Abstract
Diabetes mellitus is a chronic disease affecting all age groups. It is one of the leading causes of mortality and morbidity worldwide. Many chronic macrovascular and microvascular complications of diabetes have been reported in the literature with few reports about oral complications. This article aims to review and increase the awareness of oral manifestations and complications of diabetes mellitus and to stimulate research on the subject. It treats in depth some of the complications such as periodontal disease, fungal infection and salivary dysfunction while other complications are mentioned briefly.
Keywords: Diabetes Mellitus, Oral, Complications, Periodontitis, Taste, Fungal
Diabetes mellitus is a growing public health concern and a common chronic metabolic disease worldwide.1–4 Diabetes mellitus represents a group of metabolic diseases that are characterised by hyperglycaemia due to a total or relative lack of insulin secretion and insulin resistance or both. The metabolic abnormalities involve carbohydrate, protein and fat metabolism. Diabetes mellitus affects all age groups, but is more common in adults. The World Health Organization (WHO) has recently declared it to be a pandemic.2 Its prevalence has increased dramatically over the past few decades and it is expected to triple in the next decade. Diabetes mellitus is considered a leading cause of death due to its microvascular and macrovascular complications.5,6 The most common types of diabetes are type 1 (also known as insulin dependent) and type 2 (also known as non-insulin-dependent).7,8 Type 2 is the more prevalent type. Countries with the highest rates of diabetes in the Eastern Mediterranean region and the Middle East are the United Arab Emirates, Saudi Arabia, Bahrain, Kuwait and Oman.9 Oman is one of the countries that has a high prevalence of diabetes mellitus, especially type 2 diabetes, and its prevalence is expected to increase in the next twenty years.10,11
Various inflammatory diseases and soft tissue pathologies in oral cavities are associated with diabetes mellitus;12–14 however, awareness of these complications is lacking worldwide.15–18 Periodontal diseases have been proposed as the sixth most prevalent complication of diabetes mellitus following the other diabetic complications.19 It has been reported as a more frequent oral complication of diabetes compared to other oral manifestations such as dry mouth and caries. Periodontitis is more frequent and severe in patients with diabetes with poor glycaemic control. Early identification and/or management of these oral manifestations may help in the early diagnosis of diabetes and in attaining better glycaemic control.20 Therefore, diabetic oral complications need to be identified and included in the ultimate care of diabetes in order to fight this chronic metabolic disease effectively.
Oral Complications and Manifestations of Diabetes Mellitus
Several soft tissue abnormalities have been reported to be associated with diabetes mellitus in the oral cavity. These complications include periodontal diseases (periodontitis and gingivitis); salivary dysfunction leading to a reduction in salivary flow and changes in saliva composition, and taste dysfunction. Oral fungal and bacterial infections have also been reported in patients with diabetes. There are also reports of oral mucosa lesions in the form of stomatitis, geographic tongue, benign migratory glossitis, fissured tongue, traumatic ulcer, lichen planus, lichenoid reaction and angular chelitis.21–25 In addition, delayed mucosal wound healing, mucosal neuro-sensory disorders, dental carries and tooth loss has been reported in patients with diabetes.26 The prevalence and the chance of developing oral mucosal lesions were found to be higher in patients with diabetes compared to healthy controls.27
Periodontal Diseases
Pathophysiologylogy of Periodontitis
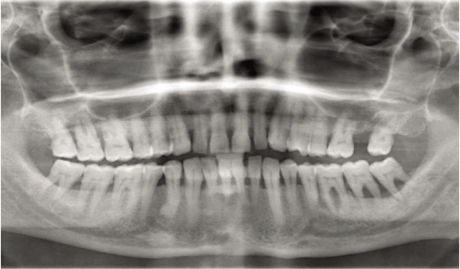
Periodontitis is one of the most widespread diseases in the world affecting the oral cavity, and is highly prevalent in both developed and developing countries.28 Periodontitis is a chronic inflammatory disorder affecting the gingivae and the periodontal tissue initiated by bacteria.29 The micro-flora in the dental plaque that forms daily adjacent to the teeth causes this inflammatory process. Eventually, the toxins that are released by the microorganisms in the dental plaque will start the gingival inflammation as a result of failure to remove the dental plaque on a daily basis. A periodontal pocket is formed as a result of the progression of the gingival inflammation causing the gingivae to detach from the tooth surface. This periodontal pocket is filled with bacteria and its toxins. As the disease worsens, the pocket will get deeper carrying the dental plaque until it reaches the alveolar bone that will eventually be destroyed with the periodontal attachment. This process is very common and causes destruction of periodontal tissues, loss of alveolar bone and, finally, tooth loss [Figure 1]. There are many factors contributing to this type of inflammation beside the presence of bacteria in dental plaque; a susceptible host is one of them.
