Abstract
Rhinitis is a global problem and is defined as the presence of at least one of the following: congestion, rhinorrhea, sneezing, nasal itching, and nasal obstruction. The two major classifications are allergic and nonallergic rhinitis (NAR). Allergic rhinitis occurs when an allergen is the trigger for the nasal symptoms. NAR is when obstruction and rhinorrhea occurs in relation to nonallergic, noninfectious triggers such as change in the weather, exposure to caustic odors or cigarette smoke, barometric pressure differences, etc. There is a lack of concomitant allergic disease, determined by negative skin prick test for relevant allergens and/or negative allergen-specific antibody tests. Both are highly prevalent diseases that have a significant economic burden on society and negative impact on patient quality of life. Treatment of allergic rhinitis includes allergen avoidance, antihistamines (oral and intranasal), intranasal corticosteroids, intranasal cromones, leukotriene receptor antagonists, and immunotherapy. Occasional systemic corticosteroids and decongestants (oral and topical) are also used. NAR has 8 major subtypes which includes nonallergic rhinopathy (previously known as vasomotor rhinitis), nonallergic rhinitis with eosinophilia, atrophic rhinitis, senile rhinitis, gustatory rhinitis, drug-induced rhinitis, hormonal-induced rhinitis, and cerebral spinal fluid leak. The mainstay of treatment for NAR are intranasal corticosteroids. Topical antihistamines have also been found to be efficacious. Topical anticholinergics such as ipratropium bromide (0.03%) nasal spray are effective in treating rhinorrhea symptoms. Adjunct therapy includes decongestants and nasal saline. Investigational therapies in the treatment of NAR discussed include capsaicin, silver nitrate, and acupuncture.
Keywords: Allergic rhinitis, nonallergic rhinitis, intranasal corticosteroids, immunotherapy, intranasal antihistamines, oral antihistamines
ALLERGIC RHINITIS
Definition
Rhinitis is defined as the presence of at least one of the following: congestion, rhinorrhea, sneezing, nasal itching, and nasal obstruction.1,2 Other reported symptoms include throat clearing, headaches, facial pain, ear pain, itchy throat and palate, snoring, and sleep disturbances.3,4 A system of rating symptom severity has been developed using a 7-point visual analog scale that includes elements of nasal symptoms, non-nasal symptoms, and the effects of medications. (See reference for copies of assessment forms).5 Allergic rhinitis is present when these symptoms are triggered by an allergen. Perennial allergic rhinitis is most often attributed to dust mites, mold spores, and animal dander, whereas seasonal allergic rhinitis is attributed to a large variety of pollens that varies based on geographical region.2
Epidemiology
Allergic rhinitis is very common condition throughout the world.6 In the United States it affects between 10-30% of the adult general population and up to 40% of children. This accounts for 30-60 million people in the United States1 and the prevalence has been increasing in recent decades,2 making it the fifth most common chronic disease in the US.7 Risk factors include an atopic family history, IgE levels above 100 IU/mL before the age of 6 years, higher socioeconomic status, and positive epicutaneous allergen testing.1 However, 44-87% of people with rhinitis have mixed allergic and non-allergic rhinitis,1 and therefore all that sneezes is not necessarily purely allergic in etiology.
While many patients downplay rhinitis symptoms as an inconvenience rather than a disease, the economic burden is quite significant. In the United States, the direct medical costs (physician services, diagnostics, medications, etc.) nearly doubled from US$6.1 billion in 2000 to US$11.2 billion in 2005.8 In Europe, it was estimated that by the late 1990s, €1.0-1.5 billion were spent on direct costs.2 Additionally, the indirect costs (travel for physician visits, decreased work productivity, missed school and loss of parents' pay from missed work to care for their children, etc.) are also considerable. In the US, there are 3.5 million lost workdays and 2 million lost school days due to allergic rhinitis. It is estimated that productivity decreases by US$600 per affected employee per year, which is a greater loss than asthma, diabetes, and coronary heart disease. Overall, allergic rhinitis was the fifth costliest chronic disease in the United States with 75% of the costs coming from decreased productivity.4,8 The indirect costs in Europe were estimated to be more than the direct costs at €1.5-2.0 billion.2
Pathophysiology
Cellular signals
Allergic rhinitis is an IgE-mediated disease resulting in inflammation of the nasal mucosa. Allergic patients have increased levels of allergen specific IgE in their nasal mucosa compared to controls. Histamine release from resident mast cells is a major mediator in the inflammation of allergic rhinitis. Eosinophilic inflammation also plays an important role. A Th2 response ensues with the release of IL-4 and IL-5. Recently, thymic stromal lymphopoietin (TSLP), IL-25 (or IL-17E), and IL-33 have also been implicated. As eosinophils produce IL-5 and granulocyte macrophage-colony stimulating factor (GM-CSF), they perpetuate their own survival. After allergen exposure, rhinitis can persist for several weeks.2,9 There is an immediate and a late phase to allergic rhinitis. Both are characterized by the same symptoms, but the late phase's predominate symptom is nasal congestion. Eosinophils release mediators that can induce tissue damage, and pre-treating with topical glucocorticoids reduces eosinophil infiltration and cytokine release.1
Neuronal aspects
The interplay between sensory nerve fibers and the efferent sympathetic and parasympathetic neurons helps to regulate the mucosal barrier of the nasal epithelium. The thinly myelinated Aδ fibers convey the sensations of pain and cold to the central nervous system. A thick mucosal lining decreases the ability of these neurons to sense passing airflow, which contributes, to the sensation of nasal obstruction and dyspnea. When menthol receptors on these nerves are stimulated, the result is a false sense of nasal patency and less dyspnea. After the initial rapid stimulation of Aδ fibers, a delayed activation of the non-myelinated slowly conducting C fibers ensues. In addition to multiple allergens, the C fibers can be stimulated by nicotine, cigarette smoke, aldehyde, formaldehyde, isocyanates, sulfur dioxide, and other toxicants. Capsaicin is the naturally occurring substance in spicy peppers that induces the sensation of heat, and it activates transient receptor potential and ion channel proteins (TRPs). A stinging sensation similar to that induced by capsaicin occurs when the osmotic tonicity rapidly changes at the cellular surface. This can happen when dry pollen and dust grains land on mucosal surfaces, causing water to efflux from epithelial cells.
