Abstract
AIM: To investigate the mechanism behind β-cell regeneration in neonatal rat pancreas treated with streptozotocin (STZ).
METHODS: Neonatal Sprague Dawley rats were intraperitoneally injected with 70 mg/kg STZ. Body weight, pancreas weight and blood glucose were recorded every two days after the treatment. To identify the expression and location of transcription factors in the rat pancreas, double immunofluorescent staining was performed using antibodies to specific cell markers and transcription factors.
RESULTS: Expression of Neurogenin 3 (Ngn3), a marker for endocrine precursor cells, was observed by immunofluorescence in a few β-cells and many α-cells. The expression reached a peak 12 d after treatment. Pax4, a transcription factor that lies downstream of Ngn3 and plays an important role in β-cell differentiation, was also expressed in the α-cells of STZ-treated rats. We did not observe significant changes in Nkx6.1, which is essential for β-cell maturation in the treated rats.
CONCLUSION: α-cells dedifferentiated into endocrine precursor cells and acquired the ability to dedifferentiate in the neonatal rat pancreas after STZ treatment.
Keywords: Pancreatic remodeling, Dedifferentiation, Endocrine precursor cells, Streptozotocin, Transcription factors
INTRODUCTION
The pancreas originates from gut endoderm. During development, the rat pancreas undergoes two transitions in embryonic days[1]. After birth, many major developmental changes occur, including β-cell apoptosis, replication, and exogenesis[2]. This stage is referred to as the remodeling of pancreas. In our previous work, we found alpha-fetoprotein and Mesothelin in embryonic rat pancreases but not in adult rat pancreases, nevertheless, we observed the expression of these two proteins during the remodeling of the rat pancreas[3,4]. These studies indicate that during the remodeling phase, the neonatal pancreas is not fully matured. Furthermore, after treatment with Streptozotocin (STZ) during this stage, the ontogeny of regeneration can be observed[5]. Conversely, after treatment with STZ during adulthood, little regeneration of β-cells was found[6]. Extensive studies have been reported on the model of STZ-induced depletion of β-cells in the neonatal rat pancreas, which showed that this model can be used to study β-cell replacement therapy for diabetes[5,7,8].
The development of β-cells is regulated by a series of transcription factors[9,10]. However, few studies have focused on the expression of these transcription factors during the regeneration of β-cells in STZ-treated neonatal rats. One of the most important upstream transcription factors is pancreas-duodenal homeobox 1 (Pdx1)[11]. The initial expression of Pdx1 (E8.5-E9.0) marks the pre-pancreatic endoderm before it is visibly thickened[12-14], and corresponds to the classically defined period of pancreatic specification[15]. Following the expression of Pdx1 is the Neurogenin 3 (Ngn3), a basic helix-loop-helix transcription factor[16] that marks endocrine pancreatic precursor cells. Among a series of transcription factors that differentiate endocrine precursors into β-cells, paired domain homeobox gene 4 (Pax4)[17] and NK family member Nkx6.1[18] lie downstream of Ngn3.
Although these factors are essential for the development and maturation of β-cells, it is unknown whether Ngn3, Pax4 and Nkx6.1 participate in the regeneration of β-cells after STZ treatment during the remodeling phase of the pancreas. Especially, there is little information on the differentiation factors that are involved in the remodeling of the rat pancreas. This study was designed to determine the expression and location of these transcription factors in the STZ-treated neonatal rat pancreas.
MATERIAL AND METHODS
Animals
Pregnant Sprague Dawley rats from the Animal Center of Nanjing Medical University, Nanjing, China, were kept under conventional conditions and provided with a 12:12 h light-dark cycle. Litters were reduced to 12 pups at birth. Four days after birth, half of the pups in each litter was intraperitoneally injected with 70 mg/kg STZ freshly dissolved in citrate buffer (0.05 mol/L, pH 4.5). The remaining pups received vehicle only. Blood glucose was measured with a OneTouch Ultra blood glucose meter (LifeScan Inc. Milpitas, CA, USA) in blood obtained by lancing the tail vein. Body weight was recorded every two days. On the day of treatment and days 4, 8, 12, 16 and 20 after treatment, animals were killed by decapitation or by overdose of anesthesia (sodium amobarbital, amytal sodium, Sigma-Aldrich 200 mg/kg body weight). Pancreases were collected immediately and frozen in liquid nitrogen or fixed. Three to five pups from at least three separate litters were studied at each time point. All experiments were conducted in accordance with the Chinese Law for Animal Protection and were approved by Nanjing Medical University Ethics Review Committee (approval No. 200913).