Figure 1.
Radiographic appearance of diabetic patient with sever periodontal destruction and bone loss.
Periodontitis and Diabetes Mellitus
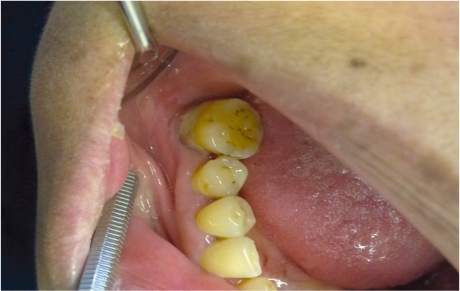
The link between diabetes mellitus and periodontal disease is not well recognised by the medical community. Periodontal disease has been reported with increased prevalence and severity in patients with type 1 and type 2 diabetes.30 The mechanism by which hyperglycaemia can induce periodontal destruction is not yet fully understood. However, there are many theories which propose factors such as advanced glycation end products, changes in collagen statue, and altered immune function that causes impaired polymorphonuclear leukocyte function which may facilitate bacterial persistence in the tissue and the accumulation of advanced glycation end products, which results from prolonged and chronic hyperglycaemia and increased secretion of pro-inflammatory cytokines such as tumour necrosis factor-α and prostaglandin E-2.31,32 The increase in collagenase activity together with the reduction in collagen synthesis will adversely influence collagen metabolism. This would result in compromised wound healing as well as periodontal tissue destruction. Recent studies indicate that periodontitis has a bidirectional effect on glycaemic control in patients with diabetes.33 There is a cluster of research studies, which support the hypothesis of periodontitis occurring more frequently in patients with diabetes with poor glycaemic control.33–38 In addition, there is enough evidence to support the hypothesis that poor periodontal conditions could worsen glycaemic control as well. Many studies report that diabetes is a risk factor for gingivitis and periodontitis and it is more severe with poor glycaemic control [Figure 2].39 The risk of developing periodontitis in patients with diabetes has been reported to be three times higher than the general population.40
Figure 2.
Clinical photograph showing periodontal abscess in poorly controlled diabetic patient.
Numerous risk factors have been reported that make patients with diabetes more susceptible to periodontal disease, especially those with poor oral hygiene, poor metabolic control, longer duration of diabetes and who are smokers.41–43 Smoking was identified in many studies as being a major preventable risk factor for periodontal disease and tooth loss in the general population and in patients with diabetes.44–48 The dentist and the physician should play an important role in advising and supporting patients with diabetes regarding smoking cessation. The dentist should be engaged in counselling these patients and referring them to a specialist organisation which deals with smoking cessation.49
Several studies showed that the treatment of periodontal disease has an influence on glycaemic control in both type 1 and type 2. A recent meta-analysis of the efficacy of periodontal treatment on glycaemic control in patients with diabetes suggested that such treatment could lead to a significant reduction in HbA1c.50 However, they also recommended that the results need to be viewed with caution due to a lack of strength and limitations in the designs of some of the studies included. Periodontitis and diabetes are related to each other therefore further larger studies are required to determine the effect of periodontal treatment on glycaemic control.
Salivary and Taste Dysfunction
Salivary Dysfunction
Saliva has a major role in maintaining a healthy oral cavity. Saliva is produced by major salivary glands (parotid, sub-mandibular and sub-lingual) and numerous minor salivary glands distributed in the oral cavity. Salivary dysfunction has been reported in patients with diabetes.51,52 A cross sectional epidemiological study was conducted in 2001 to look at the prevalence of hyposalivation and xerostomia (dry mouth) and to determine the relationship between salivary dysfunction and diabetes complications. This study was conducted in type 1 diabetics and control subjects without diabetes. They found that symptoms of reduced salivary flow rate and xerstomia were more frequently reported by patients with diabetes than the controls, especially by those diabetics who had developed neuropathy.53 Other studies conducted in type 2 diabetics also confirmed that xerostomia and hyposalivation were more prevalent in this group of patients.54 It has been shown that poorly controlled type 2 diabetics have a lower stimulated parotid gland flow rate compared to well-controlled patients and patients without diabetes.55 An increase in salivary pathogens was also reported in these patients.56 Patients with diabetes usually complain of xerostomia and the need to drink very often (polydypsia and polyuria). The constant dryness of the mouth would irritate the oral soft tissues, which in turn will cause inflammation and pain. Patients with diabetes with xerostomia are more predisposed to periodontal infection and tooth decay. The cause of this is not yet fully understood in patients with diabetes, but may be related to polydypsia and polyuria or alternation in the basement membrane of the salivary glands. It is known that diabetes mellitus is associated with chronic complications such as neuropathy, micro-vascular abnormalities and endothelial dysfunction that lead to deterioration of microcirculation and this may play a role in reduction of the salivary flow rate and composition.57,58 Sialosis is defined as asymptomatic, non-inflammatory, non-neoplastic, bilateral chronic diffuse swelling mainly affecting the parotid glands. Sialosis has been found to be more prevalent in patients with diabetes mellitus.59
