Abstract
Previous studies have shown that CXC chemokines containing Glu-Leu-Arg (ELR) in their amino-terminus stimulate hepatocyte proliferation and liver regeneration after partial hepatectomy. These ELR+CXC chemokines bind to two receptors, CXCR1 and CXCR2. Previous work has shown that CXCR2 is involved in the proliferative effects of CXC chemokines. However, the function of CXCR1 during the regenerative response has not been studied. The aim of the current study was to investigate the role of CXCR1 in liver regeneration after partial hepatectomy. C57BL/6 (wild type) or CXCR1 −/− mice were subjected to 70% partial hepatectomy or sham surgery and sacrificed on day 2 and 4 after operation. There were no significant differences in liver/ body weight ratio or hepatocyte proliferation. The data suggest that CXCR1 does not mediate the proliferative effects of ELR+ CXC chemokines during liver regeneration after partial hepatectomy.
Introduction
The regenerative capacity of the liver is vital to the restoration of functional mass after massive resection or transplantation. This unique process is still not fully understood due to the complexity of the regenerative process and the vast number of contributing cytokines, growth factors and transcription factors (1). One class of soluble mediators that have been shown to positively regulate liver regeneration is CXC chemokines (2). CXC chemokines are classified as Glu-Leu-Arg (ELR)-positive or ELR-negative CXC chemokines based on the presence or absence of these three amino residues in the amino-terminus (3). ELR+ CXC chemokines bind to the receptors, CXCR1 and CXCR2, whereas ELR-negative CXC chemokines bind to the receptors CXCR3 and CXCR4 (4). To date, only ELR+ CXC chemokines have been shown to regulate liver regeneration, focusing attention on the importance of CXCR1 and CXCR2. Previous studies have shown that blockade or gene deletion of CXCR2 diminishes the regenerative response after partial hepatectomy in a manner associated with decreased hepatocyte proliferation and subsequent recovery of liver mass (5). Other studies have shown that CXCR2 modulates hepatocyte proliferation in vitro (6). However, there have been no studies of the role of CXCR1 in liver regeneration. Therefore, we sought to determine if gene deletion of CXCR1 altered the regenerative response of the liver after partial hepatectomy.
Materials and Methods
Animal model
Male C57BL/6J mice and CXCR1−/− mice (Jackson Laboratory, Bar Harbor, ME) weighing 20–26g (wild-type: 24.0 ± 0.7 g, CXCR1−/−: 24.9 ± 0.5 g) (Table 1) were used in all experiments. This project was approved by the University of Cincinnati Animal Care and Use Committee and was in compliance with the National Institutes of Health guidelines. The animals were randomly separated into partial hepatectomy group, and sham operation group. A total of 18 wild-type and 18 CXCR1−/− mice were operated and 6 mice were included in each group. Partial hepatectomy was performed according to the method of Mitchell and Willenbring (7). Briefly, all mice were anaesthetized with sodium pentobarbital (60 mg/kg, i.p.) and a midline laparotomy was performed. A 4-0 silk suture (Ethicon, Inc., Somerville, NJ) were secured around the base of the left lateral and median hepatic lobes separately, and each lobes were resected. Mice were sacrificed 48 and 96 hours after operation, and blood and liver samples were taken for analysis. Blood was served for analysis of serum alanine aminotransferase (ALT) as an index of hepatocellular injury. Measurements of serum ALT were made using a diagnosis kit by bioassay (Wiener Laboratories, Rosario, Argentina). The liver lobes to body weight ratio was determined, and normalized to the pre-hepatectomy liver/body weight ratio.
Table 1.
Body and liver weights during experimental timecourse.