Fluorescence immunohistochemistry
Tissues were fixed in 4% paraformaldehyde for 24-36 h followed by a standard protocol of dehydration and paraffin embedding. Sections (5 μm) were cut and mounted on glass slides (Fisher Scientific, Pittsburgh, PA, USA). The paraffin sections were deparaffinized in xylene and dehydrated in graded ethanol and distilled water. The tissue sections were blocked in 1% bovine serum albumin for 1 h. For double fluorescence immunohistochemical localization of glucagon and insulin, the mouse anti-glucagon (1:100, Sigma-Aldrich, St. Louis, MO, USA) antibody was applied after blocking and revealed using goat anti-mouse IgG-FITC (1:400, Santa Cruz, Santa Cruz, CA, USA). Rabbit anti-insulin polyclonal antibody (1:100, Santa Cruz, Santa Cruz, CA, USA) was then applied and revealed by Cy3-labeled anti-rabbit IgG (1:400, Santa Cruz, Santa Cruz, CA, USA). For dual fluorescence immunohistochemical localization of Ngn3, Pax4 or Nkx6.1 and insulin, rabbit anti-neurogenin 3 (1:100, Santa Cruz, Santa Cruz, CA, USA), goat anti-Pax4 (1:100, Santa Cruz, Santa Cruz, CA, USA) or goat anti-Nkx6.1 (1:100, Santa Cruz, Santa Cruz, CA, USA) antibody were added, respectively, and revealed by rabbit anti-goat IgG-FITC (1:400, Chemicon, Temecula, CA, USA) or mouse anti-rabbit IgG-FITC (1:400, Chemicon, Temecula, CA, USA). Mouse anti-insulin (1:100, Sigma-Aldrich, St. Louis, MO, USA) was applied and revealed by Cy3 conjugated anti-mouse (1:400, Chemicon, Temecula, CA, USA) antibody. For co-localizations of Ngn3, Pax4 or Nkx6.1 and glucagon, rabbit anti-neurogenin 3 (1:100), goat anti-Pax4 (1:100) or goat anti-Nkx6.1 (1:100) antibodies were added and revealed by rabbit anti-goat IgG-FITC (1:400) or mouse anti-rabbit IgG-FITC (1:400). Mouse anti-glucagon (1:100) was applied and revealed by Cy3 conjugated anti-mouse (1:400) antibody. Sections were placed in Gel Mount Aqueous Mounting Medium (G0918, Sigma) with a cover glass, and were examined under an Olympus BX51 microscope (Olympus Optical, Tokyo, Japan) at a magnification of × 200 or × 400.
β-cell mass
β-cell mass was measured by point counting morphometry on the same stained sections as described above. Each section was covered systematically at a magnification of × 400 using a 48-point grid to obtain the number of grid intercepts over β-cells, endocrine non-β-cells, exocrine pancreatic tissues, and non-pancreatic tissues. The relative β-cell area was calculated by dividing the number of intercepts over β-cells by the number of intercepts over total pancreatic tissue; the β-cell mass was then estimated by multiplying the relative β-cell volume by the corrected pancreatic weight. Non-β-cell mass was similarly calculated. A correction factor for pancreas weight was obtained by multiplying the pancreas weight by the ratio of intercepts over non- pancreatic tissues to intercepts over total tissues. Actual pancreas weight was then calculated by subtracting this correction factor from total pancreas weight. A monogram related to the number of points, the volume density and the expected relative standard error of the mean (< 10%) was used to determine the number of intercepts needed for a representative sampling.
Statistical analysis
The experimental data was analyzed by paired Student t test using the SPSS 17.0 software. P < 0.05 was considered statistically significant. Data were presented as mean ± SD.
