Abstract
We report a young Omani male who developed severe and persistent anaemia after a kidney transplantation while being on immunosuppression therapy, standard practice to prevent rejection of the transplanted kidney. His bone marrow aspirate showed the classic morphological changes of pure red cell aplasia (PRCA), induced by parvovirus B19 infection which is the presence of giant proerythroblasts with viral inclusions. The virus was also demonstrated by polymerase chain reaction in the blood along with IgM antibodies to parvovirus B19. He responded dramatically to high dose immunoglobulin with a normalisation of his haemoglobin level in two weeks and remained normal until seven months later. Parvovirus B19 induced PRCA can be cured. This aetiology must be kept in mind especially when a chronic anaemia, refractory to treatment, is accompanied by a reticulocytopenia. The latter reflects the lysis of the proerythroblasts, preventing maturation of the erythroid cells causing anaemia. Early recognition and prompt treatment spares the patient unnecessary exposure to blood transfusions, erythropoietin and renal disease caused by the virus. PRCA secondary to parvovirus B19 infection following kidney transplantation is reported in the literature, but not in the Omani population. To the best of our knowledge, this is the first such report in Oman.
Keywords: Red cell aplasia, pure; human parvovirus B19; kidney transplantation; Immunosuppuression; Case report; Oman
Parvovirus, a single stranded DNA virus with tropism for the erythroid progenitor cells1 is known to cause lytic destruction of the proerythroblasts leading to a mild anaemia in the immunocompetent person.2 It is the only known member of the Parvoviridae family to be pathogenic to humans.3 Infections occur worldwide and especially in the spring and winter months. It is so common in the Northern European community that by the age of 50 years about 80% of this population will have been infected.4
Although it is believed that the virus infects only erythroid progenitors, the viral capsid antigen has been demonstrated inside giant granulocytes in the bone marrow in patients with pancytopenia following bone marrow transplantation.5 Viral growth can also be maintained in fetal liver cell cultures.2 In children, parvovirus B19 infection causes a transient anaemia associated with an erythematous rash called the fifth disease or Erythema infectiosum. Occasionally, in immunocompetent adults, a symmetric polyarthropathy mimicking rheumatoid arthritis occurs.3 The tropism to erythroid progenitors in the bone marrow is well known to cause a transient aplastic crisis (TAC) in individuals with an underlying haemolytic anaemia and Hydrops fetalis in intrauterine infections.6
In immunocompromised patients, the inability to generate an immune response leads to a state of persistent anaemia, reticulocytopenia caused by lytic destruction of proerythroblasts giving rise to an acquired pure red cell aplasia (PRCA). Following allogeneic stem cell transplants (ASCT), this infection is known to occur most commonly in the first year post transplant as the immune suppression is at a maximum during this period.7–11 The standard treatment for these patients is intravenous (IV) gammaglobulin (IVIG). This replaces the neutralising antibodies which the patient does not possess; however, it is unknown whether the virus is always eliminated completely by this treatment.
Case Report
An Omani man aged 30 had end stage renal disease caused by pyelonephritis of the right kidney. He was on haemodialysis for three years following which he had a kidney transplant from a live donor. He had a course of antilymphocyte globulin (ALG), commenced intraoperatively and was on tacrolimus, mycophenolate mofetil and prednisolone as a means of immunosuppression. No information was available regarding the donor’s parvovirus status as the transplant was done in another country. Three months later he was referred to our clinic for investigation of tiredness on exertion and anaemia with a haemoglobin of 5 g/dL. On presentation, his renal and liver functions were normal. He was afebrile, cheerful and, apart from being very pale, was clinically well.
He received a transfusion of two units of packed red blood cells. Despite this his anaemia persisted at an Hb of 6.9 g/dL. The doses of immunosupressive drugs (tacrolimus and mycophenolate mofetil) were reduced. Other laboratory investigations were as follows; red cell count - 2.93 × 109/L (4.5–6.6); white blood cells - 4.0 × 109/L (4–11); platelets - 353 × 109/L; absolute reticulocyte count - 3 ×109/L (5–200); haptoglobin Level - 1817 mg/L (360 – 1950mg/L); lactate dehydrogenase level - 322 (95– 190 IU/L); folate Level - 30.7 (4.8–30.5 nmol/L), vitamin B12 - 130 (139–651 pmol/L); iron profile – normal; direct and indirect antibody tests – no antibodies detected; antibodies for parvovirus B19 – positive; IgM antibodies and polymerase chain reaction (PCR) parvovirus - positive. Figure 1 shows the bone marrow aspirate, Figure 2 shows bone marrow trephine biopsy with giant proerythroblast with a megakaryocyte at the left hand end of the, and Figure 3 positive glycophorin stain for giant proerythroblasts.
