Abstract
Objective
Electronic medical records (EMRs) are a rich data source for discovery research but are underutilized due to the difficulty of extracting highly accurate clinical data. We assessed whether a classification algorithm incorporating narrative EMR data (typed physician notes), more accurately classifies subjects with rheumatoid arthritis (RA) compared to an algorithm using codified EMR data alone.
Methods
Subjects with ≥1 ICD9 RA code (714.xx) or who had anti-CCP checked in the EMR of two large academic centers were included into an ‘RA Mart’ (n=29,432). For all 29,432 subjects, we extracted narrative (using natural language processing) and codified RA clinical information. In a training set of 96 RA and 404 non-RA cases from the RA Mart classified by medical record review, we used narrative and codified data to develop classification algorithms using logistic regression. These algorithms were applied to the entire RA Mart. We calculated and compared the positive predictive value (PPV) of these algorithms by reviewing records of an additional 400 subjects classified as RA by the algorithms.
Results
A complete algorithm (narrative and codified data) classified RA subjects with a significantly higher PPV of 94%, than an algorithm with codified data alone (PPV 88%). Characteristics of the RA cohort identified by the complete algorithm were comparable to existing RA cohorts (80% female, 63% anti-CCP+, 59% erosion+).
Conclusion
We demonstrate the ability to utilize complete EMR data to define an RA cohort with a PPV of 94%, which was superior to an algorithm using codified data alone.
Electronic medical records (EMRs) used as part of routine clinical care, have great potential to serve as a rich resource of data for clinical and translational research. There are two types of EMR data: ‘codified’ (e.g., entered in a structured format) and ‘narrative’ (e.g., free-form typed text in physician notes). While the exact content will depend on an institutions’ EMR, codified EMR data often include basic information such as age, demographics, billing codes, and laboratory results. The content of narrative data, which often consists of typed information within physician notes, is usually broader in scope, providing information on a patient's chief complaint, symptoms, comorbidities, medications, physical exam, and the physician's impression and plan (1). The ability to tap into this treasure trove of clinical information has widespread appeal – from biologists who link EMR with biospecimen data (2) to epidemiologists who link codified medical record data with outcomes of interest (3). However, EMR clinical data have been underutilized for discovery research because of concerns about data accuracy and validity.
Several studies have used codified EMR data – but not the complete EMR consisting of both narrative and codified data – to classify whether or not a patient has rheumatoid arthritis (RA) (3-7). In one study, at least 3 physician diagnoses of RA according to the International Classification of Disease, 9th Revision (ICD9) was used to identify RA subjects as this method resulted in RA estimates similar to population based studies (8). A 1994 study from the Mayo clinic found that computerized diagnostic codes for RA had a sensitivity of 89%, but a positive predictive value (PPV) of only 57% (4). In the Veterans Administration (VA) database, one ICD9 code for RA was found to be 100% sensitive, but not very specific or accurate (specificity of 55%, PPV of 66%) (5). Addition of a prescription for a disease modifying anti-rheumatic drug (DMARD) increased PPV to 81%, but with a decrease in sensitivity to 85%. These rates of disease misclassification can have a profound impact on research studies that require precise disease definitions.
More recently, computational methods have been developed to extract clinical data entered in typed format from the narrative EMR using a systematic approach. The conventional method of extracting narrative information for clinical research, which requires researchers to manually review charts, is labor intensive and inefficient. In contrast, natural language processing (NLP) represents an automated method of chart review by processing typed text into meaningful components based on a set of rules. To use NLP, a concept is defined that corresponds to a specific clinical variable of interest (e.g. radiographic erosions). Clinical experts developed lists of terms to be used for each NLP query. Terms for erosions might include: ‘presence of erosions on radiographs,’ ‘erosions consistent with RA,’ or ’erosion positive.’ NLP can also incorporate abbreviations (e.g. ‘erosion+’, misspellings- ‘radeograhic erosions’), and negation terms (e.g. ’absence of erosions’). NLP has been applied to a limited number of biomedical settings – for example, mandatory reporting of notifiable diseases (9-11), definition of co-morbid conditions (12-14) and medications (15, 16), and identification of adverse events (17, 18) – but not yet for classification of diseases in an EMR.
In the current study, our objective was to classify RA subjects in our EMR with high positive predictive value. We assessed whether the combination of narrative EMR data (obtained using NLP) and codified EMR data (ICD9 codes, medications, laboratory test results), together with robust analytical methods, can more accurately classify subjects with RA than the standard approach of using codified data alone.
PATIENTS AND METHODS
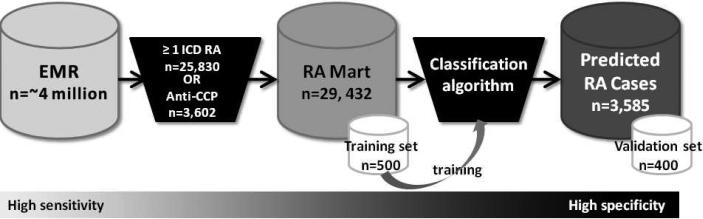
An overview of our approach is outlined in Figure 1. Starting with the complete EMR (narrative + codified data), we : (1) created an RA database (RA Mart) of all possible RA patients; (2) randomly selected 500 subjects from the RA Mart for medical record review to develop a training set of RA and non-RA cases; (3) developed and trained 3 classification algorithms on the training set; (4) applied the 3 classification algorithms to the RA Mart to obtain the predicted RA cases; and (5) validated the classification algorithm by performing medical record reviews on 400 of the predicted RA cases, a validation set, to confirm RA status to determine the positive predictive value (PPV). Steps 3-5 were conducted for each algorithm: narrative + codified EMR data (complete), codified EMR data, narrative only EMR data.
