Abstract
Cancer treatments are rapidly changing. Curative treatment for oesophageal adenocarcinoma currently involves surgery and cytotoxic chemotherapy or chemoradiotherapy. Outcomes for both regimes are generally poor as a result of tumor recurrence. We have reviewed the key signalling pathways associated with oesophageal adenocarcinomas and discussed the recent trials of novel agents that attempt to target these pathways. There are many trials underway with the aim of improving survival in oesophageal cancer. Currently, phase 2 and 3 trials are focused on MAP kinase inhibition, either through inhibition of growth factor receptors or signal transducer proteins. In order to avoid tumor resistance, it appears to be clear that targeted therapy will be needed to combat the multiple signalling pathways that are in operation in oesophageal adenocarcinomas. This may be achievable in the future with the advent of gene signatures and a combinatorial approach.
Keywords: Oesophageal adenocarcinoma, Signalling pathways, MAP and PI3 Kinase pathways, Wnt signalling, Transforming growth factor-β pathway, Nuclear factor-κB pathways, Transcription factors, Tyrosine kinase receptors
INTRODUCTION
Oesophageal adenocarcinoma is the 10th commonest malignancy in the UK yet it is the 5th commonest cause of cancer death[1]. This poor prognosis is partly attributable to a disease afflicting an elderly population. All too often the disease presents with symptoms of dysphagia which usually heralds advanced disease, typically with lymph node or distant metastases[2]. The 5 year survival, despite recent advances in neo-adjuvant chemotherapy, radiotherapy and surgery is approximately 25%. The incidence has been steadily increasing over the past 30 years[3-5]; this is thought to be due to the trend of an aging and increasingly obese population in combination with Helicobacter pylori eradication[1,6,7]. Barrett’s oesophagus has been established as a clear risk factor for oesophageal adenocarcinoma[8]. It has been demonstrated that surveillance of patients with Barrett’s oesophagus can identify early stage adenocarcinomas[9,10]. If diagnosed at an early stage, with the disease confined to the submucosa, 5 year survival rates are as high as 90%[11]. Unfortunately current strategies for surveillance of Barrett’s oesophagus are insufficient to reduce the incidence of oesophageal adenocarcinoma and most cases are diagnosed in patients that are not on Barrett’s surveillance programs[10]. This may be accounted for by the fact that a significant proportion of patients with Barrett’s oesophagus are asymptomatic. Currently, it is not economically viable to screen the whole population for Barrett’s oesophagus[12]. Until this is addressed, there does not seem to be a solution to providing an early diagnosis of oesophageal adenocarcinoma for the majority of patients. This indicates the importance of developing improved treatments for advanced disease.
CURRENT MEDICAL TREATMENTS
The medical therapies in mainstream use for the treatment of oesophageal and junctional adenocarcinomas are cytotoxic and antimetabolite agents. They target rapidly dividing cells in an non cancer cell specific manner[13]. 5-Fluorouracil (5-FU) inhibits DNA synthesis through inhibition of thymidylate synthetase[14]. The platinum agents cisplatin and oxaliplatin form DNA adducts and cross-links which prevents DNA transcription and replication[15]. The anthracyclines epirubicin and doxorubicin induce DNA damage and inhibit DNA transcription through inhibition of topoisomerase II and DNA helicase activity[16]. The cytotoxic action of taxanes are predominantly due to disruption of microtubules[17].
Cytotoxic chemotherapy is generally not very effective and side effects are common. The agents are usually contraindicated in severe cardiac and liver disease, a common occurrence in the affected elderly population. Recent advances have been made with the route of administration. A tablet form is now available, capecitabine, which is an effective alternative to infusing 5-FU. This reduces the morbidity associated with central venous catheterisation. Furthermore, oxaliplatin appears to be less toxic and more potent than cisplatin, and it can be infused over a shorter period of time[18,19]. Approximately 30% of patients with oesophageal adenocarcinoma are offered palliative chemotherapy and radiotherapy[1,20]. Prognosis is only 6-11 mo[21,22],
with a 5 year survival of 4%[22]. Surgery is beneficial in patients that present with disease localised to the oesophagus or with localised lymph node metastases. Neo-adjuvant chemotherapy modestly improves survival compared to surgery alone; 5 year survival is 23% compared to 36% with neo-adjuvant chemotherapy[23]. On subgroup analysis, patients with tumors at the gastro-oesophageal junction seemed to benefit the most and this regimen is offered in the UK[24]. In the USA, the protocol of neo-adjuvant chemo-radiotherapy is favoured[25]. 5 year survival is 8%-20% in selected patients. Curative chemo-radiation is an alternative strategy to surgery and prognosis is similar[2]. This may be due to the avoidance of postoperative mortality and morbidity. Whatever regimen is used, the poor prognosis for oesophageal adenocarcinoma is largely a result of disease recurrence and the morbidity surrounding major surgery[2]. Treatment failure is thought to be a consequence of the blanket therapy approach due to the nature of the non-specific or non-targeted mechanism of action of the medical agents described earlier. Recent evidence suggests that standard chemotherapy and radiotherapy activate signalling pathways that stimulate growth and resistance of cancer cells[26]. Prognosis may improve with agents that specifically target mitogenic signalling pathways and intense research is currently underway.
The aim of this review is to explore the key signalling pathways that are associated with oesophageal adenocarcinoma and review the clinical trials of novel therapeutic agents that are in progress. We will draw parallels from breast and colon cancer. We will address the question: by targeting key signalling pathways is personalised medicine a reality in oesophageal adenocarcinoma?