Acetylcholine is released from parasympathetic nerve fibers that innervate glands and vessels of the airway mucosa. Eosinophils interfere with the activation of the presynaptic M2 muscarinic receptor, which decreases the negative feedback on acetylcholine release. The result is an increase in bronchoconstriction and glandular secretion. To balance the effects of the parasympathetic nervous system, sympathetic neurons induce vasoconstriction in the epithelium. Stimulation of α-adrenergic receptors by nasal decongestants (discussed below) reduces mucosal thickness.
The nociceptive C fibers innervate glands and deep subepithelial vessels. Their release of substance P may lead to increased expression of E-selectin and VCAM on endothelial cells. The result is increased infiltration of leukocytes, which is a critical part of the late-phase response of allergic rhinitis. Interestingly, when substance P is administered to allergic individuals, mRNA levels of IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, TNFα, and γ-interferon vs. only an increase of IL-6 and IL-6 mRNA in non-allergic individuals. Neural plasticity also comes into play in allergic subjects. This occurs when persistent stimulation from allergens increases the sensitivity of involved neurons to depolarize. Allergic individuals' neurons will depolarize in the presence of bradykinin and endothelin, whereas these substance induce no response in non-allergic subjects.
Because it is more difficult to localize visceral compared to peripheral sensations, the activation of nerve fibers that innervate deep tissues often results in referred pain. Sinus headaches are a common example. Noxious stimulation of the inferior turbinate induces the sensation of pain in the maxillary teeth, zygoma, and eyes. The middle turbinates refer pain to the temple, zygoma, inner canthus, and forehead.10
Genetics
Monozygotic twins show a concordance of 45-60% in the development of allergic rhinitis, and dizygotic twins have a concordance rate of about 25%. These data point to a genetic link. However studies into the genetics of allergic rhinitis are lacking, and current findings are preliminary. Chromosome 3 has three regions linked to allergic rhinitis, 3q13, 3q13.31, and 3p24. A possible involved region on chromosome 4 is 4q24-q27. Certain single nucleotide polymorphisms (SNPs) have been implicated GATA3 and IL-13.9 Specific HLA haplotypes have been associated with allergic responses to particular allergens. This may be due to more than just an association since HLAs present antigens to T-cells. There is also evidence that points to genetic associations of the T-cell receptor (TCR) α-chain and the high affinity IgE receptor FcεRI with increased allergy. Other candidate genes for further investigation include those involved with the production of IgE, IL-4, IL-5, and IL-13.11
Treatment
Avoidance
Since allergic rhinitis is induced by specific allergens, it makes sense that avoiding those triggers would be an effective treatment. However, this is not always possible as in the case of pollens, and for those with mixed allergic and non-allergic rhinitis, avoidance will not completely alleviate their symptoms. Some allergens can and should be avoided as the severity of rhinitis correlates with the levels of allergens in the environment. Precautions can be taken against dust mites. Carpet removal, removal of soft toys from the bedroom, using allergen-impermeable bedding covers for the mattress and pillow, vacuuming with a high-efficiency particulate air (HEPA) filter, and washing bedclothes and bed sheets in hot water (60℃) are helpful. Any single method alone is unlikely to provide benefit, and patient should be encouraged to use multiple interventions. For those with animal allergies, ideally, removal of the pet from the home would be best along with careful vacuuming of all carpets, upholstered furniture, and mattresses. It may be impossible to clear cat dander or take up to 20 weeks for cat dander levels to decrease to cat free homes. Isolating the pet to a single room and using a HEPA filter is a second best option. Studies have been inconsistent on the benefits of regular bathing of cats.1,2 Spaying or neutering cats and dogs increases levels of their major allergens found in homes, Fel d 1 and Can f 1 respectively. Having fewer pets correlates with lower dander levels. Interestingly, keeping cats outside does not significantly reduce the presence of Fel d 1, while the less access dogs have to the home and bedroom correlates with lower amounts of Can f 1 found in the bedroom.12,13 Environmental moisture control can improve mold levels. Using pesticides and meticulous control of food debris can decrease cockroach environmental allergens. However, it may take over 6 months to remove residual cockroach allergen.1
Antihistamines
Histamine activates the H1 receptor on a distinct set of neurons to produce the sensation of itching. This leads to sneezing, nose rubbing, and the "allergic salute."10 H1-antihistamines are inverse agonists, rather than H1-antagonists, that combine with and stabilize the inactive form of the H1 receptor leading toward a shift in equilibrium to the inactive state. In addition to the inverse agonist effect at the H1 receptor, the newer second-generation agents have both anti-allergic and anti-inflammatory properties.
