Abstract
Recasting of the casting alloys affects the composition and elemental release which may have cytotoxic effect different from the pure alloy in the surrounding tissues. An Invitro study was conducted to investigate the elemental release and their cytotoxic effects from commercially available Ni–Cr dental casting alloys, commonly used for fabricating fixed partial dentures. Three Ni–Cr alloys [Wiron 99(A), Ceramet (B), and Hi Nickel CB (C)] were tested. Alloy specimens (disks 3 × 5 mm) were casted and grouped as follows: Group I (A1/B1/C1): 100% pure alloy; Group II (A2/B2/C2): 50% new with 50% recast; and Group III (A3/B3/C3): 100% recast. Disks of each alloy type from each group were transferred to Dulbecco’s modified eagle medium and left for 3 days at 37°C in an atmosphere of 5% CO2. Ni, Cr, Co, Cu and Mo elemental release from metal alloys into culture medium was investigated using Inductively Coupled Plasma Mass Spectrometry. Cytotoxicity was tested using mouse fibroblast cells and MTT Assay. Controls consisted of 6 wells containing cells with no alloy specimens. Data were analyzed by two-way analysis of variance followed by t-test. The total amount of elements released in parts per billion for various casting groups were Group I, A1-6.572, B1-6.732, C1-8.407; Group II, A2-22.046, B2-26.450, C2-29.189; Group III, A3-84.554, B3-88.359, C3-92.264. More amounts of elements were released in Hi Nickel CB than Ceramet and Wiron 99 in all the three test groups. Percentage of viable cells from MTT analysis were Group I, A1-62.342, B1-61.322 C1-60.593, Group II, A2-58.699, B2-56.494, C2-52.688, Group III, A3-53.101, B3-52.195, C3-47.586. The viable cells present in the culture media were more in Wiron 99 than Ceramet and Hi Nickel CB. Elemental release increased with amount of recast alloy. Amongst the three alloys tested Hi Nickel CB had significantly higher elements released compared to Ceramet and Wiron 99 in 100% pure alloys, 50% recast and 100% recast alloys. Wiron 99 showed least element release in 100% pure alloy, 50% recast and 100% recast specimens. 100% pure alloys of all three alloys are less cytotoxic, but their cytotoxicity is more on 50% and 100% re-casted alloys. Out of all three variations of casting Wiron 99 was least cytotoxic, followed by Ceramet and Hi Nickel CB. Recasting of alloys significantly increased the elements released and their cytotoxicity.
Keywords: Recasting, Cytotoxicity, Elemental release, Dental casting alloys, Base metal
Introduction
Advances in science and technology in the field of dental material science have lead to the invention of base metal alloys for application in dentistry [1]. Introduction of chrome alloys has provided an alternative to gold, with these resulted in Ni–Cr and Co–Cr based alloys which are considered as economically less expensive and also have the required qualities and biocompatibility for usage in dental restorative work [2, 3].
Base metal alloys are prone to various types of corrosion depending on its composition and oral environment [1]. The release of metallic elements from dental alloys is a potential health problem to dental patient [4]. Metals are known to cause toxic inflammatory allergic or mutagenic reactions. Important consequence of elemental release is cytotoxicity of adjacent tissues in cell cultures [5, 6]. Cast restorations being placed in close contact with oral tissues for various periods of time may elicit local adverse tissue reactions such as gingivitis and periodontitis adjacent to them. Degree of cytotoxicity and biocompatibility of dental casting alloys has been related to alloy composition and elements released from alloys into surrounding medium [7].
Recasting of used alloys in the form of sprues or defective casting has been practiced to prevent wastage of material after casting [3]. Identification of the elements and their concentrations responsible for cytotoxic effects is important because it will help in improving and designing newer alloys to avoid the release of elements which are harmful to oral cavity.