Figure 1.
Overview of approach for classifying RA subjects in the electronic medical record (EMR). Subjects were selected from the EMR with ≥1 ICD9 RA code or had anti-CCP checked to create the RA Mart. 500 subjects were randomly selected from the RA Mart to undergo medical record review to establish a training set. This training set of RA and non-RA cases was used to develop and train the classification algorithm. The classification algorithm was applied to the entire RA Mart to determine predicted RA cases. 400 subjects were randomly selected from the predicted RA cases to undergo medical record review to determine the PPV of the algorithm (validation set). 279×89mm (96 × 96 DPI)
Data source
We studied the Partners HealthCare EMR, which is utilized by two large hospitals, Brigham and Women's Hospital (BWH) and Massachusetts General Hospital (MGH), that combined, care for approximately 4 million patients in the Boston metropolitan area (Massachusetts, USA). The EMR began on October 1, 1996 for BWH and October 3, 1994 for MGH. To build an initial database of potential RA subjects (‘RA Mart’), we selected all subjects with ≥1 ICD9 code for RA and related diseases (714.xx) or subjects who had been tested for antibodies to cyclic citrullinated peptide (anti-CCP) (Figure 1). Subjects who were deceased or age< 18 at the time of the RA Mart creation (June 5, 2008) were excluded. In total 29,432 subjects had at least one ICD9 code for RA (714.xx) (n=25,830) or had been tested for anti-CCP (n=3,602) (4,283 subjects had at least one ICD9 code for RA and had anti-CCP checked). The Partners Institutional Review Board approved all aspects of this study.
Codified EMR data
We used the following codified data in our analysis: ICD9 codes, electronic prescriptions, and anti-CCP and rheumatoid factor (RF) laboratory values. The ICD9 codes included RA and related diseases 714.xx (excluding juvenile idiopathic arthritis/juvenile rheumatoid arthritis (JRA) codes), systemic lupus erythematosus (SLE) 710.0, psoriatic arthritis (PsA) 696, and JRA 714.3x (abbreviated as RA ICD9, PsA ICD9, SLE ICD9, and JRA ICD9). Because a single visit could result in multiple tests and notes, leading to multiple codes for the same day, we eliminated codes that occurred less than one week after a prior code. In our analysis, RA ICD9 was analyzed in two forms: (1) number of RA ICD9 codes for each subject at least one week apart (RA ICD9) and (2) number of normalized RA ICD9 codes which is the natural log of the number RA ICD9 codes for each subject at least one week apart. We determined which subjects were RF and anti-CCP positive according to the cutoffs at each hospital laboratory. The presence of a coded medication signifies that a patient was prescribed the medication by a physician using a computerized prescription program embedded within our EMR or had the medication entered onto a medication list maintained by a physician. The presence of a coded medication does not signify that the medication was actually filled as patients can take prescriptions to any pharmacy. The coded medications assessed in this study included the disease modifying anti-rheumatic medications (DMARDs): methotrexate, azathioprine, leflunomide, sulfasalazine, hydroxychloroquine, penicillamine, cyclosporine, and gold. Biologic agents included the anti-tumor necrosis factors (anti-TNF): infliximab and etanercept, and other agents including abatacept, rituximab and anakinra. Adalimumab was not available as coded data in our system. To provide an index of medical care utilization, we assessed the number of ‘facts’, which is related to the number of medical entries a subject has in the EMR. Examples of a fact include: a physician visit, a visit to the laboratory for a blood draw, a visit to radiology for an X-ray.
Narrative EMR data and natural language processing (NLP)
We used five types of notes to extract information from narrative data: health care provider notes, radiology reports, pathology reports, discharge summaries, and operative reports. We utilized natural language processing (NLP) to extract clinical variables from the narrative data entered in a typed format (no scanned hand-written notes were used). We used the Health Information Text Extraction (HITex) system (19) to extract the clinical information from narrative text. HITEx is an open source NLP tool written in Java and built on the General Architecture for Text Engineering (GATE) framework (20). The NLP application determines the structure of unstructured text records and outputs an annotated document tagging variables of interest (further details provided in Zeng et al., 2006 (19)).
The variables included broad concept terms such as disease diagnoses (RA, SLE, PsA, JRA), medications (listed above, with the addition of adalimumab), laboratory data (RF, anti-CCP, the term seropositive) and radiology findings of erosions on x-rays. We used the Health Information Text Extraction (HITEx) system (19) to extract clinical information from narrative text. We extracted the variables mentioned above from the narrative data and created coded NLP variables for the number of mentions per subject as well as dichotomous variables for each disease diagnosis, medication, laboratory test result, and erosions on x-rays. To account for variability in language usage, a variety of specific phrases can be defined which is then collapsed into a single concept term for analyses. The clinicians on the team developed lists of terms to be used for each NLP query. Further analysis was performed to determine positive or negative variables. For example, a patient was flagged as CCP positive by NLP if terms were found in their records such as ‘anti-CCP+’, ‘CCP positive RA.’ For RF, anti-CCP, seropositive and erosions, a negation finding algorithm was used to distinguish subjects who were positive or negative for the variable. For example, the algorithm could distinguish a subject who was anti-CCP positive vs. anti-CCP negative.