DEFINING SIGNALLING PATHWAYS
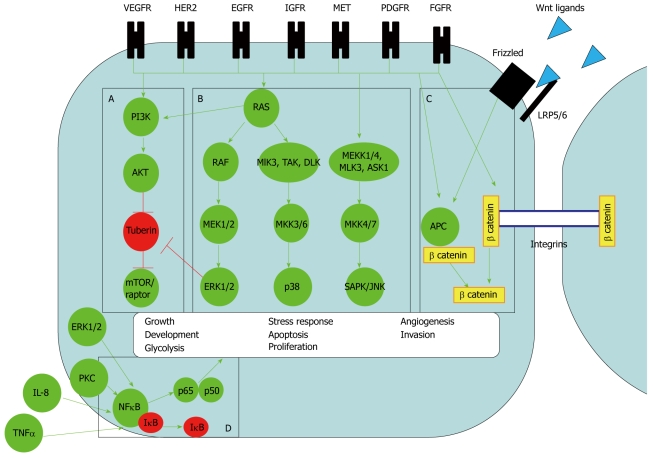
Signalling pathways are essential components in all cells; they are important to stimulate cell growth, proliferation, differentiation, invasion and apoptosis. Certain pathways are specifically important in embryonic development, inflammation and carcinogenesis. Signalling pathways have common mechanisms of action. They convey extra-cellular stimuli, usually via cell surface receptors, onto a chain of signal transducer proteins which subsequently enter the nucleus. In the nucleus the signalling proteins activate transcriptional machinery on gene promoters. Gene expression and cell phenotype are altered. In the context of cancer cells, phenotypic change may include cell growth, cell division, increased cell motility, evasion of apoptosis and sustained angiogenesis. These changes constitute the hallmarks of cancer[27]. Signalling pathways in cancer cells are usually unregulated and resistant to feedback inhibition, and this usually occurs as a consequence of sustained activation from their components. The components are commonly known as oncogenes or tumor suppressor genes[28]. Oncogenes and tumor suppressor genes are usually expressed as a result of genetic mutations. In oesophageal cancer, mutations occur as a consequence of DNA damage from bile or acid reflux, nitric oxide, alcohol and cigarette smoking. Mutations usually involve chromosomal translocations of oncogenes or tumor suppressor genes onto housekeeping genes or other genes undergoing active transcription[29,30]. This culminates in persistent activation or inhibition of specific signalling pathways. Components of signalling pathways can be potentially inhibited at a variety of levels. Inhibitors can target the cell surface receptor, signal transducer proteins or even transcription factors. Unfortunately multiple pathways and receptors are associated with oesophageal cancer and the complexity that exists between different pathways is likened to a computer circuit (Figure 1). Blockade of one pathway or component may not be sufficient.
Figure 1.
Signalling pathways in oesophageal adenocarcinoma. The pathways known to be operative in oesophageal adenocarcinoma are A: PI3-Kinase, B: Mitogen activated protein (MAP) Kinase, C: Wnt signalling and D: Nuclear factor-κB (NF-κB). The green arrows indicate activation, the red arrows indicate inhibition. Vascular epidermal growth factor (VEGF), human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor 1 (EGFR), insulin growth factor receptor (IGFR), hepatocyte growth factor receptor (MET), platelet derived growth factor receptor (PDGFR) and fibroblast growth factor receptor (FGFR) are the known receptor tyrosine kinase (RTK) associated with oesophageal adenocarcinomas. The pathways are complex and inter-connected. For example RTKs can activate all 4 known pathways. The ultimate biological response varies from cell proliferation, development, apoptosis, differentiation, inflammatory response, angiogenesis and invasion depending on which specific pathway or receptor is activated. A: PI3-Kinase pathway. RTK activates PI3-Kinase directly or through RAS. As a consequence of phosphorylation, PI3K recruits AKT. AKT inhibits tuberin, this allows activation of the Raptor/mTOR complex to activate transcriptional machinery. mTOR/raptor activates transcription factors and co-activator proteins cMyc and hypoxia inducible factor (HIF)-1α that drive the biological response of growth, proliferation, glycolysis, angiogenesis and invasion; B: MAP Kinase pathways. The 3 main MAP Kinase pathways are extracellular related kinase (ERK), p38 and JUN kinase (JNK). They are all activated by similar RTKs. The signalling pathways have a common feature of a cascade of phospho-proteins. A MAPKK Kinase (e.g. RAF), a MAPK Kinase [e.g. MAP ERK kinase (MEK)1/2] and a MAP Kinase (eg. ERK) transfer the signal onto multiple transcription factors on gene promoters[31]. ERK signalling for example can alter the gene expression of over 200 genes[32]. The subsequent biological response varies from cell proliferation, development, apoptosis, differentiation and inflammatory response, depending on the specific pathway activated; C: Wnt signalling. Wnt signalling exerts a biological response through release of β catenin into the nucleus with subsequent action on gene promoters. β catenin is released directly from RTK phosphorylation of Axin. Alternatively Wnt ligands bind to Frizzled receptors and form a complex with the LRP5/6 membrane receptor. The membrane receptor complex recruits dishevelled and axin from a cytoplasmic complex of dishevelled, Axin, adenomatous polyposis coli protein (APC) and β catenin. This allows release of β catenin; D: NF-κB pathway. NF-κB (p65p50) activates gene promoters only when released from IκB. Interleukin-8 (IL-8), tumour necrosis factor (TNF)α and radiation activate the pathway. Adding to complexity, ERK MAP Kinase and Wnt, through protein Kinase C, can also potentially activate NF-κB although this link has not been investigated in oesophageal adenocarcinoma.
KEY SIGNALLING PATHWAYS IN OESOPHAGEAL ADENOCARCINOMA
MAP-Kinase and PI3 kinase pathways
MAP-Kinase (MAPK) pathways are the most well described pathways in carcinogenesis. They are made up of three distinct pathways: ERK, SAP/JNK and p38[31] (Figure 1[31,32]). The pathways are normally activated by growth factors, temperature changes, cytokines and hypoxia via a variety of cell surface receptors[33]. In oesophageal cancer cells, gastric and bile acid[34,35] and the cytotoxic agent etoposide[36] are known to activate MAPK pathways. Cell surface receptors known to activate MAPK include receptor tyrosine kinase (RTK), G protein linked receptors and integrins[33]. Following activation of cell surface receptors a cascade of phospho-proteins is initiated via GTPase signal transducer proteins. RAS and RAF are examples of GTPase signal transducer proteins which act as a hub, receiving signals from many different cell surface receptors[28] (Figure 1). GTPase signal transducer proteins also amplify signals onward through multiple signalling pathways (Figure 1). MAPK pathways are often up-regulated in breast[37,38], ovarian[39] and prostate cancer[40]; however the impact on prognosis of MAPK signalling is sometimes conflicting. ERK MAPK is active in 60% of ERK MAPK oesophageal adenocarcinomas[36]. Tumors with active ERK MAPK signalling frequently have metastases and a worse prognosis. This suggests that blockade of ERK MAPK in oesophageal adenocarcinomas may have an important therapeutic role.
The PI3-Kinase (PI3K) pathway is activated by RTKs and RAS (Figure 1). Following RAS and/or RTK activation, AKT is phosphorylated by PI3K. Activation of the PI3K pathway stimulates cell growth, glycolysis, and proliferation[41] mainly through cMyc and hypoxia inducible factor 1α (HIF-1α) stimulation. Components of the PI3K pathway are up-regulated in oesophageal adenocarcinoma. The expression of phosphorylated AKT is increased in oesophageal adenocarcinoma tissue compared to normal epithelial and Barrett’s tissue[34]. PI3K pathway mutations are thought to occur in 6% of oesophageal adenocarcinomas[42]. Crosstalk exists between the MAPK and PI3K pathways at the levels of RAS and ERK (Figure 1). This is likely to play a role in drug resistance seen in therapies that target signal transducer proteins. Crosstalk indicates that inhibition of multiple pathways may be needed for effective anti-cancer therapy.