The first generation H1 antihistamines such as diphenhydramine, chlorpheniramine, brompheniramine and hydroxyzine are also referred to as the sedating antihistamines. These agents are effective at controlling the rhinorrhea, sneezing and pruritus associated with allergic rhinitis. Unfortunately these agents cross the blood-brain barrier thus producing undesirable side effects such as central nervous system depression, sedation leading to impaired performance at home, work and school and cardiotoxicity. There are no long-term safety studies on the first generation antihistamines. These agents have poor H1 receptor selectivity and act on muscarinic receptors causing anticholinergic effects such as dry mouth, urinary retention, constipation and tachycardia. The second-generation antihistamines developed in the early 1980's, have improved H1 receptor selectivity, absent or decreased sedation, faster onset and longer duration of action and fewer adverse effects. Their half-lives are longer (12-24 hours) compared to the first generation (4-12 hours).14 Of the second generation H1-antagonists, fexofenadine has no sedating effects even at higher than recommended doses. Loratadine and desloratadine are non-sedating at recommended doses but may cause sedation at higher doses. Cetirizine, and its purified enantiomer levocetirizine, have more sedation potential that other second generation H1-antagonists.15 All rhinitis symptoms, except for obstruction, can be alleviated by H1-antihistamines, and there does not seem to be a superiority of any one of the second generation H1-antihistmines over another.
Topical H1-antihistamines (azelastine, olopatadine) provided faster onset of action (less than 15 minutes) and similar to greater efficacy compared to oral preparations in regard to rhinitis and conjunctivitis. There has even been an association with improvement of congestion. However, their results are limited to the local organ effects, and require twice daily use to maintain a sustained response; whereas second generation oral H1-antagonists can be taken on a daily basis. Some patients may complain of a bitter taste, and intranasal H1-antagonists are less effective than intranasal steroids.1,2 In a direct comparison trial between azelastine nasal spray versus oral cetirizine, azelastine was found to have a significant improvement in nasal symptom scores for the specific symptoms of sneezing and nasal congestion over cetirizine.16
Steroids
In addition to oral H1-antihistamines, intranasal corticosteroids are a mainstay of treatment. They are the most effective medications for controlling all rhinitis symptoms. Their onset of action is from 3-12 hours. Their use on an as needed basis is not as effective as continual use1 but may not be required continually in all patients. They are generally safe, and there is little evidence to support suppression of the hypothalamic-pituitary-adrenal axis with prolonged use. Side effects are generally mild (crusting, dryness, and minor epistaxis). They can be minimized by proper nasal spray technique. Septal perforation has only been described anecdotally.2 For patients whose symptoms are not optimally controlled with intranasal steroids, adding an intranasal (but not oral) antihistamine may give some additional benefit.3
Systemic corticosteroids should be considered a last resort treatment option, but they may be necessary for severe or intractable symptoms. If they are used, then oral is preferred over parenteral because of the lower risk of systemic side effects and the ability to adjust doing. Steroids should never be injected into the turbinates. Recommendations on short courses oral steroids differ from 5-7 days1 to no more than 3 weeks.2
Decongestants
Decongestants are also available in oral and topical formulations. They are effective in relieving congestion. However, studies of H1-antihistamine in combination with oral decongestants failed to show improved benefit compared to either alone. Side effects include insomnia, anorexia, irritability, and rarely elevated blood pressure. Oral decongestants should be avoided in children less the 1-year of age, adults over 60 years of age, and any patient with a cardiac condition. The main side effect of topical decongestants is the development of rhinitis medicamentosa, which can appear in some patients after only 3 days of use or not at all in other patients after six weeks of use. European guidelines recommend a maximum of 10 days use.1,2
Cromones
Intranasal formulations of cromolyn and nedocromil have been used to treat allergic rhinitis but are less effective than topical corticosteroids. It is believed that cromones are less effective than topical antihistamines2, but adequate comparative studies have not been performed.1 Although the exact mechanism is unknown, they work mainly by inhibiting mast cell activation. Studies have shown that nedocromil inhibits the activation of neutrophils, eosinophils, monocytes, and macrophages as well. There may even be an inhibitory effect on neural signals involved in rhinitis.2 Overall, they are safe with minimal to no side effects.1,2
Miscellaneous
The anticholinergic ipratropium bromide is available in a nasal form and blocks the parasympathetic signaling that leads to watery rhinorrhea, and it has been shown effective in controlling this particular symptom. There are little to no side effects. Guidelines state it does not decrease sneezing or nasal obstruction,1,2 but one study in children showed improvement in rhinorrhea, congestion, and sneezing although to a lesser degree than intranasal steroids.17
Leukotriene receptor antagonists have been shown to be effective controlling allergic rhinitis, and they are comparably effective with oral antihistamines.1 After 2 weeks of therapy, montelukast progressively decreased symptoms scores, but still to a lesser degree than intranasal fluticasone.18 For patients whose symptoms are not controlled with intranasal corticosteroids, adding montelukast did not offer any further benefit.19
The anti-IgE antibody omalizumab may be efficacious, but it has not been shown to be superior to current allergic rhinitis treatments. Additionally, its high cost limits it use as a standard treatment.1
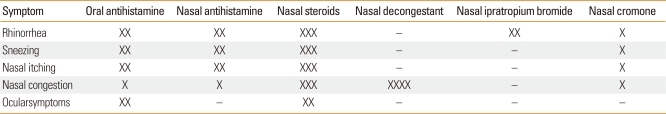
Taken as a whole, intranasal corticosteroids seem to be the most effective in controlling nasal symptoms. The next most effective are oral and intranasal antihistamines. However, it is difficult to fully stratify medication classes because of the lack of sufficient uniform data. For instance, studies on antihistamines have excluded nasal congestion as a component of symptoms scores because they are not expected to improve this symptom. There may be some differences between seasonal and perennial allergic rhinitis where, for some patients with perennial allergic rhinitis, oral antihistamines may be as effective as nasal steroids. Additionally, there is variable response to treatments among individuals.3 Table 1 lists the effectiveness of different medications in symptom control of allergic rhinitis.
Table 1.
Effectiveness in symptom control of various medications for allergic rhinitis
Adapted from van Cauwenberge et al.2.
-, no effect; X: least; XXXX: most effective.
