patients undergoing an isolated episode of rejection requiring high-dose inotrope support was 45% (10/22). Three patients (13.6%) in the high-dose group underwent retransplantion prior to hospital discharge, resulting in 59% of grafts being lost in this group (or 41% graft survival). There was an overall patient survival to hospital discharge of 55% (12/22) in patients requiring high-dose inotrope support for acute graft rejection. Among all episodes of rejection requiring high-dose inotropes, for those surviving to discharge there was no additional graft loss in the first year following the episode of rejection, resulting in an overall probability of graft survival at 1 year of 57% with a mean follow-up of 2105 days with a range from 788 to 3505 days (Table 5).
Table 5.
Graft outcome
| Variable | High-dose group | Low-dose group |
|---|---|---|
| In-hospital mortality | 10/22 (45%) | 2/32 (6.3%) |
| Retransplantation | 3/12 (25%) | 7/30 (23%) |
| Out-of-hospital mortality | 0/12 (0%) | 9/30 (30%) |
| Graft survival at 6 months | 41% | 44% |
| Mean follow-up | 5.8 years (2.2–9.6 years) | 4.4 years (1.4–8.6 years) |
| Development of new/progression of TCAD | 1 patient—continued graft survival | 8 patients—all died before 6 months |
Of the 22 patients requiring high-dose support, 8 patients (36%) were placed on extracorporeal membrane oxygenation (ECMO) support. Five of eight patients were urgently placed on ECMO following an acute decompensation or cardiac arrest and four of these five patients survived decannulation. Of these four patients, one patient was retransplanted while on ECMO, two patients had only short-term survival postdecannulation (20 days and 4 months), and one patient continued to have graft survival at last follow-up (6.5 years after decannulation). Three patients were emergently placed on ECMO while undergoing active cardiopulmonary resuscitation. One of the three patients could not be successfully cannulated in a timely manner and attempts at additional resuscitation were ceased. Neither of the two additional patients survived decannulation. Of the eight patients who required ECMO support, seven of these patients were suffering their first episode of acute graft rejection. There was no difference in time from transplant, time from previous rejection episode, or graft function by echocardiography in those patients who required ECMO (shortening fraction 22.7 ± 2.8%) compared to those who did not (24 ± 1.4%; p = 0.73).
Low-Dose Inotrope Group
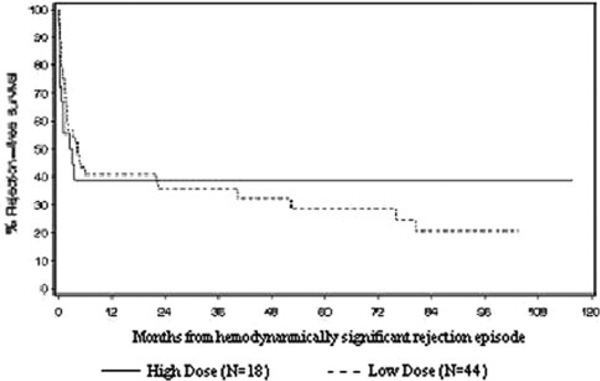
Only 2 of 32 patients in the low-dose group did not survive to hospital discharge (6.3% in hospital mortality). One additional patient in the low-dose group required retransplantation prior to hospital discharge. When patients receiving ECMO support are excluded, the in-hospital mortality for patients receiving high-dose support was significantly worse than those receiving low-dose support (p = 0.006). In contrast to the high in-hospital mortality of the patients in the high-dose group, patients who required low-dose inotropic support had an increased late mortality. Of the 30 patients who survived to hospital discharge, 9 died less than 6 months after their rejection episode (30%). Additionally, six patients in the low-dose group were retransplanted less than 6 months after discharge from their rejection episode. Therefore, for patients in the low-dose group, there is an overall 1-year graft survival of 44% with a mean follow-up of 4.4 years (range = 1.4–8.6 years; Fig. 1).
Fig. 1.
All rejection episodes to next rejection (p = 0.913)
Survivors of Hemodynamically Significant Episodes of Acute Graft Rejection
When comparing survivors to those who suffered graft loss, independent of the inotrope group in which they fell, survivors had a lower shortening fraction at time of diagnosis of graft rejection than those patients who lost their grafts (20.68 ± 1.8% vs. 25.85 ± 1.6%, p = 0.044). However, there was no difference in shortening fraction at the time of discharge for patients in the low-dose versus the high-dose group (28 ± 10.2% vs. 25.9 ± 14.3%, p = 0.23). In addition, there was no difference in shortening fraction when comparing survivors to those that suffered graft loss (28 ± 11.4% vs. 25.2 ± 13.4%, p = 0.19). Survivors in both groups demonstrated a trend toward a fewer number of total prior rejection episodes and a longer time period between episodes.