Mechanisms of sustained MAPK and PI3K activation
RTKs on the cell surface are key activators of MAPK and PI3K pathways. RTKs can be activated constitutively by dimerisation, by ligand activation or by receptor over-expression[43]. Alternatively RAS mutations can render the GTPase in its active form so that the signal is permanently switched on, resistant to the activity of cell surface receptors. RAS mutations occur in only 10% of oesophageal adenocarcinomas. Aberrant expressions of RTK are frequently associated with oesophageal adenocarcinoma and there are many different family members (Figure 1). Receptor over-expression is usually associated with disease recurrence and a poor prognosis. Human epidermal growth factor receptor 1 (EGFR) and human epidermal growth factor receptor 2 (HER2) are over-expressed in 50% of oesophageal adenocarcinomas and positive expression is associated with a poor prognosis and cytotoxic drug resistance[30,44]. High expression of the hepatocyte growth factor receptor (Met) predicts metastases and recurrence in resectable oesophageal adenocarcinoma[45]. Vascular endothelial growth factor receptors (VEGF) are commonly associated with oesophageal adenocarcinoma, VEGF is thought to be important in angiogenesis and correlates with tumor microvessel density, crucial for tumor growth. VEGF A and C expression indicates a poor prognosis[46]. The significance of insulin like growth factor receptors (IGFR) has not been studied in oesophageal adenocarcinoma[47,48], however low IFGR expression correlates with an improved survival in metastatic gastric adenocarcinoma. The platelet derived growth factor receptor (PDGF) has also been shown to be expressed in oesophageal adenocarcinoma[49]. In oesophageal squamous cell carcinoma, fibroblast growth factor receptors[50] and tropomyosin-related kinase receptors[51] are indicators of tissue invasion and chemo-resistance respectively. Each member of an RTK family may have up to 20 sub-types[52] and it has been demonstrated that oesophageal adenocarcinoma cells often co-express the different subtypes of RTKs[49]. Therefore a therapy that targets only one receptor may not be effective. Furthermore cancers are known to be heterogeneous, made up of a population of genetically different cells. Gene expression at the invasive site of a tumor is different from the centre[53] and the gene expression of primary tumors is different to those at metastatic sites[54]. The variety of RTKs and downstream MAPK signalling pathways indicates that complete blockade of such a complex and diverse system may be impossible. Growth inhibition is more pronounced in oesophageal adenocarcinoma cells treated with combined inhibition of the EGFR and IGFR compared to inhibition of either receptor in isolation[55]. Furthermore patients with co-expression of HER-2 and EGFR also have a worse outcome[56]. Even if drug inhibition of both IGFR and EGFR is successful, resistance will prevail if alternative receptors or signal transducer proteins are active. Tumor heterogeneity may explain the modest improvement in response and survival seen with agents directed towards a solitary receptor, discussed in more detail below.
Targeting the epidermal growth factor receptor 1
Gefitinib and Erlotinib: Gefitinib and Erlotinib are small molecular inhibitors of tyrosine kinase phosphorylation of EGFR (Figure 2). Gefitinib therapy has been investigated in metastatic oesophageal adenocarcinoma[57]. Two thirds of patients had prior standard cytotoxic chemotherapy of which half had received surgery. Partial response and stable disease (according to Response Evaluation Criteria of Solid Tumors) was achieved in 37% and the median survival was 4.5 mo[57]. It is difficult to compare small phase 2 clinical trials but results were not significantly different compared to treatment with combined cytotoxic chemotherapy in a similar cohort of patients. Partial response was 12.5% and a median survival of 5 mo was seen in patients treated with irinotecan with docetaxel[58]. Partial response was 29% and median survival was 6.4 mo in patients treated with irinotecan with 5-FU/leucovorin[59]. To understand the poor results seen with gefitinib, ERK MAPK and PI3K pathway activation was determined by immunohistochemistry. Staining for phospho-ERK and phospho-AKT was assessed before and after treatment in 7 patients. No differences in staining patterns were seen in tumors treated with gefitinib, suggesting that the two pathways were not inhibited by the drug. This result is mirrored in a larger study of 70 gastric adenocarcinomas treated with gefitinib[60]. This indicates ERK MAPK and PI3K pathway resistance to EGFR blockade. A further study conducted in 43 metastatic adenocarcinomas at the gastro-oesophageal junction treated with gefitinib[61] also had similar survival and response rates to the study by Ferry et al[57]. Trends for favourable outcome were more likely in tumors with expression of EGFR, ERK MAPK and PI3K signalling activation. This was assessed by immunohistochemistry prior to treatment although assessment was not made post treatment. The differences in outcome did not meet statistical significance, but this is likely due to the small sample size. Of the poor responders, 2 (9%) had k-RAS mutations. This study indicates the importance of patient selection with targeted therapy. On the contrary in gastric and oesophageal adenocarcinomas treated with a similar EGFR inhibitor, erlotinib (Figure 2), EGFR expression and PI3K signalling activation was not found to influence drug response[62]. The difference may be explained by different receptor specificity between gefitinib and erlotinib or it may indicate the activity of additional RTKs or other cell surface receptors
Figure 2.
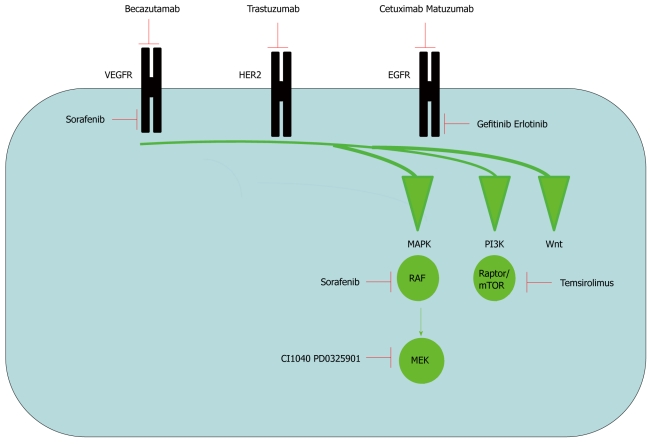
Drug inhibition of signalling pathways. Drugs that target signalling pathways can be divided into two groups. Antibodies [Becazutumab, trastuzumab, cetuximab and matuzumab are antibodies that target the receptor tyrosine kinase (RTK), vascular epidermal growth factor receptor (VEGFR), human epidermal growth factor receptor 2 (HER2) and epidermal growth factor receptor 1 (EGFR) respectively] and small molecular inhibitors (The small molecular inhibitors gefitinib and erlotinib target EGFR. Temsirolimus specifically inhibits the PI3-kinase pathway at the level of Raptor/mTOR. CI1040 and PD0325901 inhibits ERK MAP kinase pathway at the level of MEK. Sorafenib is a dual inhibitor of the VEGF receptor and ERK MAP kinase at the level of RAF).