Immunotherapy
Subcutaneous immunotherapy (SCIT) has been shown to be effective in treating allergic rhinitis in patients with identifiable IgE mediated symptom triggers. It has some advantages over the above mention treatments. Effects can be sustained for years, and it may prevent the development of new allergen sensitivities or even asthma.1 It is effective for not only control of allergic rhinitis but also of allergic conjunctivitis and allergen induced asthma.20 However, immunotherapy is underutilized with only 2 to 3 million US individuals on SCIT of the estimated 55 million people with allergic diseases.21 In regard to specific inhaled allergens, evidence supports immunotherapy for pollens, animal dander, and dust mite. Large local reactions at the injection site are the most common adverse reaction. The risk of severe systemic reactions during subcutaneous immunotherapy is rare but present in less than 1% of those receiving standard immunotherapy. Near fatal events occurred at a rate of 5.4 per million injections. High ambient pollen levels and dosing errors were the two main risk factors for such a reaction. It is advised that patients receive immunotherapy injections in a setting with staff and equipment that can handle anaphylaxis, and that patient be observed for 30 minutes after each injection.20 Other disadvantages include injection discomfort, the frequency of shot visits, and the total cost. However, immunotherapy is the only treatment that can modify the disease. When the direct costs of symptomatically managed allergic rhinitis are compared to the cost of immunotherapy, the values are virtually the same.8 When indirect costs are factored, immunotherapy may be much more economical.
Subcutaneously is the most common way to deliver immunotherapy, but sublingual immunotherapy (SLIT) is also used. SLIT has been reported to cause oral itching and gastrointestinal side effects, but in most studies, these rates seem to be the same as those observed in the placebo arm.2 There is a lack of standardization in SLIT with timothy grass pollen extracts being the only commercially available therapy (Grazax by ALK-Abelló Hørsholm. Denmark), and there are no SLIT therapies approved for the US by Food and Drug Administration. Advantages of SLIT include an extremely low risk of anaphylaxis and the ability to begin therapy at the maintenance dose without a build-up phase.22 SLIT for dust mite allergy has been specifically studied in the Korean population and found to be effective in reducing symptom scores.23,24 Although anaphylaxis has not been noticed in studies on SLIT, there are case reports of anaphylaxis occurring during treatment, even with the first dose.21 SLIT is not as well established as SCIT, and further investigation is required to determine the optimal dose and patient selection.2
A meta-analysis25 done in January of 2010 reviewed the past 20 years of studies on SLIT. Nineteen studies were included with a total of 2,971 study subjects. SLIT was found to improve both symptom scores and medication use for allergic rhinitis. It appears that a minimal dose of 450 µg of antigen per treatment was necessary and that using higher doses produced to benefit. Upon subgroup analysis, SLIT was far less effective in children than adults. This conclusion may have been confounded by the fact that most of the pediatric studies used doses of less than 276 µg and the only pediatric study that showed statistically significant benefit used a dose of 600 µg. Along these lines, the meta-analysis showed that SLIT tablets were more effective than drops in reducing symptom scores with the caveat that this difference is mostly noticed in pediatric studies where drops administered a lower dose than tablets. Additionally, some of the pediatric studies included allergens other than grass. Other pertinent conclusions were: that SLIT was more effective when given for 12 months or less compared to over 1 year of use; SLIT was not more effective for rhinitis control in allergic asthmatics than in subjects without allergic asthma; and the more important that the length of treatment was the timing of beginning SLIT with initiation at least three months before grass season being optimal.
NONALLERGIC RHINITIS
Definition
Nonallergic rhinitis (NAR) is generally described as chronic nasal symptoms, such as obstruction and rhinorrhea that occur in relation to nonallergic, noninfectious triggers such as change in the weather, exposure to caustic odors or cigarette smoke, barometric pressure differences, etc. There is a lack of concomitant allergic disease, determined by negative skin prick test for relevant allergens and/or negative allergen-specific antibody tests.26 The term vasomotor is often used which suggests involvement of neural, glandular, and vascular pathways; however, this term is misleading because it implies a true understanding of the underlying pathophysiology of the disease when this has not been definitively established.27
In December of 2008, a roundtable conference which included 8 expert physicians on rhinitis convened to establish a consensus on the clinical definition of nonallergic vasomotor rhinitis and to develop appropriate inclusion and exclusion criteria for the enrollment of subjects in future clinical studies. From this NAR Consensus Panel Proceedings, there were at least 8 subtypes that filled the criteria for NAR (Table 2).1,26,28 Nonallergic rhinopathy (formerly known as vasomotor rhinitis) accounts for the majority of NAR. It is a diverse group of patients that have chronic nasal symptoms with a lack of nasal eosinophilia and an etiology that is neither immunologic nor due to infection. NAR with eosinophilia is characterized by patients who have year-round nasal symptoms but eosinophils are found on nasal smear though they lack positive skin tests and/or specific IgE antibodies in the serum. Atrophic rhinitis, as the name implies, refers to a chronic condition in which there is progressive atrophy of the nasal mucosa with crusting and dryness as the most prominent features. It is typically not inflammatory mediated.1 Senile rhinitis occurs most commonly in the elderly, presents mostly with watery rhinorrhea that may worsen after certain foods or environmental irritants. Gustatory rhinitis occurs after eating, especially hot or spicy foods. Rhinitis medicamentosa is included in drug-induced rhinitis, though a variety of medications have been implicated in causing chronic nasal congestion. Rhinitis medicamentosa most commonly occurs after repeated use of topical nasal decongestants such as oxymetzaoline or phenylephrine. Hormone induced rhinitis refers to the congestion and nasal symptoms that occur in response to endogenous female hormones, such as seen in pregnancy. Cerebrospinal fluid (CSF) leak should be considered in patients with a history of cranio-facial trauma or past facial surgery that have persistent, clear rhinorrhea.26
Table 2.