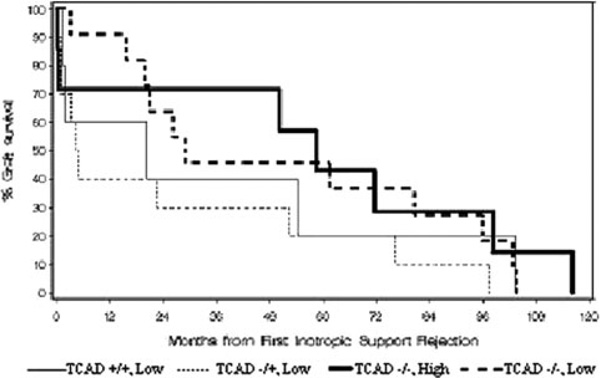
There was no difference in graft survival between those with and without documented TCAD at presentation with acute rejection (Fig. 2). Following treatment for the rejection episode and resolution of symptoms, eight of the nine patients in the low-dose group who died within 6 months of their episode of rejection had evidence of TCAD either prior to death or on autopsy. On autopsy, five of the nine patients in the low-dose group who died after discharge had previously undiagnosed TCAD ranging from one- to two-vessel disease of <50% to 75–100% occlusion of two major vessels. Patients in the low-dose group who suffered graft loss within 6 months of the rejection episode had progression of TCAD, compared to patients whose grafts survived (p = 0.0001).
Fig. 2.
From first rejection treatment to graft failure (no ECMO) (p = 0.316)
Effects of Era on Hemodynamically Significant Rejection Episodes
To determine if there was an era-based difference in outcome, patients were separated into two groups: those transplanted prior to 1999 (early era) and those transplanted in 1999 or later (late era). Thirty-eight of the 70 (54.3%) rejection episodes occurred in 31 patients transplanted prior to 1999. There was a significant difference in graft age at the time of rejection (5.5 ± 3.5 years early era vs. 2.3 ± 1.7 years late era; p < 0.001). The younger age at time of rejection in the late era is due to the increased number of infant recipients in that era. Although there was a trend toward increased use of ECMO in the late era, this difference was not significant (2/31 early era vs. 6/23 late era, p = 0.06). There was no difference between eras in graft loss prior to discharge from an episode of hemodynamically significant rejection (8/31 early era vs. 7/23 late era, p = 0.71) or graft loss at 6 months (17/31 early era and 12/23 late era 6-month graft loss, p = 0.85). There also was no apparent change in annual rate of those having at least one episode of hemodynamically significant rejection, according to year of transplant [slope of 1.0, 95% confidence interval (CI) = 0.92–1.08, p = 0.95], with a 20% rate for those transplanted in 1991, 13.6% for those transplanted in 2004 and rates between 5.6% and 33.3% for intermediate years.
Discussion
Hemodynamically significant acute graft rejection in pediatric heart transplant patients is associated with a mortality rate as high as 50% in previously published data [8, 12]. Therefore, early recognition and treatment of acute rejection is important in order to circumvent severe graft dysfunction necessitating inotropic support. Whether the degree of inotropic support required during an episode of acute graft rejection correlates with graft survival and long-term outcome in children is not known. The purpose of this study was to determine if outcome after an episode of hemodynamically significant rejection was dependent on the type and amount of cardiovascular support needed, characteristics of the rejection episode including echocardiographic, invasive hemodynamic or biopsy data, and presence or severity of TCAD.
There was no difference in the number of prior rejection episodes for patients in the low-dose versus high-dose inotrope group in this study. There was also no difference found in echocardiographic or invasive hemodynamic measurements for those requiring low-dose versus high-dose inotropic support. When comparing the survivors and those who had graft loss, independent of their inotrope dose group, survivors had a lower shortening fraction at diagnosis of graft rejection than those patients who lost their grafts (20.7 ± 1.8% vs. 25.9 ± 1.6%, p = 0.044), but shortening fraction at hospital discharge (or at time of graft loss) was no different (28 ± 11.4% survivors vs. 25.2 ± 13.4% graft loss group, p = 0.23). Although statistically significant, the systolic function of these two groups at diagnosis of rejection was not sufficiently different to warrant clinically diverse approaches to their support during treatment for rejection.