Taste Dysfunction
There are many factors that have been implicated in altered taste sensation in the oral cavity. Metabolic and endocrine diseases were proposed as causative factors for this disturbance; nevertheless, salivary dysfunction can contribute to altered taste sensation or elevation of detection thresholds.60, 61 Taste dysfunction has been reported to occur more frequently in patients with poorly controlled diabetes compared to healthy controls.62 Diabetic patients who suffer from neuropathy have a higher taste threshold. Taste disturbance has also been reported to lead to poor glycaemic control by inhibiting the ability to maintain a good diet.63
Oral Infection
Fungal Infections
Oral candidosis is an opportunistic infection frequently caused by Candida albicans species. Many predisposing factors can lead to this infection; these include smoking, xerostomia and endocrine and metabolic diseases.64 Other factors were also implicated such as old age, medications, Cushing’s syndrome, malignancies, and the use of dentures.65 Oral candidosis has been classified into primary and secondary. Primary oral candidosis is subclassified into acute (pseudomembranous and erythematous), chronic (pseudomembranous, erythematous and hyperplastic) and candida associated lesions.
Pseudomembranace candidosis is also known as oral thrush. It is characterised by the presence of a creamy white patch which, when wiped, reveals underlying erythematous and bleeding oral mucosa. The soft palate is the most commonly affected area followed by the cheek, tongue and gingivae. It could be chronic in immuno-compromised patients.66 Erythematous candidosis can present as acute or chronic infection. It is believed to result from the usage of steroid and broad spectrum antibiotics and mainly affects the tongue.66 Hyperplastic candidosis is known as candidal leukoplakia. It appears as an irregular whitish raised plaque-like lesion commonly seen in the buccal mucous membrane near the commissures.
Candida associated lesions include denture induced stomatitis, angular chelitis and median rhomboid glossitis which have mixed bacterial and fungal etiology. Denture induced stomatitis is mainly seen in full denture wearers in the underlying surface of the upper denture. Angular chelitis is seen in the lip commissures as an erythematous crusting lesion. The lesion has been reported to occur in diabetics with poor glycaemic control. Median rhomboid glossitis is seen on the dorsal surface of the tongue as adepopulated erythematous diamond-shaped patch at the midline.
The incidence of fungal infections in patients with diabetes mellitus has been recognised for many years.67 Candidal infection is reported to be more prevalent in patients with diabetes especially in those patients who smoke, wear dentures, have poor glycaemic control and use steroids and broad spectrum antibiotics.68 In addition, salivary dysfunction in patients with diabetes can also contribute to higher carriage of fungi in this group of patients. It is clear from these studies that both local and systemic predisposing factors might increase candidal carriage rate and hence increase the risk of oral candidal infection in patients with diabetes.69–71
Bacterial Infections
Patients with diabetes are more susceptible to developing oral bacterial infections. They are well known to have an impaired defense mechanism hence considered to be immuno-compromised. Diabetics with diabetic complications and poor metabolic control are more prone to spreading and recurrent bacterial infection. Several studies have reported that patients with diabetes are more prone to deep neck bacterial infection compared to patient without diabetes.72,73 A four-year prospective study by Rao et al. investigated the severity of maxillofacial space infection of odontogenic origin, the type of micro-organism, the sensitivity of the micro-organisms to antibiotics, and the length of hospital stay of patients with diabetes compared with patients without diabetes. They concluded that the spread of the bacterial infection to the submandibular space was more common in patients and controls and that the second commonest area was the buccal space. Streptococcus species was more commonly isolated in both groups. Patients with diabetes were found to stay longer in hospital due to more severe infection and required more time to control their blood glucose levels.74
Poor Oral Wound Healing
Poor soft tissue regeneration and delayed osseous healing in patients with diabetes are known complications during oral surgery. Therefore, the management and treatment of patients with diabetes undergoing oral surgery is more complex. It was reported that delayed vascularisation, reduced blood flow, a decline in innate immunity, decreased growth factor production, and psychological stress may be involved in the protracted wound healing of the oral cavity mucosa in patients with diabetes.75
Non-Candidal Oral Soft Tissue Lesion
Oral lesions that are not caused by candidal infection have been reported to occur in patients with diabetes such as fissured tongue, irritation fibroma and traumatic ulcer. These lesions were more prevalent in diabetes compared to the controls.27 Altered or delayed wound healing may play a role in traumatic ulcer.