Matuzumab: Matuzumab is a humanised monoclonal antibody that binds with the EGFR (Figure 2). Phase 1 trials have been conducted in metastatic oesophageal adenocarcinoma treated with conventional therapy with matuzumab[63]. EGFR was evident in 80%-100% of tumor specimens; however MAPK activity was not measured post treatment. This makes it difficult to assess the efficacy of the medication in the absence of survival data from this phase 1 study.
Cetuximab: Cetuximab is a monoclonal antibody directed against the EGFR (Figure 2), utilised in the treatment of advanced colorectal adenocarcinoma[64]. Trials have shown an improvement in average survival to 9 mo. RAS mutations occur commonly in colon cancer and account for resistance seen with cetuximab. When taken into account, colorectal carcinoma patients without k-RAS mutations have a significantly improved response to cetuximab compared to patient with k-RAS mutations which have survival times comparative to that of supportive care alone[65]. RAS mutations are less commonly seen in oesophageal adenocarcinomas and occur in less than 10% of cases so this is unlikely to account for the poor response seen with tyrosine kinase receptor inhibition[66]. Phase 2 clinical trials with cetuximab in advanced oesophageal adenocarcinoma have shown modest results similar to that seen with gefitinib[67].
Targeting the epidermal growth factor receptor 2
A Phase II trial has been conducted with trastuzumab, a monoclonal antibody targeted to targeting the epidermal growth factor receptor 2 (HER2) (Figure 2). Trastuzumab was tested in combination with cisplatin, paclitaxel and radiotherapy in locally advanced oesophageal adenocarcinoma[68]. Patients were selected and included those with HER2 expression on immunohistochemistry. 74% of patients had positive HER2 expression. Median survival was 24 mo and 50% survived for 2 years. The patient population was different to the patients treated with gefitinib and cetuximab. None of the patients had organ metastases and distant lymph node metastases were present in only 37%, which makes it difficult to make direct comparisons.
Targeting the vascular epidermal growth factor receptor
Becazutumab is a monoclonal antibody directed against the targeting of vascular epidermal growth factor receptor (VEGFR) (Figure 2). A phase 2 trial in metastatic gastric adenocarcinomas with 23 oesophageal junctional adenocarcinomas showed a response rate of 65% and median survival time of 12.3 mo[69]. Most patients were inoperable and the results were an improvement on standard cytotoxic therapies. Although VEGFR is frequently over-expressed in oesophageal adenocarcinomas[49], an assessment of VEGFR expression was not made prior to treatment. This may suggest that an improved outcome could be achieved by selecting tumors with high VEGFR expression.
Taken together, this may indicate that tailored RTK inhibition has a role in the treatment of selected patients with oesophageal adenocarcinomas. Initial trials have yet to make a significant impact and this may be down to poor patient selection and the use of growth factor receptor inhibitors in isolation.
Wnt signalling
Wnt signalling is important in cell growth, motility, angiogenesis, differentiation and other important phenotypic characteristics of cancer cells. Wnt ligands activate the Frizzled cell membrane receptor; Wnt is under feedback control from Wnt ligand inhibitors. Once activated, Frizzled forms a complex with another receptor LRP5/6 and recruits Dishevelled and Axin. The complex of APC, Axin and GSK and β-catenin is disrupted releasing unphosphorylated β-catenin (Figure 1). β-catenin can then enter the nucleus, and activate genes that stimulate growth, angiogenesis, invasion and cell cycle progression (c-Myc, COX2, MMP7 and Cyclin D1). Alternatively β-catenin can also be released by RTK phosphorylation of E-cadherin or Axin (Figure 1). Furthermore Wnt ligands can also directly activate calmodium kinase II and protein kinase C in turn releasing intracellular calcium or increasing JNK. Components of the pathway are altered in oesophageal adenocarcinoma. APC, Axin and Wnt ligand inhibitors are silenced by loss of heterozygosity or DNA methylation and collectively these events increase β-catenin activity. Although APC mutations are less commonly seen than in colorectal cancer, β-catenin or Wnt ligands are over-expressed in up to 77% of oesophageal adenocarcinomas[70,71]. This makes components of this pathway a potential target for drug inhibition.
Transforming growth factor-β pathway
Transforming growth factor-β (TGF-β) is a tumor suppressor gene and a potent inhibitor of cell growth. TGF-β binds to serine/threonine kinase type 1 and type 2 receptors. Upon binding to receptors, TGF-β forms a complex and phosphorylates intracellular signalling mediators called SMAD2/3. SMAD2/3 dissociates from the receptors and forms a complex with SMAD4 allowing it to enter the nucleus and regulate a large number of target genes. One target is SMAD7 which targets ubiquitin to the membrane receptor complex resulting in feedback inhibition of the pathway. Down regulation of SMAD4 has been shown in the progression of Barrett’s oesophagus to adenocarcinoma. TGF-β is anti-proliferative in some oesophageal cancer cell lines[72]. In contrast TGF-β expression has been demonstrated at the invasive margin of oesophageal adenocarcinomas and promotes cell invasion[73]. This may be explained by cross talk between the TGF-β pathway with PI3K, Wnt, PKC and the MAP-Kinase pathways. One potential mechanism is via SMAD7 inhibition leading to loss/diminished feedback inhibition of the pathway.
Nuclear factor-κB pathways
Nuclear factor-κB (NF-κB) is a proinflammatory transcription factor. It exists as a heterodimer p50/p65, situated in the cytoplasm under inhibitory control by IκB (Figure 1). Numerous activators have been identified including ERK MAPK signalling, cytokines (IL-8, TNF-α) and radiation. Specifically for oesophageal adenocarcinoma, bile salts and gastric acid have been shown to activate NF-κB. Gastrin has been shown to activate NF-κB through PKC signalling in gastric cancer cells[74]. Once activated, NF-κB enters the nucleus and through chromatin re-modelling it becomes a central regulator of many genes including cell cycle regulators (cyclin D1, cMyc, p53), inhibitors of apoptosis (Bcl-2), cytokines (interleukins, TNF-α), angiogenic mediators (COX2) and the growth factor receptor EGFR. Increased NF-κB expression is seen in Barrett’s oesophagus and adenocarcinoma. In oesophageal adenocarcinoma the expression correlates with chemo-radiation resistance[75].
Wnt signalling, TGF-β pathway and NF-κB pathways all activate important mediators in oesophageal adenocarcinoma. However there are no trials investigating the impact of specific inhibitors of these pathways outside the laboratory setting. RTK inhibitors have been investigated in oesophageal adenocarcinoma; however their role in Wnt signalling inhibition of β catenin has not been evaluated. The development of agents that inhibit alternative components of the Wnt, TGF-β and NF-κB signalling pathways are needed to avoid resistance and improve the modest responses seen with current therapies in clinical trials in oesophageal adenocarcinoma that focus on RTKs and MAPK.