RESULTS
Body and pancreatic weight, blood glucose and islets in STZ-treated neonatal rat pancreases
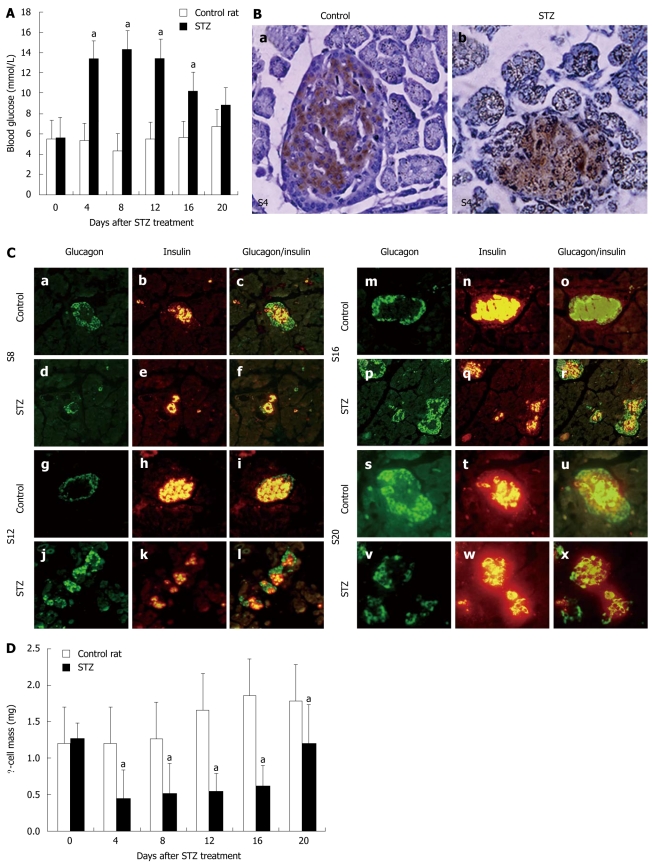
After STZ treatment, body and pancreas weight did not change significantly (Table 1). Blood glucose concentrations significantly increased within 2 d after STZ treatment (Figure 1A). However, on day 20 after treatment, there was no longer a difference in blood glucose concentrations between the two groups.
Table 1.
Body and pancreas weight in control and streptozotocin-treated animals after streptozotocin treatment on postnatal day 4 (mean ± SD)
|
Days after STZ treatment |
|||||
| 4 | 8 | 12 | 16 | 20 | |
| Body weight (g) | |||||
| Control | 13.6 ± 0.3 | 24.4 ± 1.5 | 30.5 ± 1.3 | 34.5 ± 1.5 | 50.0 ± 1.5 |
| STZ | 12.4 ± 0.3 | 22.3 ± 1.4 | 26.4 ± 1.3 | 32.4 ± 1.7 | 43.9 ± 1.5 |
| Pancreas weight (g) | |||||
| Control | 24.4 ± 2.3 | 49.8 ± 3.2 | 59.8 ± 1.4 | 60.5 ± 3.4 | 187.5 ± 6.9 |
| STZ | 18.7 ± 1.4 | 46.8 ± 3.3 | 57.1 ± 1.9 | 57.3 ± 3.2 | 162.5 ± 8.4 |
n = 3 litters/18 animals per group. STZ: Streptozotocin.
Figure 1.
Body and pancreatic weight, blood glucose, islets and β-cell mass in streptozotocin-treated neonatal rat pancreas. A: Concentrations of fasting blood glucose in control rats or rats treated with streptozotocin (STZ) between day 0 and day 20 after STZ treatment. Data were obtained from 12-18 animals at each time point. aP < 0.005 vs control; B: Immunohistochemical location of insulin in sections of rat pancreas in the control (a) and 4 d after STZ treatment; (b). Original magnification, × 400; C: Structure of islets in STZ-treated rats (d-f, j-l, p-r and v-x) and control rats (a-c, g-i, m-o and s-u) on d 8 (a-f), 12 (g-l), 16 (m-r) and 20 (s-x) after STZ treatment. Pancreatic sections were immunostained with anti-glucagon antibody (green) and anti-insulin antibody (red). Original magnification, × 400. Mean ± SD; D: β-cell mass in control rats or rats treated with STZ between day 0 and day 20 after STZ treatment. Data were obtained from three rats per time point. aP < 0.05 vs control rats.
Histological analysis showed that approximately 60% of insulin immunoreactive cells within the islets were lost 4 d after STZ treatment (Figure 1B). On day 8 after treatment, an increased number of small islets was observed (Figure 1C). On day 20 after treatment, more large islets were found, which may indicate that islet function had also recovered. Similarly, calculation of β-cell mass showed a reduction in β-cell mass from 4 d after STZ treatment onwards (Figure 1D). While β-cell mass was still reduced in STZ-treated rats on day 20 after treatment, blood glucose levels were not significantly different.