Chronic rhinitis subtypes not associated with allergies, infection, or anatomic abnormalities
Epidemiology
The exact prevalence and impact of NAR is not as established as it is for allergic rhinitis. It is estimated that it affects more than 19 to 20 million patients in the United States, with vasomotor rhinitis being the most common subtype seen.26,29 European studies evaluating the prevalence of NAR found that approximately 1 in 4 patients with nasal symptom complaints had "pure" NAR and it is estimated that 50 million Europeans have NAR, with a total prevalence of more than 200 million worldwide.26
With many subtypes of disease, the true economic burden of NAR is most likely grossly underestimated. Schatz et al.29 reviewed the records of more than 1 million patients enrolled in the Kaiser Permanente Southern California Medical care program from 2002-2005 and found that 15% had at least 1 encounter with the diagnosis. Another 14% received rhinitis medication with no medical encounter. They also found that patients from either group had significantly more health care visits per year for asthma (2-4 times as many), acute sinusitis (6-8 times as many) and all other diagnoses (almost twice as many). They also found that patients with rhinitis or treated for rhinitis had a higher prevalence of comorbid diseases such as asthma, acute and chronic sinusitis, nasal polyposis, conjunctivitis, acute otitis media, chronic serous otitis media, sleep apnea, and fatigue. When reviewing the patient demographics, those with NAR were significantly older, mean age of 42.6 vs. 35.8 and more likely to be female than the patients with the diagnosis of allergic rhinitis.29
Importance of treatment
As Ledford30 points out in his symposium on assessing the damage of inadequately diagnosed NAR, patients are often empirically treated with oral second generation antihistamines, which are usually not sufficient in relieving their symptoms. The patients are then subjected to multiple rounds of treatment failures that lead to frustration towards seeking medical care and medication use. They must incur additional expenditures for doctor appointments, medication prescriptions, and lost time from work on top of their reduced quality of life. Besides this decrease in quality of life, untreated rhinitis does significantly increase the risk of other comorbid conditions such as obstructive sleep apnea, fatigue, headache, malaise, poor appetite and weakness. This effect is not limited to impaired work performance in adults but can also manifest as learning disabilities, behavioral, and psychological effects in children. Children are also at risk for permanent facial changes from untreated rhinitis such as increased facial length, retrognathic maxilla and mandible, and dental malocclusions from obstructed breathing.30
Beyond these physical and emotional impacts on patients there is also an economic burden from the incomplete diagnosis and treatment of rhinitis. Recent evidence shows that asthma and rhinitis are often coexisting in atopic and nonatopic patients and that effective treatment of rhinitis frequently improves asthma.31
Treatment
Avoidance
Avoidance of environmental triggers such as strong odors (perfumes, soaps, paint, etc.) and air pollutants (smoke fumes, tobacco smoke) that are respiratory irritants is recommended in those who find these worsen their rhinitis symptoms.1,32
Antihistamines
Oral second generation antihistamines are not as effective in the treatment of NAR, though first generation oral antihistamines may haves some benefit due to anticholinergic activity.33 Topical antihistamines on the other hand have been found to be very effective for the overall treatment of NAR. Of the two topical antihistamines on the market in the United States (azelastine and olopatadine), azelastine is the only one that has been shown to be efficacious for nonallergic rhinitis.1,32,34 Banov and Lieberman32 evaluated the efficacy of the azelastine nasal spray in patients with nonallergic vasomotor rhinitis in a multicenter, randomized, placebo-controlled trial and found a significant improvement in total vasomotor rhinitis symptom scores (TVRSS) in those patients receiving azelastine (two sprays twice a day, 1.1 mg) versus placebo. In an open label, 2-week study with azelastine 2 sprays per nostril twice daily in patients with allergic rhinitis, mixed rhinitis, and nonallergic vasomotor rhinitis it was found that azelastine had improvement in control of all rhinitis symptoms including nasal congestion, postnasal drip, sneezing, and sleeping difficulty.34 The previously mentioned metallic aftertaste that some patients describe with azelastine is dose-dependent and often dissipates over time.33
Steroids
Intranasal corticosteroids have been found to be effective in nonallergic rhinitis, especially in vasomotor rhinitis and NARES. Fluticasone propionate and beclomethasone are the only topical corticosteroids approved by the FDA in the US for the treatment of NAR. Clinically, there does not appear to be a difference between the intranasal steroids available at this time.1 Most are dosed twice daily and patients should be informed that it may take 24 to 72 hours before symptoms start to improve though the onset of action is said to be from 3-12 hours.33 In a randomized, double-blind, placebo-controlled trial with 983 patients with perennial nonallergic rhinitis performed by Webb et al.35 patients received fluticasone propionate 200 mcg, 400 mcg or placebo for 28 days. Primary endpoint was the mean change in total nasal symptom score (TNSS), which was a sum of patient ratings of nasal obstruction, postnasal drip, and rhinorrhea. Patients that were found to have NARES as well as those that did not, were shown to have similar statistically significant improvement on either dose of fluticasone propionate compared with placebo.33 However, there is a subgroup of NAR patients that fail to respond to intranasal corticosteroids and further study is warranted in these nonresponders.36
Decongestants
Currently there are no specific studies looking at the effectiveness of oral decongestants in the treatment of NAR. Thus, they should be considered adjunctive therapy, which is used on an as needed basis for nasal congestion that is not responsive to intranasal corticosteroids, topical antihistamines, or a combination of both.