Oral Mucosal Disease
Both lichen planus and recurrent apthous stomatitis have been reported to occur in patients with diabetes.76,77 Oral lichen planus (OLP) is a skin disorder that produces lesions in the mouth. OLP is reported to occur more frequently in patients with type1 diabetes compared to type 2 diabetes.76 The reason for this is that type 1 diabetes is considered an autoimmune disease, and OLP has been reported to have an underlying autoimmune mechanism. Patients with diabetes are subjected to a prolonged state of chronic immune suppression especially in type 1 diabetes. In addition, acute hyperglycaemia causes alteration in the immune responsiveness in diabetes mellitus. Atrophic-erosive oral lesions are more common in patients with diabetes with OLP.77
Neuro-Sensory Oral Disorder
Oral dysesthesia or burning mouth syndrome (BMS) is a painful condition affecting the oral cavity (palate, tongue, throat and gingivae).78,79 Other abnormal oral sensations may co-exist with the burning mouth sensation such as tingling, numbness, dryness or sore mouth at the same time. The exact cause of BMS is unknown, but it has been attributed to several conditions such as dry mouth, menopause, candidal infection, diabetes mellitus, cancer therapy, psychological problems and acid reflux. BMS is classified into two types: primary idiopathic, and secondary as a result of a systemic process; secondary BMS has been reported to occur with diabetes mellitus. It could adversely affect the ability to maintain good oral hygiene in patients with diabetes. Diabetic neuropathy could be the underlying cause of BMS in patients with diabetes. The nerve damage in diabetic neuropathy has been reported to show an increase in the Langerhans cells that are associated with immune disturbance.80, 81 Therefore, it is crucial to screen patients who have symptoms of BMS for diabetes mellitus.
Dental Caries and Tooth Loss
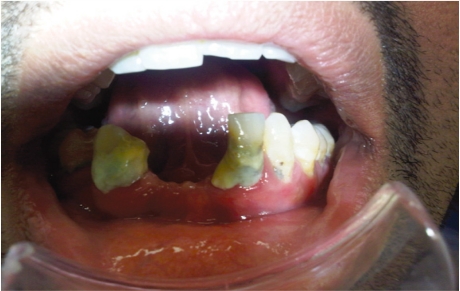
It is well known that patients with diabetes are susceptible to oral infections that lead to tooth decay and loss.82 Salivary secretion dysfunction, periodontal and sensory disorders could increase the likelihood of developing new and recurrent dental caries and tooth loss [Figure 3]. The relationship between diabetes and development of dental caries is still unclear. It is well-known that the cleansing and buffering capacity of the saliva is diminished in patients with diabetes mellitus resulting in increased incidence of dental caries, especially in those patients who suffer from xerostomia.
Figure 3.
Clinical photograph of diabetic patient with poor oral hygiene, showing dental carries and tooth loss.
Conclusion
Diabetes mellitus is a chronic, non-communicable and endemic disease. Type 2 compared to type 1 diabetes mellitus is more prevalent worldwide and increasing, especially in Oman. Oral manifestations and complications in patients with diabetes mellitus have been recognised and reported recently as a major complication of diabetes mellitus. There is increasing evidence that chronic oral complications in patients with diabetes adversely affect blood glucose control. Prevention and management of oral complications, especially periodontal disease, in patients with diabetes is important due to their possible adverse effect on glycaemic control. Promotion of a healthy oral cavity in patients with diabetes is paramount. Epidemiological and research data on this problem in Omani patients with diabetes should be expanded by further studies.
There are several clinical implications from this review. These include: 1) a lack of awareness of oral complications among both diabetics and health providers; 2) an understanding of the way diabetes affects oral health is necessary for both clinicians and patients, therefore research in this field should be encouraged; 3) the need for regular follow-up of patients with diabetes mellitus by both dentist and physicians; 4) the major role that dentists should play in recognising the signs and symptoms of diabetes and their oral complications; 5) advice and counselling for diabetic smokers regarding smoking cessation, and 6) vigorous treatment of oral infection either bacterial or fungal in these patients, especially if they have poor glycaemic control.