Two reviewers (KPL and RMP) assessed the precision of select NLP concepts: anti-CCP positive, RF positive, seropositive, methotrexate and etanercept. For each concept, one sentence containing the concept was selected from each of 150 randomly selected subjects with records containing the concept. The reviewers assessed whether the concept extraction was correctly described in the context of the sentence. We assessed two categories of NLP concepts. The first assessment for precision identifies whether a concept was identified appropriately from the physician note within a specific sentence. Concepts in this group include disease diagnoses and medications. A patient was scored as ‘correct’ for methotrexate by NLP if the term methotrexate was present in the sentence extracted from the medical record. This includes instances where subjects were prescribed the medication, the medication was held, contemplated or if the subject had taken the medication in the past. The second assessment for precision requires that the patient have a positive result. This pertains to the concepts RF, anti-CCP, seropositive, and erosions. We scored the NLP as correct for ‘RF positive’ only if the patient was also found to be RF positive on review from the sentence extracted from the medical record. We scored NLP as incorrect if RF was mentioned with no evidence that the patient was RF positive (RFpos) in the sentence. An example of how precision (with respect to positive predictive value) was calculated as follows:
Precision= (# sentences RFpos by NLP and confirmed as RFpos on review)/(#sentences RFpos by NLP) The precision of NLP concepts was high: erosions, 88% (95% CI: 84, 91%); seropositive, 96% (95% CI: 95, 97%); CCP+, 98.7% (95% CI: 98, 99%); RF+, 99.3% (95% CI: 99.1, 99.4%); methotrexate, 100%; and etanercept, 100%.
Training set of 500 subjects
We established a training set of 500 subjects randomly selected from the RA Mart for medical record review. To establish the gold standard diagnosis, two rheumatologists (KPL and RMP) reviewed the medical records for the presence of the 1987 American College of Rheumatology Classification Criteria for RA (21) and classified subjects as definite, possible/probable and not RA. Definite RA was defined as subjects who had a rheumatologists’ diagnosis of RA and supporting clinical data such as records describing synovitis, erosions, or greater than one hour of morning stiffness. Possible RA was defined as subjects with persistent inflammatory arthritis with RA in the differential diagnosis by a physician. Subjects with a diagnosis of RA by a physician, but insufficient supporting information of clinical signs and symptoms of the disease, were also classified as possible RA. Finally, subjects with an alternate rheumatologic diagnosis or whose diagnosis was unclear were considered to not have RA.
For our training set, subjects classified as definite RA were considered ‘RA cases’, while subjects classified as possible and as not having RA were classified as ‘controls’. Eighty-one percent of RA cases had sufficient information from the EMR to fulfill the 1987 ACR Classification Criteria for RA (21). This is consistent with the published specificity of the 1987 ACR criteria which ranges from 80-90% when compared to the gold-standard of rheumatologists’ diagnosis of RA (21, 22). Both RMP and KPL reviewed the same 20 subjects to assess percent agreement, and were in 100% agreement on the final diagnosis.
Classification algorithm: selecting informative variables and assigning parameters
We used penalized logistic regression to develop a classification algorithm to predict the probability of having RA (23, 24). To avoid over-fitting the model, we used the adaptive LASSO procedure which simultaneously identifies influential variables and provides stable estimates of the model parameters (25). The optimal penalty parameter was determined based on Bayes’ Information Criterion (BIC). We developed three different algorithms using (i) codified EMR variables only; (ii) narrative EMR variables only; and (iii) complete variables (narrative + codified). All three models were adjusted for age and gender and all predictors were standardized to have unit variance. The predicted probabilities based on these models were used to classify subjects as having RA.
We selected the threshold probability value for classifying RA by setting the specificity level at 97% for all 3 algorithms. Subjects whose predicted probability exceeds the threshold value were classified as having RA, denoted by Alg. To assess the overall accuracy of these algorithms in classifying RA with the training data and to estimate the threshold value for Alg, we used three-fold cross-validation repeated 50 times to correct for potential over-fitting bias. Furthermore, we used the bootstrap method to estimate the standard error and obtain confidence intervals for the accuracy measures. The predictive accuracy of the algorithm to classify RA vs. non-RA was subsequently validated using a separate validation set.
Validation of classification algorithm and assessment of sensitivity, specificity and PPV
Once the classification algorithm was established, we applied it to the remaining RA Mart and assigned a probability of RA to each subject. To validate the performance of the classification algorithm, we randomly sampled an independent set of 400 subjects (validation set) from the subset of subjects who were classified as RA (Alg by any of the three algorithms). These cases were then validated through a blinded medical record review by two rheumatologists, KPL and RMP for RA. The sensitivity, specificity and PPV were calculated using the following formulas:
PPV= (Number of Alg subject confirmed as RA on medical record review)/(Number of Alg subjects)
Sensitivity= (PPV × PAlg)/PRA
Specificity= 1- [((1-PPV) × PAlg)/(1- PRA)]
PAlg is the proportion of subjects identified by the algorithm as having RA in the RA Mart, and PRA is the RA prevalence estimated from the training set. Sampling the validation set from the subset of subjects who were classified as RA can improve the precision in estimating PPV which is the primary accuracy parameter and outcome of interest.