Hypothesis of the study was that recasting of base metal alloys would change the chemical properties of the alloys and thus affect their elemental release and subsequently elicit cytotoxic effects. The aim of this invitro study was to investigate the effects of recasting of Ni–Cr base metal alloys and their potential cytotoxic effects and elemental release into culture media. Objectives of study were to assess the elemental release and cytotoxicity of Ni–Cr alloys and correlate elemental release with cytotoxicity of pure alloy (Group I), 50% pure and 50% re-casted (Group II) and 100% re-casted (Group III) alloy.
Materials and Methods
The invitro study was undertaken with Ni–Cr alloy for metal ceramic from three different manufacturers (Fig. 1; Table 1). Alloys have been coded for simplicity and reference purposes.
Fig. 1.
Ni–Cr alloys for metal ceramic
Table 1.
Description of Ni–Cr dental cast alloys
| Serial No. | Trade name | Manufacturer | Composition (wt %)* | Code |
|---|---|---|---|---|
| 1 | Wiron 99 | Bego (Germany) | Nickel-65 | A |
| Chromium-22.5 | ||||
| Molybdenum-9.5 | ||||
| Niobium-1 | ||||
| Silica-1 | ||||
| Iron-0.5 | ||||
| Cesium-0.5 | ||||
| 2 | Ceramet | Laboline (Europe) | Nickel-62 | B |
| Chromium-26 | ||||
| Molybdenum-10 | ||||
| Silica-1.5 | ||||
| Others-0.5 | ||||
| 3 | Hi Nickel CB | Hikari (Japan) | Nickel-60 | C |
| Chromium-10 | ||||
| Copper-15 | ||||
| Manganese-7.9 | ||||
| Others-7.1 |
* Composition as provided by manufacturer
Preparation of Alloy Specimens
An aluminium metal die with a circular space of 5 mm radius and 3 mm depth was milled in the centre portion of 1 cm thick aluminium block using computerized milling machine (Fig. 2). Wax patterns 10 mm in diameter × 3 mm thickness were prepared with green inlay casting wax (Fig. 3) (Bego-Germany) using the metal die. A total of 108 wax patterns were made for three alloys for Group I: 100% pure alloy (A1/B1/C1), Group II: 50% pure 50% recast alloy (A2/B2/C2), and Group III: 100% re-casted alloy (A3/B3/C3).
Fig. 2.

Milled die
Fig. 3.
Wax patterns
Casting of the Pattern
The wax patterns of the three groups were segregated as given in Table 2. The sprued wax patterns were invested in phosphate bonded investment in metal casting ring with dry cellulose paper ring liner. Following this they were casted by lost wax technique in an induction casting machine (Fig. 4) (Bego-Germany) with the respective alloys. Metals used for recasting was obtained from cleaned left over sprues and buttons of the alloys that had been previously cast in group I. After bench cooling the casting ring was divested and sandblasted using 250 μm aluminium oxide to remove the remnant investment material. Sprues were cut off and specimens were finished and polished using carborundum discs, metal trimmers, rubber wheels, sandpapers and polishing cake using hand motor instruments. The polished disks were soaked in a detergent solution for 5 min and then scrubbed using a soft bristle brush and rinsed under tap water for 5 min. Specimens were then replaced in sterile distilled water and cleaned by sonification and autoclaved at a temperature of 150°C for 60 min.
Table 2.
Distribution of specimens
| Group | Type | No. of alloy specimen | ||
|---|---|---|---|---|
| (A) Wiron 99 | (B) Ceramet | (C) Hi Nickel CB | ||
| I | 100% New | 12A1 | 12B1 | 12C1 |
| II | 50% New–50% recast | 12A2 | 12B2 | 12C2 |
| III | 100% Recast | 12A3 | 12B3 | 12C3 |
Fig. 4.