References
- 1.Diabetes—a global threat Lancet. 2009;373:1735. doi: 10.1016/S0140-6736(09)60954-5. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Geneva: World Health Organization; 2009. [Google Scholar]
- 3.Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, et al. Diabetes trends in the US: 1990 to 1998. Diabetes Care. 2000;23:1278–83. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 4.Abegunde DO, Mathers CD, Taghreed A, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370:1929–38. doi: 10.1016/S0140-6736(07)61696-1. [DOI] [PubMed] [Google Scholar]
- 5.Moore PA, Zgibor JC, Dasanayake AP. Diabetes: A growing epidemic of all ages. J Am Dent Assoc. 2003;134:11–15. [PubMed] [Google Scholar]
- 6.Shelesh J, Swarnlata S. Type 2 diabetes mellitus – Its global prevalence and therapeutic strategies. Diabetes Metab Syndr. 2010;4:48–56. [Google Scholar]
- 7.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 8.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26:S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 9.Saadi H, Carruthers SG, Nagelkerke N, Al-Maskari F, Afandi B, Reed R, et al. Prevalence of diabetes mellitus and its complications in a population-based sample in Al Ain, United Arab Emirates. Diabetes Res Clin Pract. 2007;78:369–377. doi: 10.1016/j.diabres.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Asfour MG, Lambourne A, Soliman A, Al-Behlani S. High prevalence of diabetes mellitus and impaired glucose tolerance in the Sultanate of Oman: Result of the 1991 national survey. Diabet Med. 1995;12:1122– 5. doi: 10.1111/j.1464-5491.1995.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Lawati JA, Al Riyami AM, Mohammed AJ, Jousilahti P. Increasing prevalence of diabetes mellitus in Oman. Diabet Med. 2002;19:954–7. doi: 10.1046/j.1464-5491.2002.00818.x. [DOI] [PubMed] [Google Scholar]
- 12.Bell G, Large D, Barclay S. Oral health care in diabetes mellitus. Dent Update. 1999;26:322–30. doi: 10.12968/denu.1999.26.8.322. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin E. Oral health. Lancet. 2009;373:628–9. doi: 10.1016/S0140-6736(09)60392-5. [DOI] [PubMed] [Google Scholar]
- 14.Vernillo AT. Dental considerations for the treatment of patients with diabetes mellitus. Am Dent Assoc. 2003;134:24–33. doi: 10.14219/jada.archive.2003.0366. [DOI] [PubMed] [Google Scholar]
- 15.Yuen HK, Wolf BJ, Bandyopadhyay D, Magruder KM, Salinas CF, London SD. Oral health knowledge and behavior among adults with diabetes. Diabetes Res Clin Pract. 2009;86:239–46. doi: 10.1016/j.diabres.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Habashneh R, Khader Y, Hammad MM, Almuradi M. Knowledge and awareness about diabetes and periodontal health among Jordanians. J Diabetes Complications. 2010;24:409–414. doi: 10.1016/j.jdiacomp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Mirza KM, Khan A, Ali MM, Chaudhry S. Oral health knowledge, attitude, and practices and sources of information for diabetic patients in Lahore, Pakistan. Diabetes Care. 2007;30:3046–7. doi: 10.2337/dc07-0502. [DOI] [PubMed] [Google Scholar]
- 18.Moore PA, Orchard T, Guggenheimer J, Weyant RJ. Diabetes and oral health promotion: A survey of disease prevention behaviors. J Am Dent Assoc. 2000;131:1333–41. doi: 10.14219/jada.archive.2000.0388. [DOI] [PubMed] [Google Scholar]
- 19.Löe H. Periodontal disease: The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 20.Teeuw WJ, Gerdes VEA, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: A systemic review and meta-analysis. Diabetes Care. 2008;33:421–7. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandberg GE, Sundberg HE, Fjellstrom CA, Wikblad KF. Type 2 diabetes and oral health: A comparison between diabetic and non-diabetic subjects. Diabetes Res Clin Pract. 2000;50:27–34. doi: 10.1016/s0168-8227(00)00159-5. [DOI] [PubMed] [Google Scholar]
- 22.Chomkhakhai U, Thanakun S, Khovidhunkit S-P, Khovidhunkit W, Thaweboon S. Oral health in Thai patients with metabolic syndrome. Diabetes Metab Syndr. 2009;3:192–7. [Google Scholar]
- 23.Collin HL, Niskanen L, Uusitupa M, Töyry J, Collin P, Koivisto A-M, et al. Oral symptoms and signs in elderly patients with type 2 diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:299–305. doi: 10.1067/moe.2000.107536. [DOI] [PubMed] [Google Scholar]