To assess and compare the difference in accuracy between the 3 algorithms, we compared their PPV values and obtained confidence intervals (CIs) using the validation data:
Difference in PPV= PPV complete algorithm- PPV codified variables only algorithm;
Difference in PPV= PPV complete algorithm- PPV narrative variables only algorithm
The differences in PPV were significant if the 95% CI did not include zero. Although the PPV values between the 3 algorithms can be compared, the 95% CIs associated with the PPV values (in contrast to the difference in PPV) cannot since these estimates were derived from the same validation set of 400 subjects for all 3 algorithms.
For comparison, we also assessed the accuracy of criteria used in administrative database studies: ≥3 ICD codes for RA (8) and ≥ 1 RA ICD9 code + at least one DMARD (5). We used the training set to generate these data as it allows for unbiased estimates of these simple criteria. To compare differences in accuracy between our algorithms and the simple criteria above, we also used the difference in PPV and 95% CI.
Descriptive statistics
We assessed differences in characteristics between RA cases and controls in the training set using the t-test and the Wilcoxon Rank Sum test to compare differences between means and medians respectively. P-values are two-sided. Chi-square was used for between-group comparisons expressed as proportions and analysis of variance (ANOVA) for comparison of multiple groups.
Case only analysis
To assess whether our EMR RA cohort can replicate known associations among clinical variables, we performed a case only analysis to compare the risk of erosions in anti-CCP+ vs. CCP- subjects and RF+ vs. RF- subjects. We assessed the association between anti-CCP and radiographic erosions by including only those subjects in our database who have had anti-CCP tested in the clinical laboratory (e.g., autoantibody status was derived from codified data). Similarly, we assessed the relationship between RF and erosions only among those who had RF tested. Odds ratios and 95% CI were calculated using 2×2 contingency tables. All analyses were conducted with SAS software, version 9.2 (SAS Institute) and the R package (The R project for Statistical Computing, http://www.r-project.org/).
RESULTS
Classification algorithm
An overview of our approach is shown in Figure 1. Out of 500 subjects sampled from the RA Mart (training set), 96 (19%) subjects with a single ICD9 code for RA were determined to have a diagnosis of RA by medical record review; the remaining 404 subjects had either possible RA (n=84) or no evidence of RA (n=320). The clinical characteristics extracted from the codified compared to narrative EMR data are shown in Tables 1a and 1b for the 500 subjects in our training set. There was a strong correlation between identification as an RA case on medical record review and having a higher number of RA ICD9 codes and a higher number of NLP mentions of RA (P<0.0001). Methotrexate was the most common medication prescribed for RA cases (34.7% in the codified EMR data ) and was also the most commonly mentioned medication in the text notes (82% in the narrative EMR data).
Table 1.
(a) Characteristics of the training set; (b) Comparison of the distribution of codified compared to narrative data extracted using NLP in the training set (n=500).
| Characteristic | RA Cases | Controls* | p-value |
|---|---|---|---|
| Total, n (%) | 96 (19%) | 404 (81%) | |
| | |||
| Age, mean (SD) | 60.4 (16) | 56.1 (18.6) | 0.04 |
| Female, n (%) | 74 (78) | 300 (74.6) | 0.6 |
| Race, n (%) | 0.0003 | ||
| White | 62 (66) | 285 (70.9) | |
| Black | 3 (3.2) | 45 (11.2) | |
| Other | 36 (8.9) | 7 (7.5) | |
| Unknown | 36 (8.9) | 22 (23.4) | |
| # Facts, median (IQR**) | 750 (2159) | 952 (1722) | 0.5 |
| Rheumatologist dx RA, n (%) | 95 (100) | 21 (5) | <.0001 |
| Fulfills ACR criteria, n (%) | 77 (81) | 9 (2.2) | <.0001 |
| Codified data | Narrative data | |||||
|---|---|---|---|---|---|---|
| RA Cases | Controls* | p-value | RA cases | Controls* | p-value | |
| Total, n (%) | 96 (19%) | 404 (81%) | 96 (19%) | 404 (81%) | ||
| | ||||||
| Disease codes per subject, median (range) | ||||||
| | ||||||
| RA codes | 11 (141) | 1 (60) | <.0001 | 10 (111) | 0 (77) | <.0001 |
| PsA codes | 0 (1) | 0 (110) | 0.2 | 0 (3) | 0 (137) | 0.18 |
| SLE codes | 0 (9) | 0 (67) | 0.007 | 0 (13) | 0 (115) | 0.007 |
| JRA codes | 0 (4) | 0 (39) | 0.82 | 0 (15) | 0 (60) | 0.55 |
| Medications, n (%) | ||||||
| | ||||||
| MTX | 33 (34.7) | 39 (9.7) | <.0001 | 78 (82) | 100 (24.9) | <.0001 |
| Anti-TNF | 30 (31.3) | 20 (5) | <.0001 | 47 (49) | 47 (11.6) | <.0001 |
| Autoantibody studies, n (%) | ||||||
| | ||||||
| CCP + | 19 (20) | 8 (2) | <.0001 | 15 (15.8) | 7 (1.7) | <.0001 |
| RF+ | 43 (45.3) | 115 (28.6) | 0.0021 | 33 (34.7) | 36 (8.9) | <.0001 |
| Seropositive** | 45 (47.4) | 116 (28.6) | <.0001 | 31 (32.6) | 7 (1.7) | <.0001 |
| Radiology, n (%) | ||||||
| | ||||||
| Erosions | NA | NA | NA | 42 (44.2) | 35 (8.7) | <.0001 |
Controls= subjects with possible and no RA
IQR= interquartile range
Controls= subjects with possible and no RA
Seropositive in codified data= RF or anti-CCP+, in narrative data= term “seropositive”
To select the most informative variables that differentiate the RA cases from the controls in our training set, we used a statistical method based on penalized logistic regression. This method identified 14 variables for the narrative and codified (complete) classification algorithm shown in Table 2 in order of predictive value. The features selected for the codified EMR variables only algorithm included in order of predictive value, for positive predictors: ICD9 RA, normalized ICD9 RA, anti-TNF, RF positive, methotrexate; negative predictors included: ICD9 JRA, ICD9 SLE, ICD9 PsA. The features selected for narrative (NLP) EMR variables only algorithm included, for positive predictors: RA, seropositive, anti-TNF, positive erosions, methotrexate, CCP positive, other DMARDs, age; negative predictors included: SLE, PsA, JRA.