| body weight (g) | |||
|---|---|---|---|
| genotype | day 0 | day 2 | day 4 |
| wild-type | 23.99±0.71 | 22.12±0.92 | 21.91±0.60 |
| CXCR1 −/− | 24.93±0.52 | 22.99±0.85 | 23.27±0.42 |
| liver weight (mg) | |||
| genotype | day 0 | day 2 | day 4 |
| wild-type | 373.98±32.67 | 613.57±21.96 | 819.55±31.74 |
| CXCR1 −/− | 444.55±21.66 | 663.77±22.68 | 833.48±42.40 |
Proliferating Cell Nuclear Antigen (PCNA) Staining
Immunohistochemical staining for PCNA was performed on paraffin-embedded liver tissue with anti-PCNA antibody using DakoCytomation ARK kit (Dako, Copenhagen, Denmark). Briefly, a three-step peroxidase method was performed according to the manufacturer’s instruction. PC-10 monoclonal antibody (Santa Cruz Biotechnology) was used at a dilution of 1:50, for 15 minutes at room temperature. The sections were counterstained with hematoxylin. Evaluation of PC-10 immunostaining was performed based on the percentage of positive nuclei of 400–600 hepatocytes from 4–6 highest positive fields at high power (400X), and was expressed as PCNA labeling index.
Statistical Analysis
All data are expressed as mean ± standard error of the mean (SEM). Data were analyzed with a one-way analysis of variance with subsequent Student-Newman-Keuls test. Differences were considered significant when P < 0.05.
Results
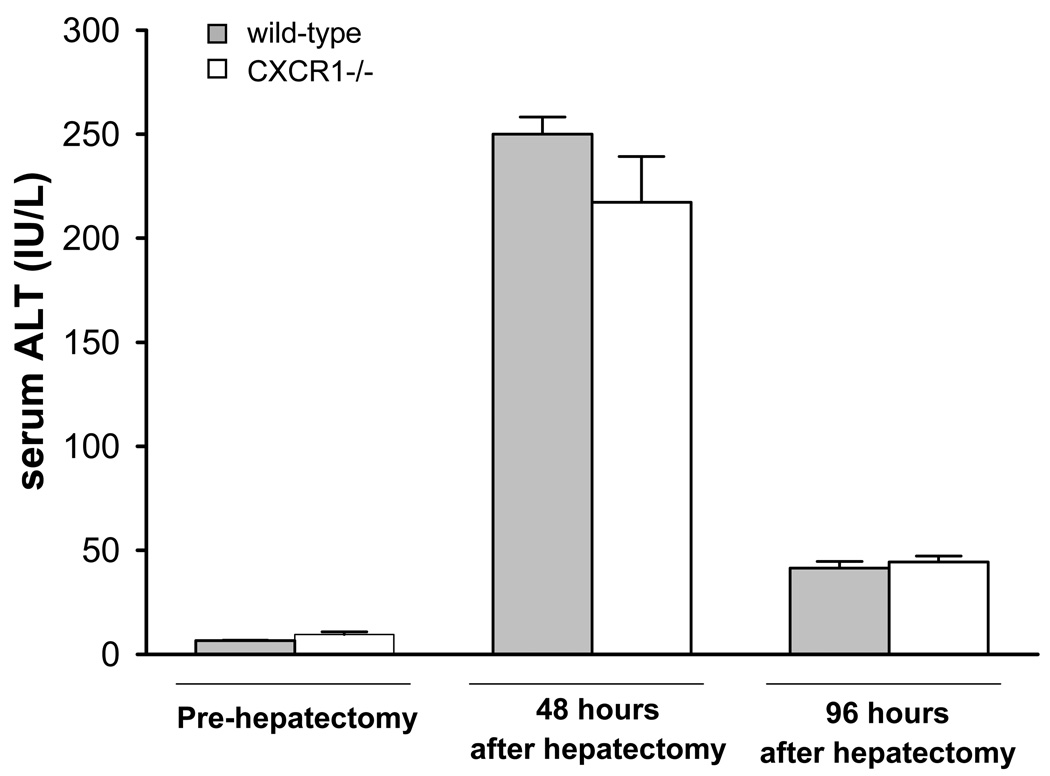
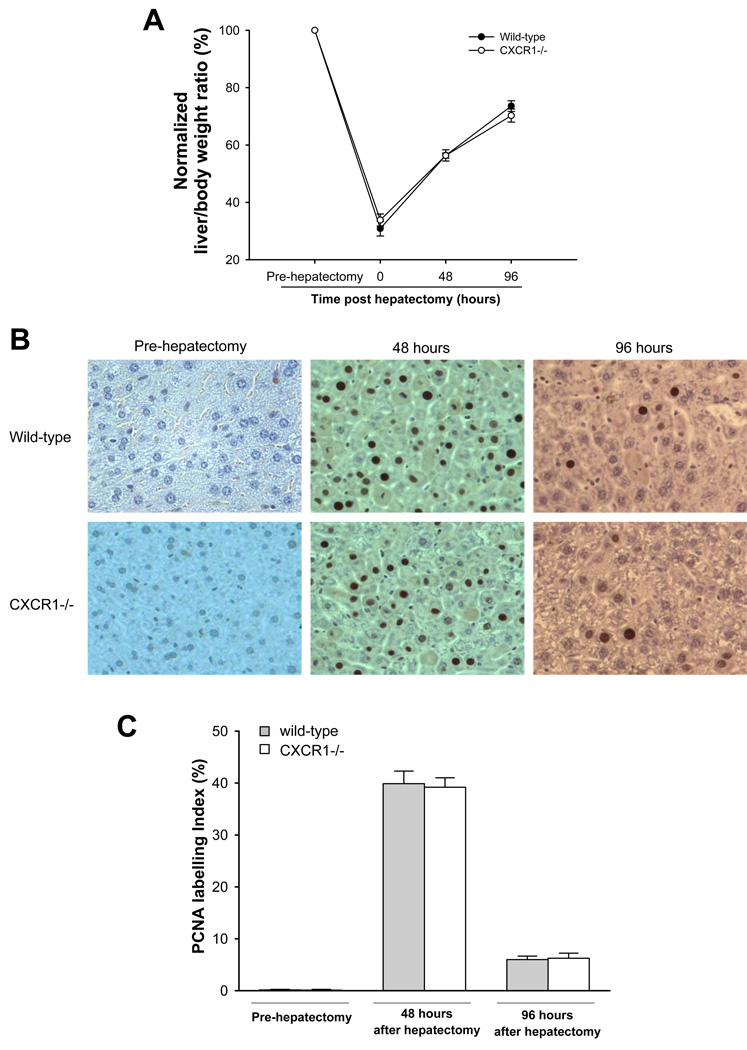
In order to determine if genetic deletion of CXCR1 would alter the regenerative response after partial hepatectomy, we assessed the recovery of liver mass in wild-type and CXCR1−/− mice after 70% hepatectomy. No surgical related complications or deaths were observed