Expression and location of Ngn3
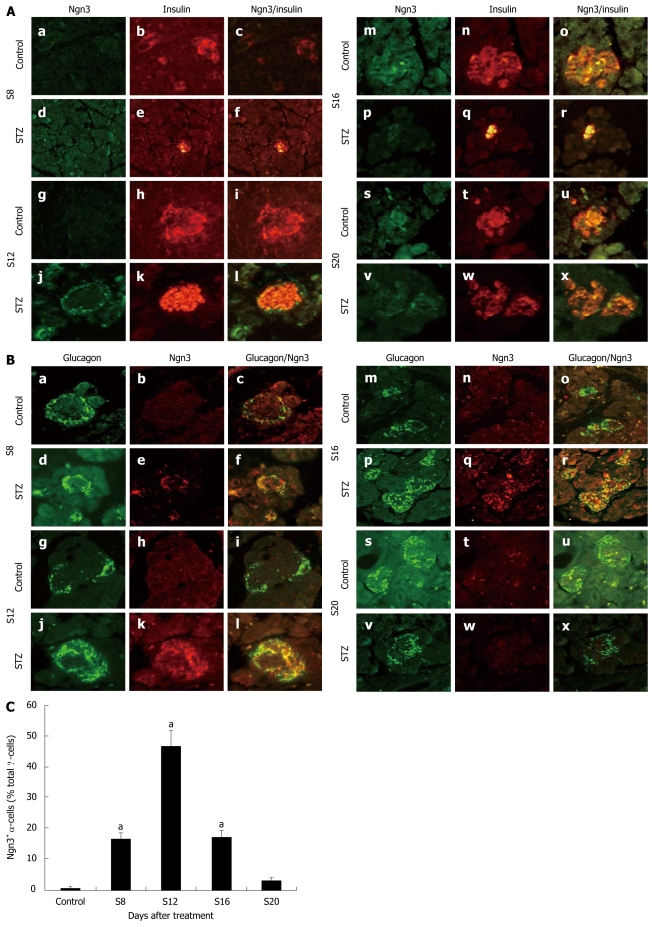
We used double immunofluorescence to stain Ngn3 and insulin or glucagon at different time points after STZ treatment. We did not find Ngn3 co-located with insulin in either treated or control rats (Figure 2A). By analyzing the coexpression of Ngn3 and glucagon, we observed abundant expression of Ngn3 in the treated rat islet α-cells (Figure 2B). In the STZ group, expression of Ngn3 could be detected on day 8 and reached a peak on day 12 after treatment (Figure 2C). However, no significant changes were observed in the signal from Ngn3 in α-cells 20 d after treatment compared with the control rats. In contrast, few α-cells expressed Ngn3 in control rats at each time point.
Figure 2.
Expression and location of Ngn3. A: Immunofluorescent colocalization of Ngn3 and insulin in streptozotocin (STZ)-treated rats (d-f, j-l, p-r and v-x) and control rats (a-c, g-i, m-o and s-u) on d 8 (a-f), 12 (g-l), 16 (m-r) and 20 (s-x) after STZ treatment. Pancreatic sections were immunostained with anti-Ngn3 antibody (green) and anti-insulin antibody (red). Original magnification, × 400; B: Immunofluorescent colocalization of Ngn3 and glucagon in STZ-treated rats (d-f, j-l, p-r and v-x) and control rats (a-c, g-i, m-o and s-u) on d 8 (a-f), 12 (g-l), 16 (m-r) and 20 (s-x) after STZ treatment. Pancreatic sections were immunostained with anti-glucagon antibody (green) and anti-Ngn3 antibody (red). Original magnification, × 400; C: Proportion of Ngn3+ / glucagon+ cells in total α-cells after treatment (Y). aP < 0.05 vs control rats.
Expression and location of Nkx6.1
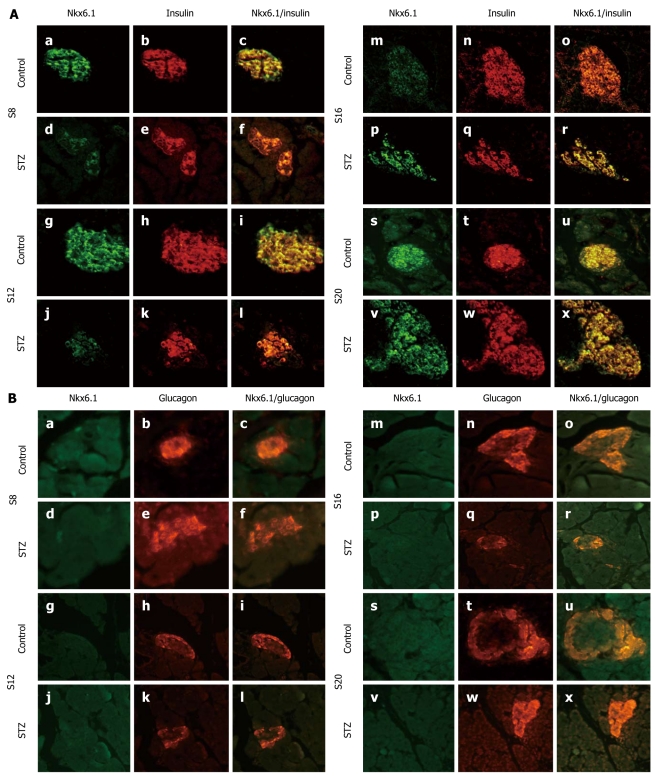
We stained Nkx6.1 and glucagon or insulin by immunofluorescence. Consistent with previous work, we found coexpression of Nkx6.1 and insulin in both the controls and the treated group (Figure 3A), while no Nkx6.1 expression was found in α-cells at any time point (Figure 3B) when we studied the coexpression of glucagon and Nkx6.1.