Table 2.
Variables selected for the complete algorithm (narrative and codified EMR data) from the logistic regression in order of predictive value.
| Variable | Standardized regression coefficient | Standard error |
|---|---|---|
| Positive predictors | ||
| NLP RA | 1.11 | 0.48 |
| NLP seropositive | 0.74 | 0.26 |
| ICD9 RA normalized* | 0.71 | 0.23 |
| ICD9 RA | 0.66 | 0.44 |
| NLP erosion | 0.46 | 0.29 |
| Codified RF negative | 0.36 | 0.36 |
| NLP methotrexate | 0.3 | 0.34 |
| codified anti-TNF** | 0.29 | 0.3 |
| NLP anti-CCP positive | 0.27 | 0.25 |
| NLP anti-TNF*** | 0.2 | 0.36 |
| NLP other DMARDs | 0.13 | 0.34 |
| Negative predictors | ||
| ICD9 JRA | -0.98 | 0.9 |
| ICD9 SLE | -0.57 | 1.09 |
| NLP PsA | -0.51 | 0.74 |
ICD9 RA normalized= ln (# ICD9 RA codes per subject at least one week apart)
codified anti-TNF= etanercept, infliximab (adalimumab was not available in our EMR)
NLP anti-TNF= adalimumab, etanercept, infliximab
We applied the three algorithms to the entire RA Mart of 29,432 subjects (excluding the 500 subjects from the training set). The narrative and codified (complete) classification algorithm classified 3,585 subjects as having RA (Table 3). The codified-only and narrative-only algorithms classified 3,046 and 3,341 subjects, respectively, as having RA.
Table 3.
Comparison of performance characteristics from validation of the complete classification algorithm (narrative + codified) to algorithms containing codified-only and narrative-only data; the complete classification algorithm was also compared to criteria for RA used in published administrative database studies.
| Model | RA by algorithm or criteria, n | PPV (%) (95% CI) | Sens (%) (95% CI) | Difference in PPV* (95% CI) |
|---|---|---|---|---|
| Algorithms | ||||
| Narrative + codified (complete) | 3585 | 94 (91, 96) | 63 (51, 75) | reference |
| Codified only | 3046 | 88 (84, 92) | 51 (42, 60) | 6 (2,9)** |
| NLP only | 3341 | 89 (86, 93) | 56 (46, 66) | 5(1,8)** |
| Published administrative codified criteria | ||||
| ≥ 3 ICD9 RA | 7960 | 56 (47,64) | 80 (72,88) | 38 (29, 47)** |
| ≥1 ICD9 RA + DMARD | 7799 | 45 (37, 53) | 66 (57,76) | 49 (40, 57)** |
Difference in PPV= (PPV of complete algorithm) – (comparison algorithm or criteria)
Significant difference in PPV compared to complete algorithm
Validation of the classification algorithm
The narrative and codified (complete) classification algorithm performed significantly better than algorithms using either codified or narrative data alone (Table 3). There was a 6% (95% CI 2, 9%) difference in PPV between the complete algorithm compared to the codified-only algorithm, and a 5% (95% CI 1, 8) difference in PPV between the complete algorithm and the narrative-only algorithm. The estimated sensitivities were also lower in the codified-only and narrative-only algorithms (51% and 56%, respectively, compared to 63% for the complete algorithm). Examples of the diagnoses of the subjects misclassified in the complete algorithm were: erosive osteoarthritis, psoriatic arthritis, a “spondylytic variant,” and “right knee monoarthritis.”
We also applied criteria used in administrative database studies for comparison: ≥3 ICD codes for RA (8) and ≥ 1 RA ICD9 code + at least one DMARD (5) (Table 3). The PPV of the former was 56% (95% CI: 47, 64%) and the latter was 45% (95% CI: 37, 52%). Using the complete classification algorithm resulted in an increase in PPV of 38% (95% CI 29, 47%) when compared to ≥3 RA ICD codes, and an increase in PPV of 49% (95% CI: 40, 57%) when compared to ≥1 RA ICD9 code + at least one DMARD.