- 24.Guggenheimer J, Moore PA, Rossie K, Myers D, Mongelluzzo MB, Block HM, et al. Insulin-dependent diabetes mellitus and oral soft tissue pathologies: I. Prevalence and characteristics of non-candidal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:563–9. doi: 10.1067/moe.2000.104476. [DOI] [PubMed] [Google Scholar]
- 25.Guggenheimer J, Moore PA, Rossie K, Myers D, Mongelluzzo MB, Block HM, et al. Insulin-dependent diabetes mellitus and oral soft tissue pathologies. II. Prevalence and characteristics of Candida and candidal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:570–6. doi: 10.1067/moe.2000.104477. [DOI] [PubMed] [Google Scholar]
- 26.Lamster IB, Lalla E, Borgnakke WS, Taylor GW. The relationship between oral health and diabetes mellitus. J Am Dent Assoc. 2008;139:19–24. doi: 10.14219/jada.archive.2008.0363. [DOI] [PubMed] [Google Scholar]
- 27.Saini R, Al-Maweri SA, Saini D, Ismail NM, Ismail AR. Oral mucosal lesions in non oral habit diabetic patients and association of diabetes mellitus with oral precancerous lesions. Diabetes Res Clin Pract. 2010;89:320–6. doi: 10.1016/j.diabres.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Poul EP. Priorities for research for oral health in the 21st Century - the approach of the WHO Global Oral health program. Community Dental Health. 2005;22:71–4. [PubMed] [Google Scholar]
- 29.Kuo L, Polson AM, Kang T. Associations between periodontal diseases and systemic diseases: A review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health. 2008;122:417–33. doi: 10.1016/j.puhe.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Preshaw PM. Periodontal disease and diabetes. J Dent. 2009;37:575–7. doi: 10.1016/j.jdent.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Ritchie CS. Mechanistic links between type 2 diabetes and periodontitis. J Dent. 2009;37:578–9. doi: 10.1016/j.jdent.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Moore PA, Weyant RJ, Mongelluzzo MB, Myers DE, Rossie K, Guggenheimer J, et al. Type 1 diabetes mellitus and oral health: Assessment of periodontal disease. J Periodontol. 1999;70:409–17. doi: 10.1902/jop.1999.70.4.409. [DOI] [PubMed] [Google Scholar]
- 33.Teeuw WJ, Gerdes VEA, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: A systemic review and meta-analysis. Diabetes Care. 2008;33:421–7. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 35.De Silva NT, Preshaw PM, Taylor JJ, Jayaratne SD, Heasman PA, Fernando DJS. Periodontitis: A complication of type 2 diabetes in Sri Lankans. Diabetes Res Clin Pract. 2006;74:209–10. doi: 10.1016/j.diabres.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–92. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 37.Davies RM, Davies GM. Periodontal disease and general health. Dent Update. 2005;32:438–42. doi: 10.12968/denu.2005.32.8.438. [DOI] [PubMed] [Google Scholar]
- 38.Taylor JW. Periodontal treatment and its effects on glycemic control a review of the evidence. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:311–6. doi: 10.1016/s1079-2104(99)70214-3. [DOI] [PubMed] [Google Scholar]
- 39.Taylor GW, Borgnakke WS. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 40.Ryan ME, Carnu O, Kamer AA. The influence of diabetes on the periodontal tissues. J Am Dent Assoc. 2003;134:34–40. doi: 10.14219/jada.archive.2003.0370. [DOI] [PubMed] [Google Scholar]
- 41.Irwin BC, Mullally B, Ziada H, Allen E, Byrne PJ. Periodontics: 2. Risk factors and susceptibility in periodontitis. Dent Update. 2007;34:270–6. doi: 10.12968/denu.2007.34.5.270. [DOI] [PubMed] [Google Scholar]
- 42.Katz PP, Wirthlin MR, Szpunar SM, Selby JV, Sepe SJ, Showstack JA. Epidemiology and prevention of periodontal disease in individuals with diabetes. Diabetes Care. 1991;14:375–85. doi: 10.2337/diacare.14.5.375. [DOI] [PubMed] [Google Scholar]
- 43.Kibayashi M, Tanaka M, Nishida N, Kuboniwa M, Kataoka K, Nagata H, et al. Longitudinal study of the association between smoking as a periodontitis risk and salivary biomarkers related to periodontitis. J Periodontol. 2007;78:859–67. doi: 10.1902/jop.2007.060292. [DOI] [PubMed] [Google Scholar]