Figure 3.
Expression and location of Nkx6.1. A: Immunofluorescent colocalization of Nkx6.1 and insulin in streptozotocin (STZ)- treated rats (d-f, j-l, p-r and v-x) and control rats (a-c, g-i, m-o and s-u) on d 8 (a-f), 12 (g-l), 16 (m-r) and 20 (s-x) after STZ treatment. Pancreatic sections were immunostained with anti-Nkx6.1 antibody (green) and anti-insulin antibody (red); B: Immunofluorescent colocalization of Nkx6.1 and glucagon in STZ-treated rats (d-f, j-l, p-r and v-x) and control rats (a-c, g-i, m-o and s-u) on d 8 (a-f), 12 (g-l), 16 (m-r) and 20 (s-x) after STZ treatment. Pancreatic sections were immunostained with anti-Nkx6.1 antibody (green) and anti-glucagon antibody (red). Original magnification, × 400.
Expression and location of Pax4
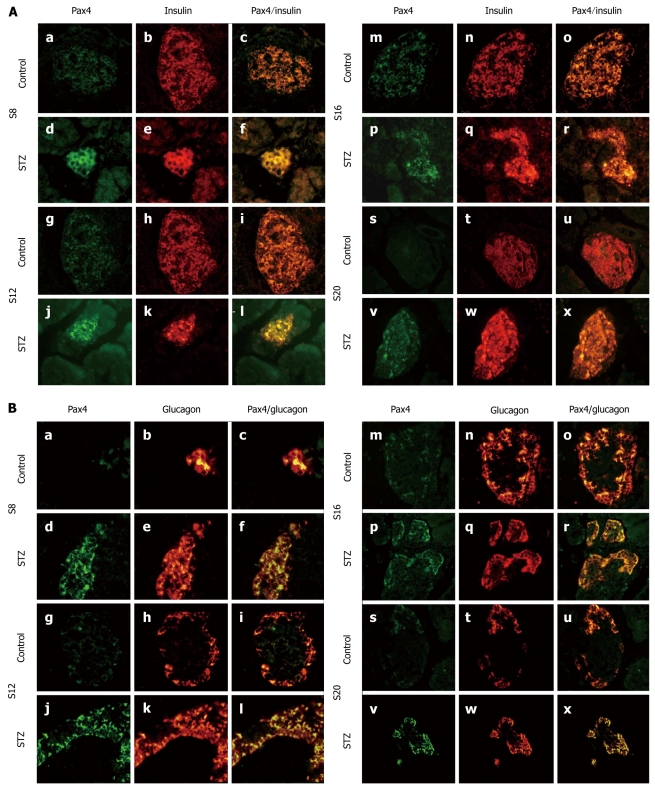
We studied the colocation of Pax4 and insulin or glucagon by dual immunofluorescence. Consistent with previous work, we observed coexpression of insulin and Pax4 in both the control group and the treated group (Figure 4A). We also found enhanced expression of Pax4 in STZ-treated rat pancreases compared with control rats (Figure 4A). Eight days after treatment, we observed expression of Pax4 in α-cells of the treated rats but little expression in the control rats. However, we found coexpression of glucagon and Pax4 in both treated and control rats on day 12 after treatment (Figure 4B). On day 20 after STZ treatment, we could still observe a signal of Pax4 in the α-cells. However, in the control rats, few α-cells expressed Pax4 on day 20.
Figure 4.
Expression and location of Pax4. A: Immunofluorescent colocalization of Pax4 and insulin in streptozotocin (STZ)-treated rats (d-f, j-l, p-r and v-x) and control rats (a-c, g-i, m-o and s-u) on d 8 (a-f), 12 (g-l), 16 (m-r) and 20 (s-x) after STZ treatment. Pancreatic sections were immunostained with anti-Pax4 antibody (green) and anti-insulin antibody (red); B: Immunofluorescent colocalization of Pax4 and glucagon in STZ-treated rats (d-f, j-l, p-r and v-x) and control rats (a-c, g-i, m-o and s-u) on d 8 (a-f), 12 (g-l), 16 (m-r) and 20 (s-x) after STZ treatment. Pancreatic sections were immunostained with anti-Pax4 antibody (green) and anti-glucagon antibody (red). Original magnification, × 400.