We used the PPV estimates to determine the increase in the total number of RA subjects classified by our three algorithms. The complete algorithm with a PPV of 94% would identify 3,370 RA subjects, the codified data-only algorithm with a PPV of 88% would identify 2,680 RA subjects, and a narrative data-only algorithm would identify 2973 RA subjects. This represents a 26% increase in the identification of true RA subjects if the complete algorithm were used compared to the codified-only algorithm.
Clinical characteristics of EMR RA cohort
We assessed the clinical characteristics of the 3,585 subjects classified as having RA (EMR cohort). The clinical characteristics of our EMR cohort were similar to published data from the Consortium of Rheumatology Researchers of North America (CORRONA) (26), an independent cohort of RA subjects assembled through traditional patient recruitment (Table 4).
Table 4.
Characteristics of patients classified as RA by the complete classification algorithm from our EMR compared to published data from CORRONA.
| Characteristics | EMR cohort, n=3,585 | CORRONA, n=7,971 |
|---|---|---|
| Mean age (SD) | 57.5 (17.5) | 58.9 (13.4) |
| Female (%) | 79.9 | 74.5 |
| Anti-CCP positive (%) | 63 | N/A |
| RF positive (%) | 74.4 | 72.1 |
| Erosions (%) | 59.2 | 59.7 |
| MTX (%) | 59.5 | 52.8 |
| Anti-TNF (%) | 32.6 | 22.6 |
We also assessed whether we could reproduce known associations between clinical features within our EMR cohort. Consistent with previous reports, we found that anti-CCP+ subjects (defined using codified EMR data) have an elevated risk of erosions (defined using NLP from narrative data) compared to anti-CCP- subjects, OR 1.5 (95% CI 1.2, 1.9) (27). We observed a similar relationship when RF+ were compared to RF- subjects, OR 1.3 (95% CI 1.1, 1.6). The trend towards higher risk of erosions in RA subjects seen in anti-CCP+ compared to those who are RF+ is consistent to those seen in the published literature (28, 29).
DISCUSSION
With the increasing adoption of electronic medical records (30, 31) and the high cost of maintaining large cohort studies, harnessing the complete EMR (narrative and codified data) for use in biomedical research offers an untapped resource for clinical and translational research. We have demonstrated that it is possible to accurately identify a cohort of RA subjects within an EMR with characteristics comparable to those of large cohort studies recruited using conventional methods. This represents a novel approach for establishing large patient registries in a high-throughput and cost-effective manner.
A major criticism of EMR data is accuracy. In our study, we provide convincing evidence that complete EMR data (narrative and codified data), together with robust analytical methods, can be used to identify subjects with RA with a high PPV of 94% for the complete algorithm. There was a significant increase in the PPV of 6% when narrative data was included to an algorithm containing only codified data. This degree of accuracy is substantially higher than previous studies that use EMR data (5). In our study the PPV of a single ICD9 code was only 19% (prevalence of RA in the training set). Published studies using a combination of codified data only, had lower PPVs when applied to our dataset; ≥3 ICD codes for RA (8) had a PPV of 56%, and ≥ 1 RA ICD9 code + one DMARD (5) had a PPV of 45% in our dataset. Moreover, incorporation of narrative data into a classification algorithm containing codified data only not only increased the PPV, but also the sensitivity, thereby increasing the sample size by 26%. The increase in PPV and sample size can have a profound impact on the power of the study, particularly those requiring precise disease phenotypes.
There are at least two reasons why our approach outperformed previous methods. First, we used NLP to incorporate the narrative EMR data into our classification algorithm. Narrative EMR data are increasingly accessible, with an estimated 20-30% of physicians maintaining electronic notes on their subjects (31-33). In our RA Mart, some clinical data were available in narrative notes but not in the codified EMR (e.g., radiographic erosions), and some clinical data were more detailed in the narrative notes than in the codified EMR data. For example, codified data for methotrexate is present only if it was prescribed, whereas narrative methotrexate data were available if a subject was on methotrexate, if it was taken in the past, or if it was considered. Second, we developed a robust algorithm that selected the most informative variables from an expanded list of potential clinical variables. We did not rely on a pre-specified set of rules to categorize subjects as is often used in administrative database studies. In our complete model, using both narrative and codified EMR data, the selected variables were quite diverse, including diagnostic codes of RA (NLP for rheumatoid arthritis and codified ICD9 codes for RA), concurrent medication (NLP for methotrexate), absence of diseases that mimic RA (SLE, JRA and psoriasis), and presence of RA-specific autoantibodies. This technique using a multivariable model can result with a counterintuitive direction of influence for a particular variable due to collinearity; in our model using both codified and NLP data, the codified RF negative variable had a positive influence on selecting RA subjects. This was likely due to collinearity of the codified RF negative variable with the NLP anti-CCP positive variable. Overall, it is the combined influence of all the variables in the model that are important for prediction of RA. In the algorithm using codified data alone, RF positive had a positive influence and RF negative was not included into the model.