Anticholinergics
The only topical anticholinergic medication approved in the United States for topical application is ipratropium bromide. Ipratropium bromide (0.03%) nasal spray is recommended when rhinorrhea is the predominant or only symptom, as in the case of gustatory rhinitis. From the updated rhinitis practice parameters, its use in combination with an intranasal corticosteroid is more effective than either drug alone for the treatment of rhinorrhea. This is not only effective, but safe as well since there is not an increased incidence of adverse events.1
Nasal saline
Nasal lavage with saline solution has also been found to be a helpful alone or as an adjuvant therapy in patients with chronic rhinorrhea and rhinosinusitis.1 It is best performed immediately prior to intranasal corticosteroids or azelastine and may be especially helpful in reducing postnasal drip, sneezing, and congestion.37 A 2007 Cochrane database review found 8 randomized controlled trials in which saline was evaluated in comparison with either no treatment, placebo, as an adjunct to other treatments or against treatments. There was no evidence that saline alone was beneficial in the treatment of chronic rhinosinusitis nor was it more effective than an intranasal corticosteroid. However, there was favorable evidence for saline as an adjunct treatment. The final conclusion was that saline irrigations are a well tolerated with very minor side effects that can be included as a treatment adjunct for chronic rhinosinusitis symptoms.38 In a prospective, randomized controlled trial with 121 adults with chronic nasal and sinus symptoms, Pynnonen et al.39 looked to determine if isotonic sodium chloride nasal irrigations performed with large volume and low positive pressure was more effective than saline sprays at improving quality of life and decreasing medication use. The primary outcomes measured were a change in symptom severity measure by a mean 20-item Sino-Nasal Outcome Test (SNOT-20) score, medication use, and symptom frequency. The outcomes were looked at 3 different time points (2, 4, and 8 weeks). The high volume, low positive pressure group had lower SNOT-20 scores at all time points. They also had a lower frequency of "often or always" nasal symptom reporting compared to the spray group (40% of subjects versus 61%). A significant difference was not found in sinus medication use in either group.39
The exact mechanism of how saline is helpful in allergic rhinitis and rhinosinusitis has not been confirmed but it is postulated that it may improve mucus clearance; remove antigen, inflammatory mediators, or biofilm; enhance ciliary beat; and protect the nasal mucosa. Side effects from its use are typically minor and consist of burning, irritation, and nausea. There is not an established consensus regarding method of delivery, volume to use, ratio of isotonic to hypertonic, or frequency.1
Investigational therapies
1. Capsaicin
Capsaicin is the chemical contained within the oil of Capsicum pepper and while it is initially irritating to the applied area, it eventually desensitizes the sensory neural fibers. It has been used intranasal to try and decrease nasal hyperreactivity responsible for rhinorrhea, sneezing, and congestion.37 A placebo-controlled studies using intranasal capsaicin in patient with nonallergic, noninfectious perennial rhinitis found a significant and long-term reduction in the visual analogue scale (VAS) scores in the treatment group but no difference objective measures of inflammation such as concentration of leukotriene C4/D4/E4, prostaglandin D2, and tryptase.40
2. Silver nitrate
Topically applied silver nitrate was found to be effective in a trial comparing silver nitrate, flunisolide, and placebo in patients with NAR. Improvement was found in patient reported rhinorrhea, sneezing and nasal congestion.41 Two prospective studies in patients with vasomotor rhinitis also found significant improvement in nasal symptoms.42,43
3. Acupuncture
From a systematic review of complementary and alternative medicine for rhinitis and asthma published in the Journal of Allergy and Clinical Immunology in 2006, the majority of studies on acupuncture were in allergic rhinitis and were not randomized, controlled, or descriptive. There was 1 nonrandomized study in NAR that showed no difference in nasal airflow and symptoms between acupuncture and electrostimulation.44 However, in 2009, a random, placebo-controlled study by Fleckenstein et al.45 was published that showed a significant change in nasal sickness score (NSS, max 27 points) in patients with vasomotor rhinitis treated with acupuncture versus those who had sham laser acupuncture treatment. The treatment group had a NSS that went from 9.3±3.89 to 4.1±3.2 (P<0.001) while the sham groups NSS went from 5.6±2.74 to 3.7±2.4.45
Surgery
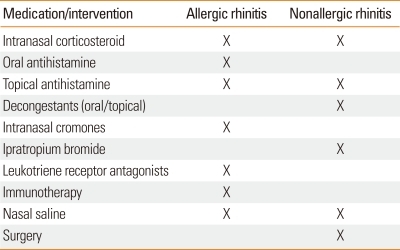
After 6-12 months of failed medical therapy (intranasal corticosteroid with azelastine and/or decongestants and/or ipratropium bromide) then surgical options may be considered. It may also be indicated if the patient has comorbid conditions such as nasal obstruction from severe nasal septal deviation or inferior turbinate hypertrophy, adenoidal hypertrophy, or refractory sinusitis.1 Treatment similarities and differences in allergy and nonallergic rhinitis are outlined in Table 3.
Table 3.
Treatment regimens for allergic and nonallergic rhinitis
X, medication/intervention recommended.
SUMMARY
Rhinitis is a prevalent disease worldwide that causes a significant impact on patient quality of life, can affect multiple comorbid conditions, and is a substantial economic burden on society. It is important to note that a majority of rhinitis patients experience significant non-allergic triggers and thus may non-allergic or mixed (allergic and non-allergic) rhinitis. An improved consensus criterion for defining rhinitis subtypes is essential. This will allow for better understanding the prevalence and epidemiology of chronic rhinitis subtypes and for selecting the appropriate study populations to investigate mechanisms and specific therapies of these disorders.