Cast specimens
Elemental Release
Six disks of each alloy type from each group were transferred to 6 ml of Dulbecco’s modified eagle medium (DMEM) and incubated for 3 days at 37°C in an atmosphere of 5% CO2. DMEM is prepared by supplementing with 5% new born calf serum, 100 U/ml of Penicillin, Streptomycin and 0.25 μ gm/l Amphotericin B (Fig. 5). The media was analyzed for presence of Ni, Cr, Mo, Co, and Cu using an Inductively Coupled Plasma Mass Spectrometry (ICPMS). Medium was diluted with de ionized water and kept in ICPMS and the readings were recorded. Triplicate absorbance was used to determine the mean concentration of different elements in parts per billion (ppb) released from alloys.
Fig. 5.

Dulbecco’s modified eagle medium (DMEM)
Cell Culture and Cytotoxicity Test
For Cytotoxicity test, the remaining 6 disks from various casting alloys in three groups were placed in center of a 24 well tissue culture plate and 0.5 ml of mouse fibroblast cell suspension was added to cell well (Fig. 6) and were left for 2 h. Following this 0.5 ml of DMEM was added and cells were incubated for 3 days at 37°C in an atmosphere of 5% CO2. The controls consisted of 6 wells containing cells with no alloy specimens. After the incubation period the plates were removed from incubator and cytotoxicity of materials was assessed by measuring Succinic Dehydrogenase activity (SDH) of the cells by using MTT assay. MTT reagent is added to the culture plate with alloy specimens and it is transferred to 96 well plates for readings in Enzyme Linked Immunosorbent Assay (ELISA) reader, the protocol followed for the MTT Assay is summarized in Table 3.
Fig. 6.
Tissue culture plate with 0.5 ml of mouse fibroblast cell suspension
Table 3.
Protocol for performing MTT assay
| Step | Action |
|---|---|
| 1 | Plate cells at 1,000–100,000 per well |
| 2 | Incubate for 6–24 h |
| 3 | Add 10 μl MTT reagent |
| 4 | Incubate for 2–4 h until purple precipitate is visible. |
| 5 | Add 100 μl detergent reagent |
| 6 | Leave at room temperature in the dark for 2 h |
| 7 | Record absorbance at 570 nm |
The readings were calculated by the formula:
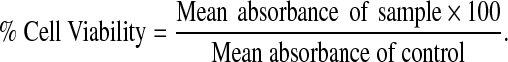 |
Statistical Analysis
The results obtained from the MTT analysis and ICPMS analysis was subjected to statistical analysis. One way ANOVA was used to calculate the P-value. Multiple range tests by Tukey–HSD procedure were employed to identify the significant groups at 5% level.
Results
Elemental Release
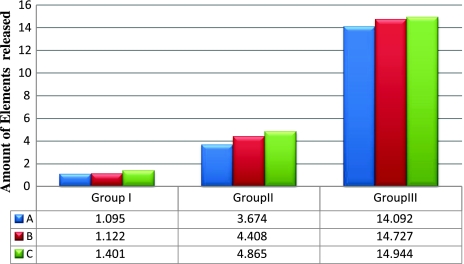
Elemental release was seen in all three casting alloy groups. Elements released were Ni, Cr, Co, Cu and Mo. Tables 4, and 5 summarize the elemental release by different casted alloys in three casting procedures. Hi Nickel CB had the highest amount of elemental release among the three casting alloys followed by Ceramet and Wiron 99. Elemental release significantly increased with the percentage of recast material used in for all elements analyzed in the study with P < 0.05 (Graph 1).
Table 4.
Comparison of mean values of elemental release between different re-casted alloys in three casting groups
| Group | Variable | Material | Mean ± SD | P-value | Significant groups at 5% level |
|---|---|---|---|---|---|
| I | 100% Pure alloy | A1 | 1.095 ± 0.052 | <0.0001 (sig) | C1 Vs A1 and B1. |
| B1 | 1.122 ± 0.081 | ||||
| C1 | 1.401 ± 0.082 | ||||
| II | 50% Pure–50% re-casted alloy | A2 | 3.674 ± 0.288 | <0.0001 (sig) | C2 Vs A2 and B2. |
| B2 | 4.408 ± 0.231 | ||||
| C2 | 4.865 ± 0.296 | ||||
| III | 100% Re-casted alloy | A3 | 14.092 ± 0.739 | <0.0001 (sig) | C3 Vs A3 and B3 |
| B3 | 14.727 ± 2.028 | ||||
| C3 | 14.944 ± 2.301 |
Table 5.