An exciting prospect is implementation of our approach in EMRs at other institutions to demonstrate portability of our EMR algorithm to classify RA patients. The tools and techniques utilized in building our EMR database, such as the program used for NLP are open source and are freely available to the rheumatology community (www.i2b2.org). Similarly, institutions with primarily codified EMR data can implement our codified-only EMR algorithm (sensitivity of 51%, PPV of 88%). Institution specific expertise from clinicians, statisticians and bioinformaticians would be required to optimize the performance of any EMR algorithm.
Increasingly, efforts have been made to link EMR data with biospecimen repositories (2). At our institution (Partners HealthCare), a concerted effort has been made to link discarded blood samples to EMR data, thereby enabling serologic and genetic studies (34). Once this infrastructure is in place, collection of biospecimen is affordable on a large scale (and across multiple diseases). A similar infrastructure at other institutions would create a large national RA registry with access to biospecimen linked to detailed EMR clinical data.
An important limitation of conducting studies based in EMRs is access to only clinical data that are collected as part of routine patient care at the institution(s). For our study, we used an EMR with comprehensive outcomes and clinical information for subjects who obtained care at two tertiary care academic medical centers; other centers may have more limited EMR clinical data. Without additional institutional review board approval, we cannot re-contact subjects to employ detailed questionnaires on exposures or other clinical variables.
In conclusion, creating clinical research databases from an EMR is an efficient and powerful tool for clinical and translational research. If successfully implemented across multiple institutions, it is theoretically possible to establish large patient registries with detailed clinical outcome data, where each institution could maintain local control of confidential patient clinical data. Biomedical research – and ultimately patients with RA – have much to gain by utilization of EMRs for discovery research.
ACKNOWLEDGMENTS
Partners Research Patient Database Registry (RPDR)
T. Cai, S. Churchill, S. Goryachev, E. Karlson, I. Kohane, S. Murphy, R. Plenge, P. Szolovits and Q. Zeng-Treitler are supported by the i2b2 grant (NIH U54 LM008748); S. Murphy is also supported by NIH grants UL1 RR02578-01 and R01 HL091495-01A1; E. Karlson is supported by NIH grants R01 AR049880, P60 AR047782, K24 AR0524-01; K. Liao is supported by NIH T32 AR055885; R. Plenge holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund; S. Raychaudhuri is supported by NIH K08 AR 055688-01A1; Q. Zeng-Treitler is also supported by NIH grants R01 LM007222, R01 DK075837, R01 LM009966, R21 NR0101710-01, R21 NS067463.
REFERENCES
- 1.Poon EG, Jha AK, Christino M, Honour MM, Fernandopulle R, Middleton B, et al. Assessing the level of healthcare information technology adoption in the United States: a snapshot. BMC Med Inform Decis Mak. 2006;6:1. doi: 10.1186/1472-6947-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi B. Biomedical science: betting the bank. Nature. 2008;452(7190):926–9. doi: 10.1038/452926a. [DOI] [PubMed] [Google Scholar]
- 3.Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65(12):1608–12. doi: 10.1136/ard.2005.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabriel SE. The sensitivity and specificity of computerized databases for the diagnosis of rheumatoid arthritis. Arthritis Rheum. 1994;37(6):821–3. doi: 10.1002/art.1780370607. [DOI] [PubMed] [Google Scholar]
- 5.Singh JA, Holmgren AR, Noorbaloochi S. Accuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum. 2004;51(6):952–7. doi: 10.1002/art.20827. [DOI] [PubMed] [Google Scholar]
- 6.Katz JN, Barrett J, Liang MH, Bacon AM, Kaplan H, Kieval RI, et al. Sensitivity and positive predictive value of Medicare Part B physician claims for rheumatologic diagnoses and procedures. Arthritis Rheum. 1997;40(9):1594–600. doi: 10.1002/art.1780400908. [DOI] [PubMed] [Google Scholar]
- 7.Losina E, Barrett J, Baron JA, Katz JN. Accuracy of Medicare claims data for rheumatologic diagnoses in total hip replacement recipients. J Clin Epidemiol. 2003;56(6):515–9. doi: 10.1016/s0895-4356(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 8.Schneeweiss S, Setoguchi S, Weinblatt ME, Katz JN, Avorn J, Sax PE, et al. Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007;56(6):1754–64. doi: 10.1002/art.22600. [DOI] [PubMed] [Google Scholar]
- 9.Effler P, Ching-Lee M, Bogard A, Ieong MC, Nekomoto T, Jernigan D. Statewide system of electronic notifiable disease reporting from clinical laboratories: comparing automated reporting with conventional methods. Jama. 1999;282(19):1845–50. doi: 10.1001/jama.282.19.1845. [DOI] [PubMed] [Google Scholar]