POTENTIAL FUTURE TREATMENT TARGETS IN OESOPHAGEAL ADENOCARCINOMA
ERK MAPK inhibition by targeting MEK
An alternative approach is to target signal transducer proteins which may be downstream of many different RTKs. Theoretically this may reduce resistance of RTK co-expression. MEK is a downstream signal transducer protein of the ERK MAP Kinase pathway (Figure 1). No clinical trials have explored the role of MEK inhibition in oesophageal adenocarcinoma but lessons may be learned from trials in other cancers. Phase 2 clinical trials of the MEK inhibitor CI1040 (Figure 2) in advanced pancreatic, breast and non small cell lung cancer failed to make an impact on tumor progression[76]. Parallels can be drawn from colon cancer where RAS mutations reduced the efficacy of cetuximab. Patients treated with MEK inhibition were tested for ERK MAPK and PI3K signalling activation in archived samples, sometimes many months preceding treatment. This did not influence recruitment into the study and patients were enrolled if ERK or PI3K activation was judged to be low. Better patient selection may have resulted in a better response to treatment. ERK MAPK signalling activity was not assessed post treatment which may mean that the dosage was insufficient. More potent MEK inhibitors, such as PD0325901, have been evaluated in hepatocellular carcinomas[77]. Alternatively the poor responses with MEK inhibition may be explained by “cross talk” between different signalling pathways (Figure 2). Resistance to MEK inhibition may be explained by PI3-Kinase activation. The combination of MEK inhibition and PI3-Kinase inhibition is superior to treatments in isolation for inhibiting the growth of breast cancer cells[78]. This suggest that dual therapy is needed to combat both pathways.
ERK MAPK inhibition by targeting RAF and VEGF
A combined targeted approach may be beneficial in oesophageal adenocarcinoma. Sorafenib, a multifunctional kinase inhibitor, which acts on several growth regulatory pathways including VEGF and RAF (Figure 2), has been shown to be of benefit in renal cell carcinoma and hepatocellular carcinomas[78,79]. Sorafenib has been shown to inhibit key signalling pathways in SEG-1 lung adenocarcinoma cells[80]. This method of inhibition of both receptor and signalling protein such as RAF may prove beneficial due the diversity of growth factor receptors displayed by tumors and this approach may have a future role in the treatment of oesophageal adenocarcinoma.
PI3 kinase by targeting mTOR
No inhibitors of the PI3 kinase have been evaluated in oesophageal adenocarcinoma. Cell line studies in oesophageal adenocarcinoma have identified that the PI3 kinase pathway is important for cell growth. Mutations of the PI3 kinase pathway occur infrequently in oesophageal adenocarcinoma. However, activation of the pathway is known to occur from RTK, a common occurrence in oesophageal adenocarcinomas. Indeed in breast cancer, the PI3 kinase pathway has been proposed as a mechanism of drug resistance to MEK inhibition[55]. Inhibition of the PI3 kinase pathway has been used with success in metastatic renal cell carcinoma. Analogues of rapamycin have been developed to target mTOR (Figure 2). The agent temsirolimus has been evaluated in stage 3 clinical trials[81] (Figure 2). In this trial, 626 patients were divided into 3 groups; temsirolimus alone, interferon alone, and interferon in combination with temsirolimus. Overall survival was 10.9, 7.3 and 8.4 mo respectively in favour of temsirolimus. In view of this, a strategy of mTOR inhibition may have a future role in oesophageal adenocarcinoma.
Targeting transcription factors
Targeting an activated transcription factor or central regulator such as NF-κB would theoretically reduce the chance of the development of drug resistance from the activity of multiple surface receptors and multiple signalling pathways. This is not without problems. Firstly transcription factors are difficult to target. Interference RNA technology involves the insertion of an oligonucleotide into the nucleus of a cancer cell, usually using a viral vector. Oligonucleotides can be manufactured to complement the sequence and therefore dimerize with any RNA of interest such as NF-κB. This allows the targeting of transcription factor RNA with the prevention of protein translation. Interference RNA technology may be the answer to transcription factor inhibition but the technology remains in its infancy. The major hurdle appears to be the development of an efficient delivery system of oligonucleotides into cancer cells. Phase 1 trials are currently underway targeting VEGF in macular degeneration using direct ocular injection[82,83]. If this technology is developed in oesophageal cancer then gene expression profiling of tumors would be required to ensure that specific targeted therapy is delivered. The identification of more central regulators of carcinogenesis, such as HIF-1α and PEA3/ETV4 transcription factors, is likely to increase treatment options. The advent of gene expression profiling will certainly increase the number of potential targets.
CONCLUSION
An international effort is underway with the aim of improving survival in oesophageal adenocarcinoma by targeting key signalling pathways. Clinical trials using receptor tyrosine kinase inhibitors in oesophageal adenocarcinomas have so far only recruited patients with advanced or metastatic cancer. Studies utilised agents that inhibit solitary receptor tyrosine kinase, sometimes in an unselected manner. This strategy is problematic. At an advanced stage the heterogeneity within the tumor is extensive, which increases the likelihood of alternative signalling pathways resistant to receptor blockade. Secondly the pathway or receptor of interest may not be active or expressed, culminating in ineffective treatment. Tumor growth is immensely complex and this is emphasised in a study of 75 oesophageal adenocarcinoma specimens. Micro array studies identified 4 genes important in disease progression[84]. The genes independently predicted prognosis independent from traditional radiological methods. Unfortunately a further 115 genes were also indicators of survival. It is not clear what role the genes play in carcinogenesis; however this study indicates the complexity and diversity of the factors implicated in oesophageal adenocarcinoma development. Taken together, this suggests that a tailored combinatorial approach for treatment that inhibits multiple genes may be useful; therapies targeting either receptors, hub signalling proteins or even transcription factors, is likely to be necessary to deliver effective responses. By tailoring therapy to tumors that express specific gene and protein signatures and prescribing a regimen of treatments that act in fundamentally different mechanisms, then further improvements in survival are likely to be possible in oesophageal adenocarcinoma.