References
- 1.Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph CC, Schuller D, Spector SL, Tilles SA. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1–S84. doi: 10.1016/j.jaci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 2.van Cauwenberge P, Bachert C, Passalacqua G, Bousquet J, Canonica GW, Durham SR, Fokkens WJ, Howarth PH, Lund V, Malling HJ, Mygind N, Passali D, Scadding GK, Wang DY. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55:116–134. doi: 10.1034/j.1398-9995.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- 3.Benninger M, Farrar JR, Blaiss M, Chipps B, Ferguson B, Krouse J, Marple B, Storms W, Kaliner M. Evaluating approved medications to treat allergic rhinitis in the United States: an evidence-based review of efficacy for nasal symptoms by class. Ann Allergy Asthma Immunol. 2010;104:13–29. doi: 10.1016/j.anai.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007;28:3–9. doi: 10.2500/aap.2007.28.2934. [DOI] [PubMed] [Google Scholar]
- 5.Spector SL, Nicklas RA, Chapman JA, Bernstein IL, Berger WE, Blessing-Moore J, Dykewicz MS, Fineman SM, Lee RE, Li JT, Portnoy JM, Schuller DE, Lang D, Tilles SA. Symptom severity assessment of allergic rhinitis: part 1. Ann Allergy Asthma Immunol. 2003;91:105–114. doi: 10.1016/s1081-1206(10)62160-6. [DOI] [PubMed] [Google Scholar]
- 6.Min YG. The Pathophysiology, Diagnosis and Treatment of Allergic Rhinitis. Allergy Asthma Immunol Res. 2010;2:65–76. doi: 10.4168/aair.2010.2.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein JA. Allergic and mixed rhinitis: Epidemiology and natural history. Allergy Asthma Proc. 2010;31:365–369. doi: 10.2500/aap.2010.31.3380. [DOI] [PubMed] [Google Scholar]
- 8.Blaiss MS. Allergic rhinitis: Direct and indirect costs. Allergy Asthma Proc. 2010;31:375–380. doi: 10.2500/aap.2010.31.3329. [DOI] [PubMed] [Google Scholar]
- 9.Broide DH. Allergic rhinitis: Pathophysiology. Allergy Asthma Proc. 2010;31:370–374. doi: 10.2500/aap.2010.31.3388. [DOI] [PubMed] [Google Scholar]
- 10.Kim D, Baraniuk JN. Neural aspects of allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2007;15:268–273. doi: 10.1097/MOO.0b013e328259c372. [DOI] [PubMed] [Google Scholar]
- 11.Bousquet J, Van Cauwenberge P, Khaltaev N Aria Workshop Group; World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147–S334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 12.Nicholas C, Wegienka G, Havstad S, Ownby D, Johnson CC. Influence of cat characteristics on Fel d 1 levels in the home. Ann Allergy Asthma Immunol. 2008;101:47–50. doi: 10.1016/S1081-1206(10)60834-4. [DOI] [PubMed] [Google Scholar]
- 13.Nicholas C, Wegienka G, Havstad S, Zoratti E, Ownby D, Johnson CC. Dog characteristics and allergen levels in the home. Ann Allergy Asthma Immunol. 2010;105:228–233. doi: 10.1016/j.anai.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzung BG, Trevor AJ, Masters SB. Katzung & Trevor's pharmacology: examination & board review. 6th ed. New York: McGraw-Hill; 2002. [Google Scholar]
- 15.Levocetirizine (Xyzal) for allergic rhinitis and urticaria. Med Lett Drugs Ther. 2007;49:97–99. [PubMed] [Google Scholar]
- 16.Ciprandi G. Treatment of nonallergic perennial rhinitis. Allergy. 2004;59(Suppl 76):16–23. doi: 10.1111/j.0108-1675.2004.00390.x. [DOI] [PubMed] [Google Scholar]
- 17.Milgrom H, Biondi R, Georgitis JW, Meltzer EO, Munk ZM, Drda K, Wood CC. Comparison of ipratropium bromide 0.03% with beclomethasone dipropionate in the treatment of perennial rhinitis in children. Ann Allergy Asthma Immunol. 1999;83:105–111. doi: 10.1016/S1081-1206(10)62620-8. [DOI] [PubMed] [Google Scholar]
- 18.Martin BG, Andrews CP, van Bavel JH, Hampel FC, Klein KC, Prillaman BA, Faris MA, Philpot EE. Comparison of fluticasone propionate aqueous nasal spray and oral montelukast for the treatment of seasonal allergic rhinitis symptoms. Ann Allergy Asthma Immunol. 2006;96:851–857. doi: 10.1016/S1081-1206(10)61349-X. [DOI] [PubMed] [Google Scholar]
- 19.Esteitie R, deTineo M, Naclerio RM, Baroody FM. Effect of the addition of montelukast to fluticasone propionate for the treatment of perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2010;105:155–161. doi: 10.1016/j.anai.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Cox L, Li T, Nelson H, Lockey R. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120:S25–S85. doi: 10.1016/j.jaci.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Mohapatra SS, Qazi M, Hellermann G. Immunotherapy for allergies and asthma: present and future. Curr Opin Pharmacol. 2010;10:276–288. doi: 10.1016/j.coph.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frew AJ. Sublingual immunotherapy. N Engl J Med. 2008;358:2259–2264. doi: 10.1056/NEJMct0708337. [DOI] [PubMed] [Google Scholar]
- 23.Chang H, Han DH, Mo JH, Kim JW, Kim DY, Lee CH, Min YG, Rhee CS. Early compliance and efficacy of sublingual immunotherapy in patients with allergic rhinitis for house dust mites. Clin Exp Otorhinolaryngol. 2009;2:136–140. doi: 10.3342/ceo.2009.2.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim ST, Han DH, Moon IJ, Lee CH, Min YG, Rhee CS. Clinical and immunologic effects of sublingual immunotherapy on patients with allergic rhinitis to house-dust mites: 1-year follow-up results. Am J Rhinol Allergy. 2010;24:271–275. doi: 10.2500/ajra.2010.24.3501. [DOI] [PubMed] [Google Scholar]