The amount of various element released from casting alloys for three casting groups. (mean ± SD)
| Element | Alloy | Group I | Group II | Group III |
|---|---|---|---|---|
| Ni | Wiron 99 | 0.495 ± 0.010 | 2.901 ± 0.169 | 12.180 ± 0.633 |
| Ceramet | 0.402 ± 0.012 | 3.664 ± 0.399 | 13.580 ± 2.088 | |
| Hi Nickel CB | 0.487 ± 0.090 | 3.354 ± 0.299 | 30.294 ± 24.225 | |
| Cr | Wiron 99 | 0.071 ± 0.015 | 0.090 ± 0.021 | 0.222 ± 0.077 |
| Ceramet | 0.346 ± 0.044 | 0.084 ± 0.014 | 0.108 ± 0.076 | |
| Hi Nickel CB | 0.265 ± 0.092 | 0.458 ± 0.091 | 0.471 ± 0.104 | |
| Co | Wiron 99 | 0.029 ± 0.008 | 0.074 ± 0.007 | 0.062 ± 0.036 |
| Ceramet | 0.048 ± 0.021 | 0.035 ± 0.007 | 0.065 ± 0.013 | |
| Hi Nickel CB | 0.027 ± 0.010 | 0.436 ± 0.128 | 0.051 ± 0.015 | |
| Mo | Wiron 99 | 0.501 ± 0.053 | 0.621 ± 0.118 | 1.629 ± 0.195 |
| Ceramet | 0.343 ± 0.051 | 0.524 ± 0.084 | 0.941 ± 0.055 | |
| Hi Nickel CB | 0.416 ± 0.029 | 0.044 ± 0.031 | 0.143 ± 0.042 | |
| Cu | Wiron 99 | |||
| Ceramet | ||||
| Hi Nickel CB | 0.207 ± 0.056 | 0.573 ± 0.156 | 4.918 ± 1.047 |
Graph 1.
Comparison of element release for three alloys
Cytotoxicity Results
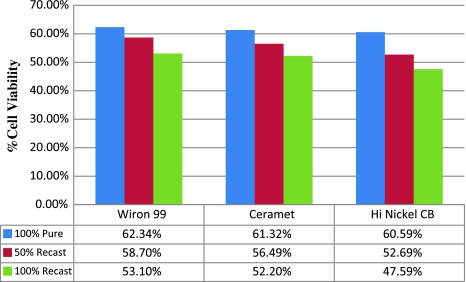
The percentage of viable cells in three groups is tabulated in Table 6. Mean percentages of cell activity of three base metal alloys for three casting conditions were analyzed and cytotoxicity was observed to be more in Hi Nickel CB, followed by Ceramet and Wiron 99. Results showed that 100% recast alloys had more cytotoxicity than 50% pure and 50% re-casted and 50% pure and 50% re-casted alloy had more cytotoxicity than pure alloy (Graph 2).
Table 6.
Mean values for percentage of cell viability for three casting groups
| Group | % of alloy | Material | Percentage of viable cells (%) |
|---|---|---|---|
| I | 100% pure | A1 | 62.342 |
| B1 | 61.322 | ||
| C1 | 60.593 | ||
| II | 50% pure–50% re-casted | A2 | 58.699 |
| B2 | 56.494 | ||
| C2 | 52.668 | ||
| III | 100% re-casted | A3 | 53.101 |
| B3 | 52.195 | ||
| C3 | 47.556 |
Graph 2.