during the experimental period. Hepatocellular injury was measured by serum ALT levels and was similar in wild-type and CXCR1−/− mice at 48 and 96 hours after partial hepatectomy (250.0 ± 8.2 IU/L versus 217.2 ± 20.1 IU/L, 41.5 ± 3.1 IU/L versus 44.4 ± 2.9 IU/L; Figure 1). Both wild-type and CXCR1−/− mice had increased liver mass 48 hours after partial hepatectomy (wild-type: 613.6 ± 32.7 g, CXCR1−/−: 663.8 ± 22.7 g). Liver mass continued to increase and reached to almost 75% of its original volume 96 hours after partial hepatectomy (wild-type: 819.6 ± 31.7 g, CXCR1−/−: 833.5 ± 42.4 g; Table 1). However, there were no differences in regenerated liver mass between the groups at any time point (Figure 2A). To evaluate whether there was any change in proliferative capacity of hepatocytes between the groups, liver sections were stained for PCNA, a marker of entry into S-phase. Similar to our results of liver mass recovery, we observed no difference between the groups in the amount of proliferating hepatocytes (Figure 2B, C).
Figure 1.
Liver injury after 70% hepatectomy in wild-type and CXCR1 −/− mice. Liver injury was measured by serum levels of ALT. Data are mean ± SEM with n=6 per group.
Figure 2.
Liver regeneration and hepatocyte proliferation after 70% hepatectomy in wild-type and CXCR1 −/− mice. (A) Recovery of functional liver mass after partial hepatectomy (normalized liver to body weight ratio. Data are mean ± SEM with n=6 per group. (B) Liver immunohistochemical staining for proliferating cell nuclear antigen (PCNA). Original magnification was 400x. (C) Quantitative analysis of PCNA labeling. Data are mean ± SEM with n=6 per group.