- 25.Di Bona D, Plaia A, Scafidi V, Leto-Barone MS, Di Lorenzo G. Efficacy of sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2010;126:558–566. doi: 10.1016/j.jaci.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Kaliner MA. Classification of nonallergic rhinitis syndromes with a focus on vasomotor rhinitis, proposed to be known henceforth as nonallergic rhinopathy. World Allergy Organiz J. 2009;2:98–101. doi: 10.1097/WOX.0b013e3181a9d55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaliner MA, Farrar JR. Consensus review and definition of nonallergic rhinitis with a focus on vasomotor rhinitis, proposed to be known henceforth as nonallergic rhinopathy: part 1. introduction. World Allergy Organiz J. 2009;2:97. doi: 10.1097/WOX.0b013e3181a8e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scarupa MD, Kaliner MA. Nonallergic rhinitis, with a focus on vasomotor rhinitis: clinical importance, differential diagnosis, and effective treatment recommendations. World Allergy Organiz J. 2009;2:20–25. doi: 10.1097/WAO.0b013e318196ca1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schatz M, Zeiger RS, Chen W, Yang SJ, Corrao MA, Quinn VP. The burden of rhinitis in a managed care organization. Ann Allergy Asthma Immunol. 2008;101:240–247. doi: 10.1016/S1081-1206(10)60488-7. [DOI] [PubMed] [Google Scholar]
- 30.Ledford D. Inadequate diagnosis of nonallergic rhinitis: assessing the damage. Allergy Asthma Proc. 2003;24:155–162. [PubMed] [Google Scholar]
- 31.Settipane RA. Rhinitis: a dose of epidemiological reality. Allergy Asthma Proc. 2003;24:147–154. [PubMed] [Google Scholar]
- 32.Banov CH, Lieberman P. Efficacy of azelastine nasal spray in the treatment of vasomotor (perennial nonallergic) rhinitis. Ann Allergy Asthma Immunol. 2001;86:28–35. doi: 10.1016/S1081-1206(10)62352-6. [DOI] [PubMed] [Google Scholar]
- 33.Meltzer EO. An overview of current pharmacotherapy in perennial rhinitis. J Allergy Clin Immunol. 1995;95:1097–1110. doi: 10.1016/s0091-6749(95)70213-x. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman P, Kaliner MA, Wheeler WJ. Open-label evaluation of azelastine nasal spray in patients with seasonal allergic rhinitis and nonallergic vasomotor rhinitis. Curr Med Res Opin. 2005;21:611–618. doi: 10.1185/030079905X41408. [DOI] [PubMed] [Google Scholar]
- 35.Webb DR, Meltzer EO, Finn AF, Jr, Rickard KA, Pepsin PJ, Westlund R, Cook CK. Intranasal fluticasone propionate is effective for perennial nonallergic rhinitis with or without eosinophilia. Ann Allergy Asthma Immunol. 2002;88:385–390. doi: 10.1016/S1081-1206(10)62369-1. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs R, Lieberman P, Kent E, Silvey M, Locantore N, Philpot EE. Weather/temperature-sensitive vasomotor rhinitis may be refractory to intranasal corticosteroid treatment. Allergy Asthma Proc. 2009;30:120–127. doi: 10.2500/aap.2009.30.3206. [DOI] [PubMed] [Google Scholar]
- 37.Settipane RA, Lieberman P. Update on nonallergic rhinitis. Ann Allergy Asthma Immunol. 2001;86:494–508. doi: 10.1016/S1081-1206(10)62896-7. [DOI] [PubMed] [Google Scholar]
- 38.Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007:CD006394. doi: 10.1002/14651858.CD006394.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Pynnonen MA, Mukerji SS, Kim HM, Adams ME, Terrell JE. Nasal saline for chronic sinonasal symptoms: a randomized controlled trial. Arch Otolaryngol Head Neck Surg. 2007;133:1115–1120. doi: 10.1001/archotol.133.11.1115. [DOI] [PubMed] [Google Scholar]
- 40.Blom HM, Van Rijswijk JB, Garrelds IM, Mulder PG, Timmermans T, Gerth van Wijk R. Intranasal capsaicin is efficacious in non-allergic, non-infectious perennial rhinitis. A placebo-controlled study. Clin Exp Allergy. 1997;27:796–801. doi: 10.1046/j.1365-2222.1997.670842.x. [DOI] [PubMed] [Google Scholar]
- 41.Erhan E, Külahli I, Kandemir O, Cemiloglu R, Yigitbasi OG, Cüreoglu S. Comparison of topical silver nitrate and flunisolide treatment in patients with idiopathic non-allergic rhinitis. Tokai J Exp Clin Med. 1996;21:103–111. [PubMed] [Google Scholar]
- 42.al-Samarrae SM. Treatment of 'vasomotor rhinitis' by the local application of silver nitrate. J Laryngol Otol. 1991;105:285–287. doi: 10.1017/s0022215100115622. [DOI] [PubMed] [Google Scholar]
- 43.Bhargava KB, Shirali GN, Abhyankar US, Gadre KC. Treatment of allergic and vasomotor rhinitis by the local application of different concentrations of silver nitrate. J Laryngol Otol. 1992;106:699–701. doi: 10.1017/s0022215100120614. [DOI] [PubMed] [Google Scholar]
- 44.Passalacqua G, Bousquet PJ, Carlsen KH, Kemp J, Lockey RF, Niggemann B, Pawankar R, Price D, Bousquet J. ARIA update: I--Systematic review of complementary and alternative medicine for rhinitis and asthma. J Allergy Clin Immunol. 2006;117:1054–1062. doi: 10.1016/j.jaci.2005.12.1308. [DOI] [PubMed] [Google Scholar]
- 45.Fleckenstein J, Raab C, Gleditsch J, Ostertag P, Rasp G, Stör W, Irnich D. Impact of acupuncture on vasomotor rhinitis: a randomized placebo-controlled pilot study. J Altern Complement Med. 2009;15:391–398. doi: 10.1089/acm.2008.0471. [DOI] [PubMed] [Google Scholar]