Percentage of cell viability
Discussion
Metals have been an integral part of dental restorative procedures. Dental casting alloys which restore the form and function of missing tooth structure should be biologically inert. Release of metallic elements from dental casting alloys is a potential health problem [2, 8]. Metal ions leached from casting alloys are known to cause toxic inflammatory, allergic or mutagenic reactions [9, 10]. These released elements have been detected by invitro and invivo studies. An important consequence of element release is its cytotoxic effect on adjacent tissue and the amount of tissue reaction depends upon element released. [5–7, 11, 12].
The selection of an alloy for specific conditions like fixed partial dentures are influenced by three factors namely physical properties, bio-compatibility and cost. Gold considered as the high noble metal was the alloy of choice for restorative procedures and it was considered that no ions leached from it but, histological studies disproved this misconception [2]. High cost of gold has lead to the introduction of base metal dental casting alloys [2, 3]. Nickel combined with chromium forms highly corrosion resistant alloy, These base metal alloys which are primarily nickel based compared with gold alloys are cheaper, have high elastic modulus (stiffer), harder, less dense and possess comparable resistance to tarnish and corrosion [1].
Due to comparable physical properties base metals have slowly replaced gold casting alloys [2, 3, 13, 14]. Commercially available base metal alloys for fixed partial dentures are usually Ni–Cr alloys [1, 2]. The composition of these alloys may vary according to manufacturer but the basic constituents are Ni, Cr and Mo [1]. Addition of minor components significantly changes the microstructure and properties which affect the bond strength of ceramic to metal oxide layer required for achieving bonding. It is at the manufacturer’s discretion to include any of these minor components to produce the desired properties [1].
The permissible leaching of these elements should be evaluated to prevent untoward side effects (cytotoxic effects) during function. It has been a practice to further reduce the cost; the casted alloy is reused again in various proportions with new alloy [3]. The elemental release of this re-casted alloy may vary from the pure virgin alloy. The biocompatibility of the alloy depends upon the type and elemental release from it, into surrounding medium or tissues [5, 15].
To assess the element released from the alloys, the alloy is stored in a suitable medium such as artificial saliva for 3 months or in a suitable culture medium and incubated for 3 days [16–19]. Storing the specimen in culture medium and incubating it is a practical method as the procedure requires shorter span of time and the elements released were also better [16]. Further to evaluate the amount of elements released into the medium various methods have been put forth, such as Atomic Absorption spectrography, Emission spectrography, Inductively Coupled Plasma Atomic Emission Spectrophotometer (ICPAES), Inductively Coupled Plasma Mass Spectrometry (ICPMS) [20]. ICPMS is fast, sensitive, precise and accurate multi element analytical technique for determination of trace elements.
The common methods used to evaluate cytotoxicity are Millipore filter, Agar overlay test, MTT assay test. MTT assay is an easy technique; the readings can be calculated directly from ELISA reader [16]. The other advantages of MTT are sensitive and reproducibility, convenient, rapidity, elimination of need of radioactive compounds and economical.
Three Ni–Cr based alloys (Wiron 99, Ceramet and Hi Nickel CB), commonly used for the fabrication of metal ceramic fixed partial dentures from different popular manufacturing companies were chosen due to the variation in their composition claimed by the manufacturers. Cleaning and sterilization of specimens were performed according to recommended protocols. Specimens were autoclaved 150°C for 60 min prior to testing to sterilize them [1, 16]. It is important to note that this procedure may affect the corrosion pattern of these alloys, however all the alloy specimens received the same treatment, thus any differences in cytotoxicity of elemental release would be result of differences in type and composition of alloys or the casting procedures.
The findings of the current study agreed with the hypothesis that recasting of base metal alloys increases their potential cytotoxic effect and element release. In the study, element release in all the new alloy samples was significantly less than the re-casted specimens. Amongst the three alloys selected Hi Nickel CB had significantly higher elements released compared to Ceramet and Wiron 99 in pure alloy, 50% recast and 100% recast alloys. Wiron 99 showed least element release in 100% pure alloy, 50% recast and 100% recast specimens. All the base metal alloys tested in this study showed to have a cytotoxic effect.