Footnotes
Supported by UK National Institute of Health Research/Cancer Research Network (UK NIHR/UKCRN) and Research and Development Department of Wrightington Wigan and Leigh NHS Foundation Trust (to Ang YS); R Keld Wrightington Wigan and Leigh NHS Foundation Trust Cancer Therapy Fund (to Keld RR, in part)
Peer reviewers: David Ian Watson, Professor, Head, Flinders University Department of Surgery, Room 3D211, Flinders Medical Center, Bedford Park, South Australia 5042, Australia; Marco Giuseppe Patti, MD, Professor of Surgery, Director, Center for Esophageal Diseases, University of Chicago Pritzker School of Medicine, 5841 S. Maryland Avenue, MC 5095, Room G 201, Chicago, IL 60637, United States
S- Editor Wang JL L- Editor O'Neill M E- Editor Ma WH
References
- 1.Office for National Statistics. Cancer Statistics registrations: Registrations of cancer diagnosed in 2006, England. Series MB1 No. 37. 2008 [Google Scholar]
- 2.Adams R, Morgan M, Mukherjee S, Brewster A, Maughan T, Morrey D, Havard T, Lewis W, Clark G, Roberts S, et al. A prospective comparison of multidisciplinary treatment of oesophageal cancer with curative intent in a UK cancer network. Eur J Surg Oncol. 2007;33:307–313. doi: 10.1016/j.ejso.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 4.el-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am. 2002;31:421–440, viii. doi: 10.1016/s0889-8553(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 5.Newnham A, Quinn MJ, Babb P, Kang JY, Majeed A. Trends in oesophageal and gastric cancer incidence, mortality and survival in England and Wales 1971-1998/1999. Aliment Pharmacol Ther. 2003;17:655–664. doi: 10.1046/j.1365-2036.2003.01520.x. [DOI] [PubMed] [Google Scholar]
- 6.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 7.Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1413–1417, 1417.e1-2. doi: 10.1016/j.cgh.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Solaymani-Dodaran M, Logan RF, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett's oesophagus and gastro-oesophageal reflux. Gut. 2004;53:1070–1074. doi: 10.1136/gut.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubenstein JH, Sonnenberg A, Davis J, McMahon L, Inadomi JM. Effect of a prior endoscopy on outcomes of esophageal adenocarcinoma among United States veterans. Gastrointest Endosc. 2008;68:849–855. doi: 10.1016/j.gie.2008.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper GS, Kou TD, Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. Am J Gastroenterol. 2009;104:1356–1362. doi: 10.1038/ajg.2009.159. [DOI] [PubMed] [Google Scholar]
- 11.Wang VS, Hornick JL, Sepulveda JA, Mauer R, Poneros JM. Low prevalence of submucosal invasive carcinoma at esophagectomy for high-grade dysplasia or intramucosal adenocarcinoma in Barrett's esophagus: a 20-year experience. Gastrointest Endosc. 2009;69:777–783. doi: 10.1016/j.gie.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett's esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:237–249. doi: 10.1093/aje/kwn121. [DOI] [PubMed] [Google Scholar]
- 13.Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, Hughes M, Mansi J, Findlay M, Hill A, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 14.Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–1664. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- 15.Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;25:4–12. [PubMed] [Google Scholar]
- 16.Sinha BK, Politi PM. Anthracyclines. Cancer Chemother Biol Response Modif. 1990;11:45–57. [PubMed] [Google Scholar]
- 17.Rowinsky EK, Onetto N, Canetta RM, Arbuck SG. Taxol: the first of the taxanes, an important new class of antitumor agents. Semin Oncol. 1992;19:646–662. [PubMed] [Google Scholar]
- 18.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 19.Sumpter K, Harper-Wynne C, Cunningham D, Rao S, Tebbutt N, Norman AR, Ward C, Iveson T, Nicolson M, Hickish T, et al. Report of two protocol planned interim analyses in a randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer receiving ECF. Br J Cancer. 2005;92:1976–1983. doi: 10.1038/sj.bjc.6602572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.2nd Annual Report of the National Oesophago-Gastric Cancer Audit 2009. The NHS Information Centre IC23090209 [Google Scholar]
- 21.Kulke MH, Wu B, Clark JW, Enzinger PC, Lynch TJ, Vincitore M, Michelini A, Fuchs CS. A phase II study of doxorubicin, cisplatin, and 5-fluorouracil in patients with advanced adenocarcinoma of the stomach or esophagus. Cancer Invest. 2006;24:229–234. doi: 10.1080/07357900600633924. [DOI] [PubMed] [Google Scholar]
- 22.Polee MB, Hop WC, Kok TC, Eskens FA, van der Burg ME, Splinter TA, Siersema PD, Tilanus HW, Stoter G, van der Gaast A. Prognostic factors for survival in patients with advanced oesophageal cancer treated with cisplatin-based combination chemotherapy. Br J Cancer. 2003;89:2045–2050. doi: 10.1038/sj.bjc.6601364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 24.Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 25.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 26.Brozovic A, Osmak M. Activation of mitogen-activated protein kinases by cisplatin and their role in cisplatin-resistance. Cancer Lett. 2007;251:1–16. doi: 10.1016/j.canlet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 28.Alberts BJ, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4 ed. Garland Science, 2002 [Google Scholar]
- 29.Chaganti RS. Significance of chromosome change to hematopoietic neoplasms. Blood. 1983;62:515–524. [PubMed] [Google Scholar]
- 30.Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, Menon A, Jing X, Cao Q, Han B, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–599. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 31.Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem Cell Biol. 2005;83:1–14. doi: 10.1139/o04-121. [DOI] [PubMed] [Google Scholar]
- 32.Mirzoeva OK, Das D, Heiser LM, Bhattacharya S, Siwak D, Gendelman R, Bayani N, Wang NJ, Neve RM, Guan Y, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69:565–572. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 34.Beales IL, Ogunwobi OO. Leptin synergistically enhances the anti-apoptotic and growth-promoting effects of acid in OE33 oesophageal adenocarcinoma cells in culture. Mol Cell Endocrinol. 2007;274:60–68. doi: 10.1016/j.mce.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Soma T, Kaganoi J, Kawabe A, Kondo K, Tsunoda S, Imamura M, Shimada Y. Chenodeoxycholic acid stimulates the progression of human esophageal cancer cells: A possible mechanism of angiogenesis in patients with esophageal cancer. Int J Cancer. 2006;119:771–782. doi: 10.1002/ijc.21917. [DOI] [PubMed] [Google Scholar]