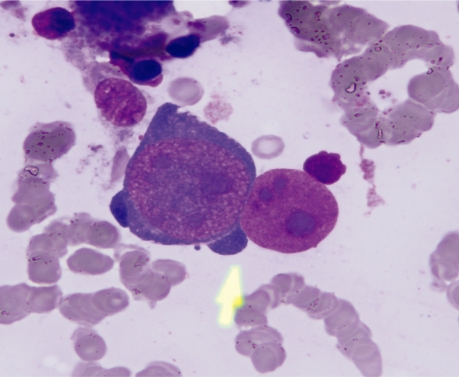
Figure 1:
Bone marrow smear showing two giant proeythroblasts. One intact with intranuclear eosinophilic inclusion body like nucleoli. Arrow points to ‘dog ear’ cytoplasmic projections. Adjacent is a denuded nucleus of a giant proerythroblast. (× 1000).
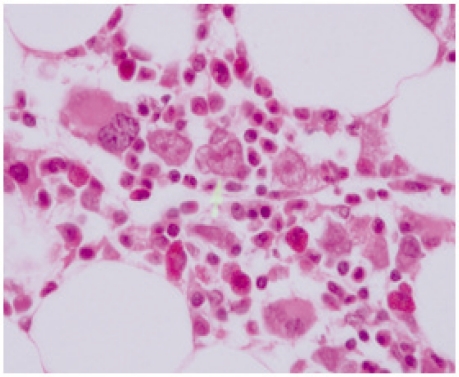
Figure 2:
Trephine biopsy. Arrow points to three giant proerythroblasts with a megakaryocyte at the left hand end of the row (×1000).
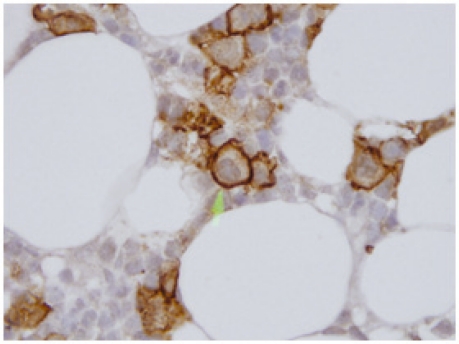
Figure 3:
Trephine biopsy - glycophorin stain giant proerythroblasts staining positive (×1000).
The above investigations show the absence of an aetiology of haemolytic anaemia (immune mediated and non immune), iron deficiency, vitamin B12 or folate deficiency for the anaemia in this patient.
The persisting anaemia, with a reticulocytopenia, no evidence of myelodysplasia, haemolysis, nutritional deficiency, the morphological evidence of parvovirus B19 infection, of gigantoblasts containing viral inclusion bodies and the absence of late normoblasts in the bone marrow confirmed the diagnosis of parvo virus B19 by morphology. Positive staining of giant proerythroblasts with the glycophorin stain, established the large cells as being erythroid in nature. Further confirmation was made by the presence of the anti parvovirus B19 immunoglobulin M (IgM) and positive polymerase chain reaction (PCR) for a high load of parvovirus B19.
The cornerstone of treatment for this disease is passive transfusion of IVIG to enrich the humoral immunity of the patient supported with packed red blood cell transfusions. In the immune competent host, 0.4g/kg/day of IVIG is given for 3 days. In the immunocompromised host, prolonged treatment with IVIG reduces the viral levels to undetectable levels by repeated PCR. A higher dose of 1g/kg/day is used for 5 days in these patients and when necessary for a longer period. Patients generally respond after two weeks of treatment. Some patients may require more than one course of immunoglobulin therapy and some may benefit from alteration of their immunosuppressive therapy.12–16
Other immunomodulatory approaches such as rituximab, steroids, (ALG), cyclophosphamide, and methotrexate have also been used in chronic PRCA. This is especially the case if it present as the primary haematological disorder, with no obvious cause, or in association with other disease ssuch as lymphorproliferative disorders, connective tissue diseases, thymoma, solid tumours, pregnancy, or following bone marrow transplantation, but rarely in patients with parvovirus B19 infection.15
Our patient was treated with 1g of IVIG/kg/day for 5 days and packed red blood cells. His haemoglobin levels recovered in two weeks. He was completely well with a normal complete blood count (CBC) seven months later, at time of writing.
Discussion
In immunocompetent persons with normal red cell life spans, parvovirus B19 infections cause a 5–10 day erythropoietic aplasia, but do not cause significant anaemia as their red cell life span is 120 days.6 However, in patients with haemolytic anaemias whose red cell life spans are shortened, for example 15 days in hereditary spherocytosis, a transient decompensated erythropoiesis leads to a severe anaemia.6 In immunocompromised patients, such as our patient, the infection can persist leading to chronic anaemia. Congenital immunodeficient states, human immunodeficiency virus (HIV) infections, post organ transplants, lymphoproliferative disorders are some of the other known immunodeficient states associated with PRCA caused by parvo virus B19.
The diagnosis rests on the presence of a persistent anaemia, with normal other blood cell counts, a reticulocytopenia, giant proerythroblasts (gigantoproerythroblasts) with prominent eosinophilic viral inclusions in the bone marrow, serum anti parvoviral IgM/IgG and positive PCR for parvoviral DNA. The neutralising antibodies are able to clear the infection usually by 5th to 10th day of infection. It is proposed that with the rising titres the virus is cleared and the giant proerythroblasts are replaced by regenerating erythroid cells.6
Parvoviridae are difficult to culture, but can be grown from the bone marrow. In the immunocompromised host, parvoviral antibodies maybe difficult to demonstrate,6 although the presence of anti parvo viral IgG makes persistent infection improbable. In PRCA caused by other aetiological causes like thymoma, large granular lymphocytic leukaemia, systemic lupus erythematosus and rheumatoid arthritis, giagantoproerythroblasts are not seen. Parvoviral infections are also associated with glomerulonephritis and a nephrotic syndrome such as protein-losing kidney disease.