DISCUSSION
It is established that neonatal β-cells are able to regenerate after subtotal β-cell damage by STZ treatment. Regeneration of neonatal β-cells after destruction mainly relies on replication of pre-existing β-cells and heterogenesis of new cells[19]. In this article, we demonstrated a series of transcription factors expressed in pancreatic α-cells, which suggested that α-cells may be a source of β-cells during the regeneration of the STZ-treated neonatal rat pancreas.
We found that β-cells were damaged 4 d after STZ treatment. On day 8 after treatment, β-cell numbers were recovered in STZ-treated rats. By day 20 after treatment, there was still a reduction in β-cell mass but the blood glucose concentrations had reverted to normal. Although the model resulted in transient hyperglycemia, no difference in the mean body weight or pancreatic weight was seen between the two groups.
The study of pancreatic development has focused on transcription factors and transcription factor hierarchies during development. A central and heavily studied transcription factor in pancreatic development is Pdx1. Although Pdx1 is a key component of pancreatic specification, we found no significant difference in α or β-cells between STZ-treated rats and control animals. This indicates that Pdx1 is not involved in the regeneration of β-cells in STZ-treated animals.
After pancreatic formation, which is mediated by Pdx1, Ngn3 regulates the differentiation of endocrine pancreas. Lack of Ngn3 leads to an absence of islets[20]; and the ectopic expression of Ngn3 in other cells converts these cells into endocrine cells[21]. We observed Ngn3 expression in the α-cells of treated rats, which reached a peak on day 12 after STZ treatment. It has been reported that Ngn3 is activated through partial duct ligation in the adult mouse pancreas[22]. However, Ngn3 is absent in the β-cells of mice that underwent partial pancreatectomy[23]. The different results among these three models indicate that the mechanism of β-cell regeneration in different pathological situations can be varied. Interestingly, we observed abundant expression of Ngn3 in STZ-treated rats but not in control rats. The expression of Ngn3 in α-cells after STZ treatment indicated that α-cells dedifferentiated into precursor cells and may be candidates for β-cell formation. It has been suggested that high Ngn3 expression at an inappropriately early time in the developing mouse pancreas may result in a pancreas entirely consisting of small clusters of glucagon-positive cells[21,24]. Furthermore, by overexpression of Ngn3 in human[25] or mouse[26,27] pancreatic duct cells, the pancreatic duct cells could become endocrine cells. Apparently, the expression of Ngn3 is necessary for the transdifferentiation of α-cells to β-cells.
Next to Ngn3 induction, a complex network of transcription factors, including Pax4, progressively and differentially promotes the particular endocrine fates[28,29]. The expression of Pax4 is first observed around E9.5 in dorsal pancreatic buds of mouse embryos and vanishes shortly after birth[29,30]. Pax4 specifies the β-cell lineage into β and δ precursor cells[31]. In mice lacking Pax4, mature pancreatic and δ-cells were absent[29]. Conversely, ectopic expression of Pax4 in the mouse pancreas converted α-cells into β-cells. Moreover, the transgenic adult mice could survive after STZ-induced hyperglycemia[32]. Activation of Pax4 in endocrine progenitor cells may be mediated by Ngn3 since it binds to the Pax4 regulatory region and is necessary for Pax4 expression[33]. Ngn3 is required for ectopic Pax4 expressing α-cells to acquire a β-cell phenotype[34]. Interestingly, we observed expression of Pax4 in α-cells of both control and STZ-treated rats. In the STZ-treated rats, the expression of Pax4 reached a peak on day 16 after the treatment. The expression of Pax4 in the control animals suggests that Pax4 expression is characteristic of the pancreatic remodeling phase. Both cell differentiation and maturation occur during remodeling of the pancreas, which explains the presence of transcription factors in islet cells. After STZ treatment, the expression of Pax4 increased and a Pax4 signal could still be observed in α-cells 20 d after the treatment. This suggests that STZ treatment exaggerates and extends the period of remodeling.
Another transcript factor which lies downstream of Ngn3 is Nkx6.1, and it is associated with the development and maturation of β-cells. Nkx6.1 appears to be a marker for multipotent pancreatic progenitor cells[35]. At later developmental stages and in the adult pancreas, Nkx6.1 becomes completely restricted to insulin-expressing cells. Consistent with previous researches, we observed coexpression of Nkx6.1 and insulin in both STZ-treated and control rats. However, we did not find any Nkx6.1-positive α-cells. Immunoblotting revealed that the expression of Nkx6.1 decreased 4 d after STZ treatment, and reached normal levels on day 12 after treatment. Although Nkx6.1 is critical for the development of β-cells, it does not affect the generation of β-cells from α-cells.