Overall survival in this group of pediatric recipients of heart transplantation suffering from an episode of hemodynamically significant acute graft rejection is poor. In-hospital mortality is particularly high for those patients requiring high-dose inotropic support and ECMO cannulation. Only a single patient (1/8) was able to be weaned from ECMO and demonstrate graft survival for greater than 6 months. These results are in contrast to the study from Texas Children’s Hospital, where a greater proportion of patients were successfully weaned from mechanical support. In their series, 88% of patients were able to be weaned from the device and 63% survived to hospital discharge. Despite the successful in-hospital support of this difficult group of patients, the 1-year survival for this cohort was 50% and 3-year survival was 38%, demonstrating the high mortality rates following an episode of hemodynamically unstable acute graft rejection [9]. As in our study, all patients successfully discharged home were placed on a form of mechanical support within 24 h of admission. The difference in outcome might in large part be due to our use of ECMO in comparison to the use of left ventricular assist devices utilized in the Morales [9] study. Additionally, ECMO was utilized in almost all of our cases as salvage with seven of the eight patients in our mechanical device arm experiencing at least one episode of cardiopulmonary collapse and resuscitation prior to cannulation. A more prompt utilization of mechanical support when indicated as well as the increasing availability and use of left ventricular assist devices in children might improve the in-hospital mortality of patients with hemodynamically significant rejection episodes.
An unexpected finding in this study was the fact that patients who required mechanical circulatory support had fewer prior episodes of rejection compared with those who were supported with inotropes. For three (38%) of the ECMO patients, this was their first rejection episode. As a result, it is possible that this led to a delay in recognizing and initiating treatment for acute rejection and might account for the fact that they presented in a severely decompensated state requiring prompt and aggressive resuscitation. However, when looked at as a separate subgroup, individuals whose first episode of rejection was hemodynamically significant did not have an increased mortality rate when compared with those who had suffered prior rejections (p = 0.47).
Although survival to hospital discharge was quite good in patients requiring only low-dose inotropic support, late graft failure was found to be significant. The diminished graft survival following hospital discharge in the low-dose group of patients was complicated by progressive or new onset TCAD. Given the high late mortality risk of patients who were supported only with low-dose inotropes, these data suggest that long-term graft function, including the coronary circulation, is compromised in these children.
Transplant coronary artery disease is an important cause of morbidity and mortality in children who have had a heart transplant and it is the leading cause of late mortality [7]. The presence of TCAD in this group of patients with hemodynamically significant acute graft rejection was associated with an increased risk of mortality. Following treatment for the rejection episode and resolution of symptoms, those who experienced graft loss had new onset or progression of TCAD, compared with patients whose grafts survived. These findings are irrespective of the degree of inotropic support required and suggest that closer TCAD surveillance might be warranted in all patients who have suffered a hemodynamically significant graft rejection episode.
There was no difference in graft outcome at hospital discharge or at 6 months postrejection when comparing patients from an early era and a late era. In addition, the annual rate of episodes of hemodynamically significant rejection has not changed over time, suggesting that this remains an important problem in the contemporary management of the pediatric recipient.
In addition to the limitations inherent in a retrospective study, there are several other limitations of this study. All patients in this study required significant inotropic support as part of their therapy for hemodynamically compromising acute graft rejection. Although the differentiation of the low- and high-dose inotropic support groups in this study is admittedly somewhat arbitrary, this allowed us the ability to assess the impact of relative inotropic support on graft outcome.
Conclusion
In conclusion, hemodynamically significant acute graft rejection in pediatric heart transplant recipients is a devastating problem with poor short- and long-term outcomes. Survival to hospital discharge is particularly low in patients who require high-dose inotropic support or ECMO cannulation. Although survival to discharge is quite good in patients who require only low-dose inotropic support, 6-month graft survival in this group is low, secondary to a high incidence of graft failure related to worsening or aggressive TCAD. We would postulate from these data that early diagnosis with prompt treatment of acute graft rejection could limit graft dysfunction, leading to better short-term outcome and successful graft survival to hospital discharge. Perhaps more sensitive tests for rejection such as gene expression assays or improved understanding of the pathophysiology of antibody mediated rejection will allow earlier and more targeted rejection therapy that could improve outcome. For those patients who require inotropic support, early consideration for elective initiation of mechanical circulatory support and subsequent increased surveillance for graft recovery and TCAD might also improve long-term outcomes in this difficult patient population.
Contributor Information
Christina M. Phelps, Email: Christina.phelps@nationwidechildrens.org, Children’s Hospital of Denver, Aurora, CO, USA.