- 36.Keld R, Guo B, Downey P, Gulmann C, Ang YS, Sharrocks AD. The ERK MAP kinase-PEA3/ETV4-MMP-1 axis is operative in oesophageal adenocarcinoma. Mol Cancer. 2010;9:313. doi: 10.1186/1476-4598-9-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gee JM, Robertson JF, Ellis IO, Nicholson RI. Phosphorylation of ERK1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. Int J Cancer. 2001;95:247–254. doi: 10.1002/1097-0215(20010720)95:4<247::aid-ijc1042>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 38.Milde-Langosch K, Bamberger AM, Rieck G, Grund D, Hemminger G, Müller V, Löning T. Expression and prognostic relevance of activated extracellular-regulated kinases (ERK1/2) in breast cancer. Br J Cancer. 2005;92:2206–2215. doi: 10.1038/sj.bjc.6602655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Givant-Horwitz V, Davidson B, Lazarovici P, Schaefer E, Nesland JM, Tropé CG, Reich R. Mitogen-activated protein kinases (MAPK) as predictors of clinical outcome in serous ovarian carcinoma in effusions. Gynecol Oncol. 2003;91:160–172. doi: 10.1016/s0090-8258(03)00434-7. [DOI] [PubMed] [Google Scholar]
- 40.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- 41.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips WA, Russell SE, Ciavarella ML, Choong DY, Montgomery KG, Smith K, Pearson RB, Thomas RJ, Campbell IG. Mutation analysis of PIK3CA and PIK3CB in esophageal cancer and Barrett's esophagus. Int J Cancer. 2006;118:2644–2646. doi: 10.1002/ijc.21706. [DOI] [PubMed] [Google Scholar]
- 43.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12:5268–5272. doi: 10.1158/1078-0432.CCR-05-1554. [DOI] [PubMed] [Google Scholar]
- 44.Gibson MK, Abraham SC, Wu TT, Burtness B, Heitmiller RF, Heath E, Forastiere A. Epidermal growth factor receptor, p53 mutation, and pathological response predict survival in patients with locally advanced esophageal cancer treated with preoperative chemoradiotherapy. Clin Cancer Res. 2003;9:6461–6468. [PubMed] [Google Scholar]
- 45.Tuynman JB, Lagarde SM, Ten Kate FJ, Richel DJ, van Lanschot JJ. Met expression is an independent prognostic risk factor in patients with oesophageal adenocarcinoma. Br J Cancer. 2008;98:1102–1108. doi: 10.1038/sj.bjc.6604251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleespies A, Bruns CJ, Jauch KW. Clinical significance of VEGF-A, -C and -D expression in esophageal malignancies. Onkologie. 2005;28:281–288. doi: 10.1159/000085198. [DOI] [PubMed] [Google Scholar]
- 47.Iravani S, Zhang HQ, Yuan ZQ, Cheng JQ, Karl RC, Jove R, Coppola D. Modification of insulin-like growth factor 1 receptor, c-Src, and Bcl-XL protein expression during the progression of Barrett's neoplasia. Hum Pathol. 2003;34:975–982. doi: 10.1053/s0046-8177(03)00354-x. [DOI] [PubMed] [Google Scholar]
- 48.Matsubara J, Yamada Y, Nakajima TE, Kato K, Hamaguchi T, Shirao K, Shimada Y, Shimoda T. Clinical significance of insulin-like growth factor type 1 receptor and epidermal growth factor receptor in patients with advanced gastric cancer. Oncology. 2008;74:76–83. doi: 10.1159/000139127. [DOI] [PubMed] [Google Scholar]
- 49.Gockel I, Moehler M, Frerichs K, Drescher D, Trinh TT, Duenschede F, Borschitz T, Schimanski K, Biesterfeld S, Herzer K, et al. Co-expression of receptor tyrosine kinases in esophageal adenocarcinoma and squamous cell cancer. Oncol Rep. 2008;20:845–850. [PubMed] [Google Scholar]
- 50.Yoshino M, Ishiwata T, Watanabe M, Matsunobu T, Komine O, Ono Y, Yamamoto T, Fujii T, Matsumoto K, Tokunaga A, et al. Expression and roles of keratinocyte growth factor and its receptor in esophageal cancer cells. Int J Oncol. 2007;31:721–728. [PubMed] [Google Scholar]
- 51.Tanaka K, Mohri Y, Nishioka J, Ohi M, Yokoe T, Miki C, Tonouchi H, Nobori T, Kusunoki M. Neurotrophic receptor, tropomyosin-related kinase B, as a chemoresistant marker in oesophageal cancer. Clin Oncol (R Coll Radiol) 2009;21:362–363. doi: 10.1016/j.clon.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 53.Tan X, Egami H, Abe M, Nozawa F, Hirota M, Ogawa M. Involvement of MMP-7 in invasion of pancreatic cancer cells through activation of the EGFR mediated MEK-ERK signal transduction pathway. J Clin Pathol. 2005;58:1242–1248. doi: 10.1136/jcp.2004.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albino AP, Le Strange R, Oliff AI, Furth ME, Old LJ. Transforming ras genes from human melanoma: a manifestation of tumour heterogeneity? Nature. 1984;308:69–72. doi: 10.1038/308069a0. [DOI] [PubMed] [Google Scholar]
- 55.Sutter AP, Höpfner M, Huether A, Maaser K, Scherübl H. Targeting the epidermal growth factor receptor by erlotinib (Tarceva) for the treatment of esophageal cancer. Int J Cancer. 2006;118:1814–1822. doi: 10.1002/ijc.21512. [DOI] [PubMed] [Google Scholar]
- 56.Kawaguchi Y, Kono K, Mimura K, Mitsui F, Sugai H, Akaike H, Fujii H. Targeting EGFR and HER-2 with cetuximab- and trastuzumab-mediated immunotherapy in oesophageal squamous cell carcinoma. Br J Cancer. 2007;97:494–501. doi: 10.1038/sj.bjc.6603885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferry DR, Anderson M, Beddard K, Tomlinson S, Atherfold P, Obszynska J, Harrison R, Jankowski J. A phase II study of gefitinib monotherapy in advanced esophageal adenocarcinoma: evidence of gene expression, cellular, and clinical response. Clin Cancer Res. 2007;13:5869–5875. doi: 10.1158/1078-0432.CCR-06-1970. [DOI] [PubMed] [Google Scholar]
- 58.Lordick F, von Schilling C, Bernhard H, Hennig M, Bredenkamp R, Peschel C. Phase II trial of irinotecan plus docetaxel in cisplatin-pretreated relapsed or refractory oesophageal cancer. Br J Cancer. 2003;89:630–633. doi: 10.1038/sj.bjc.6601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assersohn L, Brown G, Cunningham D, Ward C, Oates J, Waters JS, Hill ME, Norman AR. Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol. 2004;15:64–69. doi: 10.1093/annonc/mdh007. [DOI] [PubMed] [Google Scholar]
- 60.Rojo F, Tabernero J, Albanell J, Van Cutsem E, Ohtsu A, Doi T, Koizumi W, Shirao K, Takiuchi H, Ramon y Cajal S, et al. Pharmacodynamic studies of gefitinib in tumor biopsy specimens from patients with advanced gastric carcinoma. J Clin Oncol. 2006;24:4309–4316. doi: 10.1200/JCO.2005.04.2424. [DOI] [PubMed] [Google Scholar]