It has been established that regeneration of neonatal rat β-cells after subtotal destruction by STZ occurs by two mechanisms of equal significance. The first mechanism is the replication of surviving β-cells in the islet compartment, the second mechanism is the replication of cells from a β-cell pool outside the islet compartment. In this article, we have demonstrated that α-cells may also be a source for β-cell regeneration. Mature α-cells converted to β-cells after partial duct ligation plus alloxan treatment, and the contribution of α-cells to the emergence of new β-cells was proportional to the degree of β-cell ablation[36]. However, α-cells could only convert to β-cells when the proportion of β-cell loss reached 99%[37].
In conclusion, during the period of pancreatic remodeling, the islets are not completely matured and the dedifferentiation of α-cells into endocrine precursor cells contributes to the recovery of β-cell mass after impairment by STZ.
COMMENTS
Background
Streptozotocin-induced β-cell loss in neonatal rat pancreas can trigger transient hyperglycemia. β-cell mass recovers 20 d after treatment. It is unknown whether Ngn3, Pax4 and Nkx6.1 participate in the regeneration of β-cells after streptozotocin (STZ) treatment during the remodeling phase of the pancreas.
Research frontiers
It has been shown that mature α-cells converted to β-cells after partial duct ligation plus alloxan treatment, and that the contribution of α-cells to the emergence of new β-cells was proportional to the degree of β-cell ablation.
Innovations and breakthroughs
There is little information on the differentiation factors that are involved in the remodeling of the rat pancreas. This study was designed to determine the expression and location of these transcription factors in the STZ-treated neonatal rat pancreas. The authors for the first time found the expression of Ngn3 and Pax4 in α-cells during remodeling of rat pancreas after STZ treatment.
Applications
Insulin deficiency caused by a reduced pancreatic islet β-cell number underlies the progression of both type 1 and type 2 diabetes, prompting efforts to develop β-cell replacement therapies. This study demonstrated the dedifferentiation of α-cells into endocrine precursor cells may contributes to the recovery of β-cell mass after impairment by STZ and this may provide a alternative way for β-cell replacement therapies.
Terminology
After birth, many major developmental changes occur, including β-cell apoptosis, replication, and exogenesis. This stage is referred to as the remodeling of pancreas.
Peer review
The study is well conducted and the results are interesting. This paper puts up the fact that α-cells dedifferentiate to endocrine precursor cells.
Footnotes
Supported by The National Natural Science Foundation of China, No. 81070620
Peer reviewer: Dr. Thiruvengadam Muniraj, University of Pittsburgh Medical Center, 100 Chatham Park Drive, Apt 511, Pittsburgh, PA 15220, United States
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
References
- 1.Bernardo AS, Hay CW, Docherty K. Pancreatic transcription factors and their role in the birth, life and survival of the pancreatic beta cell. Mol Cell Endocrinol. 2008;294:1–9. doi: 10.1016/j.mce.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Guo J, Yuan L, Cheng M, Cao L, Shi H, Tong H, Wang N, De W. Alpha-fetoprotein is dynamically expressed in rat pancreas during development. Dev Growth Differ. 2007;49:669–681. doi: 10.1111/j.1440-169X.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- 4.Hou LQ, Wang YH, Liu LJ, Guo J, Teng LP, Cao LH, Shi H, Yuan L, De W. Expression and localization of mesothelin in developing rat pancreas. Dev Growth Differ. 2008;50:531–541. doi: 10.1111/j.1440-169X.2008.01047.x. [DOI] [PubMed] [Google Scholar]
- 5.Thyssen S, Arany E, Hill DJ. Ontogeny of regeneration of beta-cells in the neonatal rat after treatment with streptozotocin. Endocrinology. 2006;147:2346–2356. doi: 10.1210/en.2005-0396. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Kodama S, Toyonaga T, Kondo T, Matsumoto K, Tsuruzoe K, Kawashima J, Goto H, Kume K, Kume S, Sakakida M, et al. Enhanced expression of PDX-1 and Ngn3 by exendin-4 during beta cell regeneration in STZ-treated mice. Biochem Biophys Res Commun. 2005;327:1170–1178. doi: 10.1016/j.bbrc.2004.12.120. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson JM, Arany EJ, Hill DJ. Changes in islet microvasculature following streptozotocin-induced beta-cell loss and subsequent replacement in the neonatal rat. Exp Biol Med (Maywood) 2010;235:189–198. doi: 10.1258/ebm.2009.009316. [DOI] [PubMed] [Google Scholar]