- 44.Calsina G, Ramon J-M, Echeverrιa J-J. Effects of smoking on periodontal tissues. J Clin Periodontol. 2002;29:771–6. doi: 10.1034/j.1600-051x.2002.290815.x. [DOI] [PubMed] [Google Scholar]
- 45.Dietrich T, Maserejian NN, Joshipura KJ, Krall EA, Garcia RI. Tobacco use and incidence of tooth loss among US male health professionals. J Dent Res. 2007;86:373–7. doi: 10.1177/154405910708600414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore PA, Orchard T, Guggenheimer J, Weyant RJ. Diabetes and oral health promotion: A survey of disease prevention behaviors. J Am Dent Assoc. 2000;131:1333–41. doi: 10.14219/jada.archive.2000.0388. [DOI] [PubMed] [Google Scholar]
- 47.Berlin I. Smoking-induced metabolic disorders: Diabetes Metab Syndr. 2008;34:307–14. doi: 10.1016/j.diabet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Beziaud F, Halimi JM, Lecomte P, Vol S, Tichet J. Cigarette smoking and diabetes mellitus. Diabetes Metab. 2004;30:161–6. doi: 10.1016/s1262-3636(07)70102-7. [DOI] [PubMed] [Google Scholar]
- 49.Chestnutt I. Tobacco usage: The role of the dental team in smoking cessation. Dent Update. 2010;37:55–62. doi: 10.12968/denu.2010.37.1.55. [DOI] [PubMed] [Google Scholar]
- 50.Darr L, Vergnes JN, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: A meta-analysis of interventional studies. Diabetes Metab. 2008;34:497–506. doi: 10.1016/j.diabet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Moore PA, Guggenheimer J, Etzel KR, Weyant RJ, Orchard T. Type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:281–91. doi: 10.1067/moe.2001.117815. [DOI] [PubMed] [Google Scholar]
- 52.Lin CC, Sun SS, Kao A, Lee CC. Impaired salivary function in patients with noninsulin-dependent diabetes mellitus with xerostomia. J Diabetes Complications. 2002;16:176–9. doi: 10.1016/s1056-8727(01)00174-x. [DOI] [PubMed] [Google Scholar]
- 53.Sandberg GE, Wikblad KF. Oral dryness and peripheral neuropathy in subjects with type 2 diabetes. J Diabetes Complications. 2003;17:192–8. doi: 10.1016/s1056-8727(02)00220-9. [DOI] [PubMed] [Google Scholar]
- 54.Chávez EM, Borrell LN, Taylor JW, Ship JA. A longitudinal analysis of salivary flow in control subjects and older adults with type 2 diabetes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:166–73. doi: 10.1067/moe.2001.112054. [DOI] [PubMed] [Google Scholar]
- 55.Chavez EM, Taylor GW, Borrell LN, Ship JA. Salivary function and glycemic control in older persons with diabetes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 89:305–311. doi: 10.1016/s1079-2104(00)70093-x. [DOI] [PubMed] [Google Scholar]
- 56.Khovidhunkit SO, Suwantuntula T, Thaweboon S, Mitrirattanakul S, Chomkhakhai U, Khovidhunkit W. Xerostomia, hyposalivation, and oral microbiota in type 2 diabetic patients: a preliminary study. J Med Assoc Thai. 2009;92:1220–8. [PubMed] [Google Scholar]
- 57.Conner S, Iranfour B, Mills J. Alteration in parotid salivary flow in diabetes mellitus. Oral Surg Oral Med Oral Pathol. 1970;30:55–9. doi: 10.1016/0030-4220(70)90011-3. [DOI] [PubMed] [Google Scholar]
- 58.Chomkhakhai U, Thanakun S, Khovidhunkit S-P, Khovidhunkit W, Thaweboon S. Oral health in Thai patients with metabolic syndrome. Diabetes Metab Syndr. 2009;3:192–7. [Google Scholar]
- 59.Scully C, Bagán JV, Eveson JW, Barnard N, Turner FM. Sialosis: 35 cases of persistent parotid swelling from two countries. Br J Oral Maxillofac Surg. 2008;46:468–72. doi: 10.1016/j.bjoms.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 60.Ship JA, Chavez EM. Special Senses: Disorders of Taste and Smell. In: Silverman S Jr, Eversole LR, Truelove EL, editors. Essentials of Oral Medicine. Hamilton, London: BC Decker Inc; 2001. pp. 279–80. [Google Scholar]
- 61.Negrato CA, Tarzia O. Buccal alterations in diabetes mellitus. Diabetes Metab Syndr. 2010;2:3. doi: 10.1186/1758-5996-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lalla RV, D’Ambrossio JA. Dental management considerations for the patient with diabetes mellitus. J Am Dent Assoc. 132:1425–32. doi: 10.14219/jada.archive.2001.0059. 200. [DOI] [PubMed] [Google Scholar]
- 63.Ship JA. Diabetes and oral health: An overview. J Am Dent Assoc. 2003;134:4–10s. doi: 10.14219/jada.archive.2003.0367. [DOI] [PubMed] [Google Scholar]