Conclusion
In isolated persistent anaemia in immunocompromised adults, accompanied by a reticulocytopenia and no other aetiological evidence for the anaemia, a parvovirus B19 induced PRCA should be excluded, particularly as there is no vaccine for protecting those at risk.
References
- 1.Brown KE, Anderson SM, Young NS. Erythrocyte P antigen: Cellular receptor for B10 parvo virus. Science. 1993;262:114–17. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 2.Forea AV, Lonescu DN, Melhem MF. Parvovirus B19 infection in the immunocompromised host. Arch Pathol Lab Med. 2007;131:799–804. doi: 10.5858/2007-131-799-PBIITI. [DOI] [PubMed] [Google Scholar]
- 3.Hayes-Lattin B, Seipei J, Gatter K, Heinrich MC, Mariarz T. Pure red cell aplasia associated with parvovirus B19 infection occurring late after allogeneic bone marrow transplantation. American J Hematol. 2004;75:142–5. doi: 10.1002/ajh.10474. [DOI] [PubMed] [Google Scholar]
- 4.Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485–505. doi: 10.1128/CMR.15.3.485-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi S, Maruta A, Yamamoto T, Katayama N, Higuichi R, Sakano Y, et al. Human parvovirus B19 capsid antigen in granulocytes in parvovirus B19 induced pancytopenia after bone marrow transplantation. Acta Haematol. 1998;100:195–9. doi: 10.1159/000040903. [DOI] [PubMed] [Google Scholar]
- 6.Fisch P, Handgretinger R, Schaefer HE. Pure red cell aplasia. Br J Haematol. 2000;111:1010–22. doi: 10.1046/j.1365-2141.2000.02429.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiland HT, Salimans MM, Fibbe WE, Kluein Pm, Cohen BJ. Prolonged parvovirus B19 infection with severe anaemia in a bone marrow transplant patient. Br J Haematol. 1989;71:300. doi: 10.1111/j.1365-2141.1989.tb04276.x. [DOI] [PubMed] [Google Scholar]
- 8.Solano C, Juan O, Giemno C, Garcia-Conde J. Engraftment failure associated with peripheral blood stem cell transplantation, after B19 parvovirus infection. Blood. 1996;88:1515–17. [PubMed] [Google Scholar]
- 9.Azzi A, Fanci R, Ciappi S, Zakrezewska K, Bosi A. Human parvovirus B19 infection in bone marrow transplantation patients. Am J Hematol. 1993;44:207–9. doi: 10.1002/ajh.2830440314. [DOI] [PubMed] [Google Scholar]
- 10.Broliden K. Parvovirus B19 infection in pediatric solid organ and bone marrow transplantation. Pediatr Transplant. 2001;5:320–30. doi: 10.1034/j.1399-3046.2001.00035.x. [DOI] [PubMed] [Google Scholar]
- 11.Frickhofen N, Arnold R, Hertenstein B, Weisneth M, Young NS. Parvovirus B19 infection and bone marrow transplantation. Ann Hematol. 1992;64:121–4. doi: 10.1007/BF01715363. [DOI] [PubMed] [Google Scholar]
- 12.Waldman M, Kopp JB. Parvovirus-B19-associated complications in renal transplant recipients. Nat Clin Pract Nephrol. 2007;3:540–50. doi: 10.1038/ncpneph0609. [DOI] [PubMed] [Google Scholar]
- 13.Choi SH, Chang SP, Won JC, Lee JS, Chi HS, Yang WS, et al. A case of persistent anemia in a renal transplant recipient: Association with parvovirus B19 infection. J Infect Dis. 2002;34:71–5. doi: 10.1080/003655402753395247. [DOI] [PubMed] [Google Scholar]
- 14.Wong TY, Chan PK, Leung CB, Szeto CC, Tam JS, Li PK. Parvovirus B19 infection causing red cell aplasia in renal transplantation on tacrolimus. Am J Kidney Dis. 1999;34:1132–6. doi: 10.1016/S0272-6386(99)70021-1. [DOI] [PubMed] [Google Scholar]
- 15.Geetha D, Zachary JB, Baldado HM, Kronz JD, Kraus ES. Pure red cell aplasia caused by Parvovirus B19 infection in solid organ transplant recipients: A case report and review of literature. Clin Transplant. 2000;14:586–91. doi: 10.1034/j.1399-0012.2000.140612.x. [DOI] [PubMed] [Google Scholar]
- 16.Sawada K, Fujishima N, Hirokawa M. Acquired pure red cell aplasia: Updated review of treatment. Br J Haematol. 2008;142:505–14. doi: 10.1111/j.1365-2141.2008.07216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]