In the cytotoxic evaluation done by analyzing living cells and percentage of cell viability it was seen that in 100% pure alloy specimens Wiron 99, Ceramet and Hi Nickel CB were cytotoxic in the increasing order. With recasting of alloys either with 50% or 100% showed a definite decrease in the number of living cells in all the three alloys. In the present study in 50% new–50% re-casted and 100% re-casted, the mean of living cells and percentage of cell viability are more significant in Wiron 99 than Ceramet and Hi Nickel CB. Hi Nickel CB showed least number of viable cells.
Among Ni–Cr alloys chosen Hi Nickel CB was most cytotoxic and it was significantly more cytotoxic than Ceramet and Wiron 99. This could be related to the difference in the composition of these alloys [1, 3]. The greatest cytotoxicity showed by the Hi Nickel CB alloy for all the three casting conditions could be due to its copper content. It has been reported in the literature that the major contribution for toxicity of alloys was probably due to copper content [1]. It has been reported that only Ni–Cr alloys containing 16–27% Cr develop an adequate protective oxide layer. Molybdenum also has an active role in the formation of oxide layer. Since copper based alloys tarnish and corrode more than non copper alloys, it was observed in the present study that a remarkable amount of copper was released into culture medium from Hi Nickel CB. The content of copper in association with low content of Cr and absence of molybdenum may also explain significant cytotoxic effect of this alloy in comparison to other Ni–Cr alloys [1, 3, 17]. Thus for all of the alloys 50% recast groups were significantly more cytotoxic than 100% new alloy groups and furthermore, the 100% recast groups were significantly more cytotoxic than 50% recast group. This could be due to some effect on the chemical composition of these alloys which may increase the dissolution rate of the alloys, and thus the elements released from them will be increased as found in ICPMS analysis. From the results of present study it appears that the alloy types and the elements of these alloys become affected to a different degree when the alloy was recast and hence attributes to its cytotoxic effects.
Limitations
The results of the study need to be correlated considering the following limitations. The minerals or organic constituents in DMEM media used may have an effect on corrosion susceptibility of the alloys and thus influence the element release. Although a small sample size (n = 6) was used in the current study, significant differences were found between the different groups, indicating its sufficiently large effect size. However further studies need to be conducted with greater sample size for a more adequate representation of the alloy types.
Conclusions
The results obtained from the study prove hypothesis that recasting of base metal alloys affect their elemental release and subsequently elicit cytotoxic effects. Though it has been acceptable to reuse 50% re-casted alloy in the form of sprues removed from previous castings added to new alloy ingot to maintain physical and chemical properties, the element release from these re-casted alloys are significantly more and hence will have a definite cytotoxic effect.
Within the limitations of the current study it may be concluded that Hi Nickel CB shows the maximum elemental release and cytotoxicity effect in pure and re-casted forms and this is followed by ceramet. In the current study Wiron 99 showed the least element release and cytotoxicity.
Footnotes
Clinical Implications
Cast restorations placed in close contact with oral tissues for various periods of time may elicit local adverse tissue reactions such as gingivitis and periodontitis adjacent to them. Degree of cytotoxicity and biocompatibility of dental casting alloys has been related to alloy composition and elements released from alloys into surrounding medium. Recasting of Ni–Cr alloys increases the amount of elements released and hence the cytotoxic potential.