- 64.McIntyre G. Oral candidosis. Dent Update. 2001;28:132–9. doi: 10.12968/denu.2001.28.3.132. [DOI] [PubMed] [Google Scholar]
- 65.Samaranayake LP. Host Factors and Oral Candidiasis. In: Samaranayake LP, MacFarlane TW, editors. Oral Candidosis. 2nd ed. London: Butterworth & Co. Ltd; 1990. pp. 145–7. [Google Scholar]
- 66.Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002;78:455–459. doi: 10.1136/pmj.78.922.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lamey PJ, Darwaza A, Fisher BM, Samaranayake LP, MacFarlane TW, Frier BM. Secretor status, candidal carriage and candidal infection in patients with diabetes mellitus. J Oral Pathol. 1988;17:354–7. doi: 10.1111/j.1600-0714.1988.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 68.Willis AM, Coulter WA, Fulton CR, Hayes RJ, Bell PM, Lamey PJ. Oral candidal carriage and infection in insulin treated diabetic patients. Diabet Med. 1999;16:675–9. doi: 10.1046/j.1464-5491.1999.00134.x. [DOI] [PubMed] [Google Scholar]
- 69.Hill LV, Tan MH, Pereira LH, Embil JA. Association of oral candidiasis with diabetic control. J Clin Pathol. 1989;42:502–5. doi: 10.1136/jcp.42.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khosravi AR, Yarahmadi S, Baiat M, Shokri H, Pourkabireh M. Factors affecting the prevalence of yeasts in the oral cavity of patients with diabetes mellitus. J Mycol Med. 2008;18:83–8. [Google Scholar]
- 71.Soysa NS, Samaranayake LP, Ellepola NB. Diabetes mellitus as a contributory factor in oral candidosis. Diabet Med. 2006;23:455–9. doi: 10.1111/j.1464-5491.2005.01701.x. [DOI] [PubMed] [Google Scholar]
- 72.Huang TT, Tseng FY, Liu TC, Hsu CJ, Chen YS. Deep neck infection in diabetic patients: Comparison of clinical picture and outcomes with nondiabetic patients. Otolaryngol Head Neck Surg. 2005;132:943–7. doi: 10.1016/j.otohns.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 73.Uthkarsh L, Shrinath N. Diabetic challenge in maxillofacial infection. Int J Oral Maxillofac Surg. 2007;36:1040. [Google Scholar]
- 74.Rao DD, Desai A, Kulkarni RD, Gopalkrishnan K, Rao CB. Comparison of maxillofacial space infection in diabetic and nondiabetic patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:7–12. doi: 10.1016/j.tripleo.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 75.Abiko Y, Selimovic D. The mechanism of protracted wound healing on oral mucosa in diabetes: Review. Bosn J Basic Med Sci. 2010;10:186–91. doi: 10.17305/bjbms.2010.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amerikanou CP, Markopoulos AK, Belazi M, Karamitsos D, Papanayotou P. Prevalence of oral lichen planus in diabetes mellitus according to the type of diabetes. Oral Dis. 1998;4:37–40. doi: 10.1111/j.1601-0825.1998.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 77.Torrente-Castells E, Figueiredo R, Berini-Aytés L, Gay-Escoda C. Clinical features of oral lichen planus - A retrospective study of 65 cases. Med Oral Patol Oral Cir Bucal. 2010;15:685–90. doi: 10.4317/medoral.15.e685. [DOI] [PubMed] [Google Scholar]
- 78.ADA Division of Communications Burning mouth syndrome. J Am Dent Assoc. 2005;136:1191. doi: 10.14219/jada.archive.2005.0324. [DOI] [PubMed] [Google Scholar]
- 79.Scala A, Checchi L, Montevecchi M, Marini I, Giamberardino MA. Update on burning mouth syndrome: Overview and patient management. Crit Rev Oral Biol Med. 2003;14:275–91. doi: 10.1177/154411130301400405. [DOI] [PubMed] [Google Scholar]
- 80.Moore PA, Guggenheimer J, Orchard T. Burning mouth syndrome and peripheral neuropathy in patients with type 1 diabetes mellitus. J Diabetes Complications. 2007;21:397–402. doi: 10.1016/j.jdiacomp.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 81.Tavakoli M, Boulton AJ, Efron N, Malik RA. Increased Langerhans cell density and corneal nerve damage in diabetic patients: Role of immune mechanisms in human diabetic neuropathy. Cont Lens Anterior Eye. 2010. [Epub 2010 Sep 16] [DOI] [PMC free article] [PubMed]
- 82.Collin H-L, Uusitupa M, Niskanen L, Koivisto A-M, Markkanen H, Meurman JH. Caries in patients with non-insulin-dependent diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:680–5. doi: 10.1016/s1079-2104(98)90035-x. [DOI] [PubMed] [Google Scholar]