- 9.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Ohlsson H, Thor S, Edlund T. Novel insulin promoter- and enhancer-binding proteins that discriminate between pancreatic alpha- and beta-cells. Mol Endocrinol. 1991;5:897–904. doi: 10.1210/mend-5-7-897. [DOI] [PubMed] [Google Scholar]
- 12.Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 13.Ahlgren JD. Epidemiology and risk factors in pancreatic cancer. Semin Oncol. 1996;23:241–250. [PubMed] [Google Scholar]
- 14.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 15.Wessells NK. Differentiation of epidermis and epidermal derivatives. N Engl J Med. 1967;277:21–33. doi: 10.1056/NEJM196707062770107. [DOI] [PubMed] [Google Scholar]
- 16.Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 17.Liew CG, Shah NN, Briston SJ, Shepherd RM, Khoo CP, Dunne MJ, Moore HD, Cosgrove KE, Andrews PW. PAX4 enhances beta-cell differentiation of human embryonic stem cells. PLoS One. 2008;3:e1783. doi: 10.1371/journal.pone.0001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hald J, Sprinkel AE, Ray M, Serup P, Wright C, Madsen OD. Generation and characterization of Ptf1a antiserum and localization of Ptf1a in relation to Nkx6.1 and Pdx1 during the earliest stages of mouse pancreas development. J Histochem Cytochem. 2008;56:587–595. doi: 10.1369/jhc.2008.950675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang RN, Bouwens L, Klöppel G. Beta-cell proliferation in normal and streptozotocin-treated newborn rats: site, dynamics and capacity. Diabetologia. 1994;37:1088–1096. doi: 10.1007/BF00418372. [DOI] [PubMed] [Google Scholar]
- 20.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Lee CS, De León DD, Kaestner KH, Stoffers DA. Regeneration of pancreatic islets after partial pancreatectomy in mice does not involve the reactivation of neurogenin-3. Diabetes. 2006;55:269–272. [PubMed] [Google Scholar]
- 24.Sista AK, Knebel RJ, Tavri S, Johansson M, DeNardo DG, Boddington SE, Kishore SA, Ansari C, Reinhart V, Coakley FV, et al. Optical imaging of the peri-tumoral inflammatory response in breast cancer. J Transl Med. 2009;7:94. doi: 10.1186/1479-5876-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heremans Y, Van De Casteele M, in't Veld P, Gradwohl G, Serup P, Madsen O, Pipeleers D, Heimberg H. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol. 2002;159:303–312. doi: 10.1083/jcb.200203074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasa R, Mrejen C, Leachman N, Otten M, Barnes M, Wang J, Chakrabarti S, Mirmira R, German M. Proendocrine genes coordinate the pancreatic islet differentiation program in vitro. Proc Natl Acad Sci USA. 2004;101:13245–13250. doi: 10.1073/pnas.0405301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellitzer G, Bonné S, Luco RF, Van De Casteele M, Lenne-Samuel N, Collombat P, Mansouri A, Lee J, Lan M, Pipeleers D, et al. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO J. 2006;25:1344–1352. doi: 10.1038/sj.emboj.7601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Elghazi L, Parker SE, Kizilocak H, Asano M, Sussel L, Sosa-Pineda B. The concerted activities of Pax4 and Nkx2.2 are essential to initiate pancreatic beta-cell differentiation. Dev Biol. 2004;266:178–189. doi: 10.1016/j.ydbio.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Collombat P, Hecksher-Sørensen J, Broccoli V, Krull J, Ponte I, Mundiger T, Smith J, Gruss P, Serup P, Mansouri A. The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development. 2005;132:2969–2980. doi: 10.1242/dev.01870. [DOI] [PubMed] [Google Scholar]
- 32.Collombat P, Hecksher-Sørensen J, Serup P, Mansouri A. Specifying pancreatic endocrine cell fates. Mech Dev. 2006;123:501–512. doi: 10.1016/j.mod.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Smith SB, Gasa R, Watada H, Wang J, Griffen SC, German MS. Neurogenin3 and hepatic nuclear factor 1 cooperate in activating pancreatic expression of Pax4. J Biol Chem. 2003;278:38254–38259. doi: 10.1074/jbc.M302229200. [DOI] [PubMed] [Google Scholar]
- 34.Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schisler JC, Fueger PT, Babu DA, Hohmeier HE, Tessem JS, Lu D, Becker TC, Naziruddin B, Levy M, Mirmira RG, et al. Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol. 2008;28:3465–3476. doi: 10.1128/MCB.01791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells. 2010;28:1630–1638. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- 37.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]