Discussion
ELR+ CXC chemokines have been previously shown to regulate hepatocyte proliferation and liver regeneration after partial hepatectomy (5, 6). However, these previous studies focused on the receptor, CXCR2, as the mediator of these effects. Ren et al. showed that neutralization of CXCR2 significantly suppressed liver regeneration after partial hepatectomy, whereas, neutralization of MIP-2 alone was not effective (5). However, there is currently no information about the role of CXCR1, another CXC chemokine receptor, in liver regeneration. It has been shown that CXCR1 and CXCR2 are constitutively expressed in malignant melanoma and regulate cell proliferation and invasion (8). Recently, it was demonstrated that CXCR1 was essential to normal urothelial survival through IL-8, a prominent ELR+CXC chemokine (9). Moreover, we have recently reported that CXCR1 has divergent roles from CXCR2 and appears to facilitate recovery and regenerative response after ischemia/reperfusion injury (10). Therefore, we sought to investigate the role of CXCR1 in liver regeneration after partial hepatectomy. Our results suggest that CXCR1 is not involved in regulating the regenerative response after partial hepatectomy. In fact, there were no significant differences in the change of liver to body weight ratio or hepatocyte proliferation between wild-type and CXCR1 −/− mice.
We have recently shown that CXCR1 functions to promote liver recovery after ischemia/reperfusion injury (10). While the current study would seem to contrast with that report, the stress of the hepatocyte may play a role. Hepatocytes are injured after ischemia/reperfusion, whereas after partial hepatectomy, hepatocytes are relatively unstressed due to the lack of any manipulations in remnant liver lobes. Therefore, it appears that the role of CXCR1 in hepatocyte proliferation/regeneration is variable and may depend on the stress level of hepatocytes. These data suggest that the proliferative and regenerative effects of ELR+ CXC chemokines after partial hepatectomy are mediated solely by CXCR2 and targeted stimulation of CXCR2 may represent a rationale therapy after liver resection or transplantation.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nozomu Sakai, Email: sakainu@ucmail.uc.edu.
Satoshi Kuboki, Email: satoshi.kuboki@nifty.com.
Heather L. Van Swearingen, Email: hlewis987@gmail.com.
Amit D. Tevar, Email: amit.tevar@uc.edu.
John Blanchard, Email: john.blanchard@uc.edu.
Michael J. Edwards, Email: michael.edwards@ uc.edu.
Alex B. Lentsch, Email: alex.lentsch@uc.edu.
References
- 1.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke CN, Kuboki S, Tevar A, Lentsch AB, Edwards M. CXC chemokines play a critical role in liver injury, recovery, and regeneration. Am J Surg. 2009;198:415. doi: 10.1016/j.amjsurg.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark-Lewis I, Dewald B, Geiser T, Moser B, Baggiolini M. Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci U S A. 1993;90:3574. doi: 10.1073/pnas.90.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 5.Ren X, Carpenter A, Hogaboam C, Colletti L. Mitogenic properties of endogenous and pharmacological doses of macrophage inflammatory protein-2 after 70% hepatectomy in the mouse. Am J Pathol. 2003;163:563. doi: 10.1016/S0002-9440(10)63684-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colletti LM, Green M, Burdick MD, Kunkel SL, Strieter RM. Proliferative effects of CXC chemokines in rat hepatocytes in vitro and in vivo. Shock. 1998;10:248. doi: 10.1097/00024382-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 8.Varney ML, Li A, Dave BJ, Bucana CD, Johansson SL, Singh RK. Expression of CXCR1 and CXCR2 receptors in malignant melanoma with different metastatic potential and their role in interleukin-8 (CXCL-8)-mediated modulation of metastatic phenotype. Clin Exp Metastasis. 2003;20:723. doi: 10.1023/b:clin.0000006814.48627.bd. [DOI] [PubMed] [Google Scholar]
- 9.Tseng-Rogenski S, Liebert M. Interleukin-8 is essential for normal urothelial cell survival. Am J Physiol Renal Physiol. 2009;297:F816. doi: 10.1152/ajprenal.90733.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke C, Kuboki S, Sakai N, Kasten KR, Tevar AD, Schuster R, Blanchard J, Caldwell CC, Edwards MJ, Lentsch AB. CXC chemokine receptor-1 is expressed by hepatocytes and regulates liver recovery after hepatic ischemia/reperfusion injury. Hepatology. 53:261. doi: 10.1002/hep.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]