- 61.Janmaat ML, Gallegos-Ruiz MI, Rodriguez JA, Meijer GA, Vervenne WL, Richel DJ, Van Groeningen C, Giaccone G. Predictive factors for outcome in a phase II study of gefitinib in second-line treatment of advanced esophageal cancer patients. J Clin Oncol. 2006;24:1612–1619. doi: 10.1200/JCO.2005.03.4900. [DOI] [PubMed] [Google Scholar]
- 62.Dragovich T, McCoy S, Fenoglio-Preiser CM, Wang J, Benedetti JK, Baker AF, Hackett CB, Urba SG, Zaner KS, Blanke CD, et al. Phase II trial of erlotinib in gastroesophageal junction and gastric adenocarcinomas: SWOG 0127. J Clin Oncol. 2006;24:4922–4927. doi: 10.1200/JCO.2006.07.1316. [DOI] [PubMed] [Google Scholar]
- 63.Rao S, Starling N, Cunningham D, Benson M, Wotherspoon A, Lüpfert C, Kurek R, Oates J, Baselga J, Hill A. Phase I study of epirubicin, cisplatin and capecitabine plus matuzumab in previously untreated patients with advanced oesophagogastric cancer. Br J Cancer. 2008;99:868–874. doi: 10.1038/sj.bjc.6604622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 65.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 66.Hollstein MC, Peri L, Mandard AM, Welsh JA, Montesano R, Metcalf RA, Bak M, Harris CC. Genetic analysis of human esophageal tumors from two high incidence geographic areas: frequent p53 base substitutions and absence of ras mutations. Cancer Res. 1991;51:4102–4106. [PubMed] [Google Scholar]
- 67.Pinto C, Di Fabio F, Siena S, Cascinu S, Rojas Llimpe FL, Ceccarelli C, Mutri V, Giannetta L, Giaquinta S, Funaioli C, et al. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study) Ann Oncol. 2007;18:510–517. doi: 10.1093/annonc/mdl459. [DOI] [PubMed] [Google Scholar]
- 68.Safran H, Dipetrillo T, Akerman P, Ng T, Evans D, Steinhoff M, Benton D, Purviance J, Goldstein L, Tantravahi U, et al. Phase I/II study of trastuzumab, paclitaxel, cisplatin and radiation for locally advanced, HER2 overexpressing, esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2007;67:405–409. doi: 10.1016/j.ijrobp.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 69.Shah MA, Ramanathan RK, Ilson DH, Levnor A, D'Adamo D, O'Reilly E, Tse A, Trocola R, Schwartz L, Capanu M, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201–5206. doi: 10.1200/JCO.2006.08.0887. [DOI] [PubMed] [Google Scholar]
- 70.Clément G, Jablons DM, Benhattar J. Targeting the Wnt signaling pathway to treat Barrett's esophagus. Expert Opin Ther Targets. 2007;11:375–389. doi: 10.1517/14728222.11.3.375. [DOI] [PubMed] [Google Scholar]
- 71.Clément G, Braunschweig R, Pasquier N, Bosman FT, Benhattar J. Alterations of the Wnt signaling pathway during the neoplastic progression of Barrett's esophagus. Oncogene. 2006;25:3084–3092. doi: 10.1038/sj.onc.1209338. [DOI] [PubMed] [Google Scholar]
- 72.Onwuegbusi BA, Aitchison A, Chin SF, Kranjac T, Mills I, Huang Y, Lao-Sirieix P, Caldas C, Fitzgerald RC. Impaired transforming growth factor beta signalling in Barrett's carcinogenesis due to frequent SMAD4 inactivation. Gut. 2006;55:764–774. doi: 10.1136/gut.2005.076430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peters CR, Hardwick J, Hardwick R, Zhang J, Vowler C, Save S, O'donovan V, Caldas M, Alderson C, Fitzgerald D. On Behalf Of The OCCAMS Study Group, A seven gene signiture outperforms clinical features at predicting survival in oesophageal adenocarcinoma World Congress of Gastroenterology/UEGW, London, 2009 [Google Scholar]
- 74.Ogasa M, Miyazaki Y, Hiraoka S, Kitamura S, Nagasawa Y, Kishida O, Miyazaki T, Kiyohara T, Shinomura Y, Matsuzawa Y. Gastrin activates nuclear factor kappaB (NFkappaB) through a protein kinase C dependent pathway involving NFkappaB inducing kinase, inhibitor kappaB (IkappaB) kinase, and tumour necrosis factor receptor associated factor 6 (TRAF6) in MKN-28 cells transfected with gastrin receptor. Gut. 2003;52:813–819. doi: 10.1136/gut.52.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdel-Latif MM, Kelleher D, Reynolds JV. Potential role of NF-kappaB in esophageal adenocarcinoma: as an emerging molecular target. J Surg Res. 2009;153:172–180. doi: 10.1016/j.jss.2007.12.755. [DOI] [PubMed] [Google Scholar]
- 76.Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, Hamid O, Varterasian M, Asbury P, Kaldjian EP, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–4462. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 77.Wentz SC, Wu H, Yip-Schneider MT, Hennig M, Klein PJ, Sebolt-Leopold J, Schmidt CM. Targeting MEK is effective chemoprevention of hepatocellular carcinoma in TGF-alpha-transgenic mice. J Gastrointest Surg. 2008;12:30–37. doi: 10.1007/s11605-007-0396-4. [DOI] [PubMed] [Google Scholar]
- 78.Zhang T, Ding X, Wei D, Cheng P, Su X, Liu H, Wang D, Gao H. Sorafenib improves the survival of patients with advanced hepatocellular carcinoma: a meta-analysis of randomized trials. Anticancer Drugs. 2010;21:326–332. doi: 10.1097/CAD.0b013e3283350e26. [DOI] [PubMed] [Google Scholar]
- 79.Gollob JA, Rathmell WK, Richmond TM, Marino CB, Miller EK, Grigson G, Watkins C, Gu L, Peterson BL, Wright JJ. Phase II trial of sorafenib plus interferon alfa-2b as first- or second-line therapy in patients with metastatic renal cell cancer. J Clin Oncol. 2007;25:3288–3295. doi: 10.1200/JCO.2007.10.8613. [DOI] [PubMed] [Google Scholar]
- 80.Delgado JS, Mustafi R, Yee J, Cerda S, Chumsangsri A, Dougherty U, Lichtenstein L, Fichera A, Bissonnette M. Sorafenib triggers antiproliferative and pro-apoptotic signals in human esophageal adenocarcinoma cells. Dig Dis Sci. 2008;53:3055–3064. doi: 10.1007/s10620-008-0294-y. [DOI] [PubMed] [Google Scholar]
- 81.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 82.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grünweller A, Hartmann RK. RNA interference as a gene-specific approach for molecular medicine. Curr Med Chem. 2005;12:3143–3161. doi: 10.2174/092986705774933489. [DOI] [PubMed] [Google Scholar]
- 84.Peters CJ, Rees JR, Hardwick RH, Hardwick JS, Vowler SL, Ong CA, Zhang C, Save V, O'Donovan M, Rassl D, Alderson D, Caldas C, Fitzgerald RC. A 4-gene signature predicts survival of patients with resected adenocarcinoma of the esophagus, junction, and gastric cardia. Gastroenterology. 2010;139:1995–2004.e15. doi: 10.1053/j.gastro.2010.05.080. [DOI] [PubMed] [Google Scholar]