- 10.Klompas M, Haney G, Church D, Lazarus R, Hou X, Platt R. Automated identification of acute hepatitis B using electronic medical record data to facilitate public health surveillance. PLoS One. 2008;3(7):e2626. doi: 10.1371/journal.pone.0002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarus R, Klompas M, Campion FX, McNabb SJ, Hou X, Daniel J, et al. Electronic Support for Public Health: validated case finding and reporting for notifiable diseases using electronic medical data. J Am Med Inform Assoc. 2009;16(1):18–24. doi: 10.1197/jamia.M2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meystre S, Haug P. Improving the sensitivity of the problem list in an intensive care unit by using natural language processing. AMIA Annu Symp Proc. 2006:554–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Meystre S, Haug PJ. Natural language processing to extract medical problems from electronic clinical documents: performance evaluation. J Biomed Inform. 2006;39(6):589–99. doi: 10.1016/j.jbi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Solti I, Aaronson B, Fletcher G, Solti M, Gennari JH, Cooper M, et al. Building an Automated Problem List Based on Natural Language Processing: Lessons Learned in the Early Phase of Development. AMIA Annu Symp Proc. 2008;2008(2008):687–691. [PMC free article] [PubMed] [Google Scholar]
- 15.Levin MA, Krol M, Doshi AM, Reich DL. Extraction and mapping of drug names from free text to a standardized nomenclature. AMIA Annu Symp Proc. 2007:438–42. [PMC free article] [PubMed] [Google Scholar]
- 16.Turchin A, Morin L, Semere LG, Kashyap V, Palchuk MB, Shubina M, et al. Comparative evaluation of accuracy of extraction of medication information from narrative physician notes by commercial and academic natural language processing software packages. AMIA Annu Symp Proc. 2006:789–93. [PubMed] [Google Scholar]
- 17.Bates DW, Evans RS, Murff H, Stetson PD, Pizziferri L, Hripcsak G. Detecting adverse events using information technology. J Am Med Inform Assoc. 2003;10(2):115–28. doi: 10.1197/jamia.M1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penz JF, Wilcox AB, Hurdle JF. Automated identification of adverse events related to central venous catheters. J Biomed Inform. 2007;40(2):174–82. doi: 10.1016/j.jbi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Zeng QT, Goryachev S, Weiss S, Sordo M, Murphy SN, Lazarus R. Extracting principal diagnosis, co-morbidity and smoking status for asthma research: evaluation of a natural language processing system. BMC Med Inform Decis Mak. 2006;6(30) doi: 10.1186/1472-6947-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham H, Humphreys K, Gaizauskas R. GATE - a TIPSTER-based General Architecture for Text Engineering.. Proceedings of the TIPSTER Text Program (Phase III) 6 Month Workshop; DARPA. 1997. [Google Scholar]
- 21.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 22.Banal F, Dougados M, Combescure C, Gossec L. Sensitivity and specificity of the American College of Rheumatology 1987 criteria for the diagnosis of rheumatoid arthritis according to disease duration: a systematic literature review and meta-analysis. Ann Rheum Dis. 2009;68(7):1184–91. doi: 10.1136/ard.2008.093187. [DOI] [PubMed] [Google Scholar]
- 23.Zou H, Hastie T, Tibshirani R. On the ‘Degrees of Freedom’ of LASSO. Technical report: Stanford University Department of Statistics. 2004 [Google Scholar]
- 24.Wang H, Li R, Tsai CL. On the Consistency of SCAD Tuning Parameter Selector. Biometrika. 2008 in press. [Google Scholar]
- 25.Zou H. The adaptive lasso and its oracle properties. Journal of the American Statistical Association. 2006;101(476):1418–1429. [Google Scholar]
- 26.Greenberg JD, Reed G, Kremer JM, Tindall E, Kavanaugh A, Zheng C, et al. Association of Methotrexate and TNF antagonists with risk of infection outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis. 2009 doi: 10.1136/ard.2008.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forslind K, Ahlmen M, Eberhardt K, Hafstrom I, Svensson B. Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti-CCP). Ann Rheum Dis. 2004;63(9):1090–5. doi: 10.1136/ard.2003.014233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bukhari M, Thomson W, Naseem H, Bunn D, Silman A, Symmons D, et al. The performance of anti-cyclic citrullinated peptide antibodies in predicting the severity of radiologic damage in inflammatory polyarthritis: results from the Norfolk Arthritis Register. Arthritis Rheum. 2007;56(9):2929–35. doi: 10.1002/art.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DM, Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis. 2003;62(9):870–4. doi: 10.1136/ard.62.9.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jha AK, DesRoches CM, Campbell EG, Donelan K, Rao SR, Ferris TG, et al. Use of electronic health records in U.S. hospitals. N Engl J Med. 2009;360(16):1628–38. doi: 10.1056/NEJMsa0900592. [DOI] [PubMed] [Google Scholar]
- 31.Jha AK, Ferris TG, Donelan K, DesRoches C, Shields A, Rosenbaum S, et al. How common are electronic health records in the United States? A summary of the evidence. Health Aff (Millwood) 2006;25(6):w496–507. doi: 10.1377/hlthaff.25.w496. [DOI] [PubMed] [Google Scholar]
- 32.Berner ES, Detmer DE, Simborg D. Will the wave finally break? A brief view of the adoption of electronic medical records in the United States. J Am Med Inform Assoc. 2005;12(1):3–7. doi: 10.1197/jamia.M1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DesRoches CM, Campbell EG, Rao SR, Donelan K, Ferris TG, Jha A, et al. Electronic health records in ambulatory care--a national survey of physicians. N Engl J Med. 2008;359(1):50–60. doi: 10.1056/NEJMsa0802005. [DOI] [PubMed] [Google Scholar]
- 34.Murphy S, Churchill S, Bry L, Chueh H, Weiss S, Lazarus R, et al. Instrumenting the health care enterprise for discovery research in the genomic era. Genome Res. 2009;19(9):1675–81. doi: 10.1101/gr.094615.109. [DOI] [PMC free article] [PubMed] [Google Scholar]