References
- 1.Anusavice KJ (2003) Phillips science of dental materials, 11th edn. Saunders, St. Louis, pp 563–620
- 2.Stein RS. Pontic-residual ridge relationship: research report. J Prosthet Dent. 1966;16:251–285. doi: 10.1016/0022-3913(66)90080-1. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad Al-Hiyasat S, Darmani H. The effects of recasting on the cytotoxicity of base metal alloys. J Prosthet Dent. 2005;93:158–164. doi: 10.1016/j.prosdent.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hiyasat AS, Bashabsheh OM, Darmani H. An investigation of the cytotoxic effects of dental casting alloys. Int J Prosthodont. 2003;16(1):8–12. [PubMed] [Google Scholar]
- 5.Brune D. Mechanisms and kinetics of metal release from dental alloys. Int Endod J. 1988;21:135–142. doi: 10.1111/j.1365-2591.1988.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 6.Craig RG, Hanks CT, Brune D. Mechanisms and kinetics of metal release from dental alloys. J Dent Res. 1990;69:1539–1542. doi: 10.1177/00220345900690081801. [DOI] [PubMed] [Google Scholar]
- 7.Goehlich V, Marek M. Corrosion behavior of Pd–Cu and Ni–Cr alloys in synthetic saliva. Dent Mater. 1990;6:103–110. doi: 10.1016/S0109-5641(05)80039-9. [DOI] [PubMed] [Google Scholar]
- 8.Baum L, Philips RW, Lund MR (1995) Textbook of operative dentistry, 3rd edn. WB Saunders, Philadelphia, pp 529–571
- 9.Syrjanen S, Hensten-Pettersen K, Kangasniemi, Yli-Urpo A. Invitro and in vivo biological response to some dental alloys tested separately and in combinations. Biomaterials. 1985;6(3):169–176. doi: 10.1016/0142-9612(85)90005-5. [DOI] [PubMed] [Google Scholar]
- 10.Schmaltz G, Langer H, Schweikl H. Cytotoxicity of dental alloy extracts and corresponding metal salts solution. J Dent Res. 1998;77(10):1772–1778. doi: 10.1177/00220345980770100401. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hiyasat AS, Bashabsheh OM, Darmani H. Elements release from dental casting alloys and their cytotoxicity effects. Int J Prosthodont. 2002;15:473–478. [PubMed] [Google Scholar]
- 12.Ayad MF. Compositional stability and marginal accuracy of complete cast crowns made with as-received and recast type III gold alloys. J Prosthet Dent. 2002;87:162–166. doi: 10.1067/mpr.2002.121024. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham DM. Comparison of base metal alloys and type IV gold alloys for removable partial denture frameworks. Dent Clin N Am. 1973;17:719–722. [PubMed] [Google Scholar]
- 14.Mezger PR, Vrijhoef MM, Newman SM, Greener EH. The corrosion resistance of a new cobalt–chromium–molybdenum ceramic alloy. J Oral Rehabil. 1988;15:421–428. doi: 10.1111/j.1365-2842.1988.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 15.Grill V, Sandurucci MA, Di Lendarda R, Basa M. Invitro evaluation of the biocompatibility of dental alloy in fibronectin expression patterns and relationships to cellular proliferation rates. Quintessence Int. 2000;31(10):741–747. [PubMed] [Google Scholar]
- 16.Shillingburg HT, Hobo S, Whitsitt LD, Brackett SE (1997) Fundamentals of fixed prosthodontics, 3rd edn. Quintessence, Chicago, pp 365–383
- 17.Schedle A, Samorapoompichit P, Rausch-Fan XH, et al. Response of L-929 fibroblasts, human gingival fibroblasts, and tissue mast cells to various metal cations. J Dent Res. 1995;74:1513–1520. doi: 10.1177/00220345950740081301. [DOI] [PubMed] [Google Scholar]
- 18.Grill Vittorio, Maria A. Sanduri invitro evaluation of the biocompatibility of dental alloys. Quintessence Int. 2000;31:741–747. [PubMed] [Google Scholar]
- 19.Wang RR. Invitro evaluation of biocompatibility of experimental titanium alloys for dental restorations. J Prosthet Dent. 1998;80:495–500. doi: 10.1016/S0022-3913(98)70018-6. [DOI] [PubMed] [Google Scholar]
- 20.Moffa JP. Alternative dental casting alloys. Dent Clin N Am. 1983;27(4):733–746. [PubMed] [Google Scholar]