Cecile Tissot, Children’s Hospital of Denver, Aurora, CO, USA.
Shannon Buckvold, Children’s Hospital of Denver, Aurora, CO, USA.
Jane Gralla, Children’s Hospital of Denver, Aurora, CO, USA.
D. Dunbar Ivy, Children’s Hospital of Denver, Aurora, CO, USA.
Biagio A. Pietra, Children’s Hospital of Denver, Aurora, CO, USA
Shelley D. Miyamoto, Children’s Hospital of Denver, Aurora, CO, USA
References
- 1.Boucek MM, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: tenth official pediatric heart transplantation report-2007. J Heart Lung Transplant. 2007;26(8):796–807. doi: 10.1016/j.healun.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Boucek MM, Mathis CM, et al. Serial echocardiographic evaluation of cardiac graft rejection after infant heart transplantation. J Heart Lung Transpl. 1993;12(5):824–831. [PubMed] [Google Scholar]
- 3.Chin C, Naftel DC, et al. Risk factors for recurrent rejection in pediatric heart transplantation: a multicenter experience. J Heart Lung Transpl. 2004;23(2):178–185. doi: 10.1016/S1053-2498(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 4.Cooley DA, Frazier OH, et al. Cardiac transplantation in an 8-month-old female infant with subendocardial fibroelastosis. JAMA. 1986;256(10):1326–1329. [PubMed] [Google Scholar]
- 5.Flippin MJ, Balzer DT, et al. Rejection with heart failure after pediatric cardiac transplantation. Ann Thorac Surg. 1999;68(1):176–180. doi: 10.1016/s0003-4975(99)00476-2. [DOI] [PubMed] [Google Scholar]
- 6.Kantrowitz A, Haller JD, et al. Transplantation of the heart in an infant and an adult. Am J Cardiol. 1968;22(6):782–790. [PubMed] [Google Scholar]
- 7.Kirk R, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: eleventh official pediatric heart transplantation report-2008. J Heart Lung Transpl. 2008;27(9):970–977. doi: 10.1016/j.healun.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Mills RM, Naftel DC, et al. Heart transplant rejection with hemodynamic compromise: a multiinstitutional study of the role of endomyocardial cellular infiltrate. Cardiac Transplant Research Database. J Heart Lung Transpl. 1997;16(8):813–821. [PubMed] [Google Scholar]
- 9.Morales DL, Braud BE, et al. Use of mechanical circulatory support in pediatric patients with acute cardiac graft rejection. Asaio J. 2007;53(6):701–705. doi: 10.1097/MAT.0b013e31815d68bf. [DOI] [PubMed] [Google Scholar]
- 10.Morales DL, Dreyer WJ, et al. Over two decades of pediatric heart transplantation: how has survival changed?”. J Thorac Cardiovasc Surg. 2007;133(3):632–639. doi: 10.1016/j.jtcvs.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 11.Mulla NF, Johnston JK, et al. Late rejection is a predictor of transplant coronary artery disease in children. J Am Coll Cardiol. 2001;37(1):243–250. doi: 10.1016/s0735-1097(00)01037-8. [DOI] [PubMed] [Google Scholar]
- 12.Pahl E, Naftel DC, et al. Death after rejection with severe hemodynamic compromise in pediatric heart transplant recipients: a multi-institutional study. J Heart Lung Transpl. 2001;20(3):279–287. doi: 10.1016/s1053-2498(00)00228-x. [DOI] [PubMed] [Google Scholar]
- 13.Shaddy RE, Naftel DC, et al. Outcome of cardiac transplantation in children. Survival in a contemporary multi-institutional experience. Pediatric Heart Transplant Study. Circulation. 1996;94(9) Suppl:II69–II73. [PubMed] [Google Scholar]
- 14.Smith R, Wray J, et al. Ten year survival after paediatric heart transplantation: a single centre experience. Eur J Cardiothorac Surg. 2005;27(5):790–794. doi: 10.1016/j.ejcts.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 15.Tantengco MV, Dodd D, et al. Echocardiographic abnormalities with acute cardiac allograft rejection in children: correlation with endomyocardial biopsy. J Heart Lung Transpl. 1993;12(6 Pt 2):S203–S210. [PubMed] [Google Scholar]
- 16.Webber SA, Naftel DC, et al. Late rejection episodes more than 1 year after pediatric heart transplantation: risk factors and outcomes. J Heart Lung Transpl. 2003;22(8):869–875. doi: 10.1016/s1053-2498(02)00819-7. [DOI] [PubMed] [Google Scholar]