Abstract
Fecal examinations using the Kato Katz technique were performed on a total of 1,287 villagers (945 students and 342 general inhabitants) of Oddar Meanchey Province, Cambodia in May 2007 and November 2009. The overall intestinal helminth egg positive rate was 23.9%, and the most prevalent helminth species was hookworms (21.6%). Other helminth eggs detected included echinostomes (1.0%), Enterobius vermicularis (0.8%), small trematode eggs (0.7%), which may include Opisthorchis viverrini and Haplorchis spp., and Hymenolepis nana (0.4%). In order to recover adult echinostomes, we treated 2 patients with 10-15 mg/kg praziquantel and purged. Total 14 adult echinostomes, 1 and 13 worms from each patient, were collected. The echinostomes characteristically had 49-51 collar spines and 2 round or slightly lobated testes. They were identified as Echinostoma ilocanum (Garrison, 1908) Odhner, 1911. So far as literature are concerned, this is the first record on the discovery of human E. ilocanum infection in Cambodia.
Keywords: Echinostoma ilocanum, worm recovery, trematode, echinostome, Cambodia
Flukes of the family Echinostomatidae (=echinostomes) are medically important because they can cause severe epigastric or abdominal pain accompanied by diarrhea, malnutrition, and fatigue [1]. A total of 20 species belonging to 8 genera (Echinostoma, Echinochasmus, Acanthoparyphium, Artyfechinostomum, Episthmium, Himasthla, Hypoderaeum, and Isthmiophora) are known to infect humans worldwide [1,2]. In Cambodia, echinostomatid eggs were detected in feces of people in several provinces, including Kampong Cham, Battambang, and Pursat [3]. Different echinostome species have different life cycle and different source of infections. Therefore, the specific diagnosis of adult echinostome infections should be pursued. However, only one species, Echinostoma revolutum, has been reported in Cambodia to date [3].
The Korea Association of Health Promotion, Korea, in cooperation with The National Centre for Parasitology, Entomology, and Malaria Control, Ministry of Health, Cambodia, have been conducting an international parasite control project in Cambodia (2006-2011). In June 2007 and November 2009, we examined stools of 1,287 villagers, which consisted of 945 students and 342 general inhabitants, in 7 small villages in Oddar Meanchey Province (located in northwestern Cambodia near the border with Thailand) using the Kato-Katz technique.
The overall intestinal helminth egg positive rate was 23.9%, and the most prevalent helminth species was hookworms (21.6%) (Table 1). Other helminth eggs detected included echinostome eggs (1.0%), Enterobius vermicularis eggs (0.8%), small trematode eggs (0.7%), which may include Opisthorchis viverrini and Haplorchis spp., and Hymenolepis nana eggs (0.4%) (Table 1). Students showed a little higher rates in E. vermicularis (1.0% vs 0.3%) and small trematode egg positive rates (0.9% vs 0.3%) and slightly lower rates in echinostome egg positive rate (0.7% vs 1.8%) than general inhabitants. Echinostome egg positive cases, 13 in total number, consisted of 8 males and 5 females, and 6 students and 7 inhabitants. Some of them suffered from occasional gastrointestinal discomfort, indigestion, and diarrhea.
Table 1.
Prevalence of intestinal helminths among students and general inhabitants in 7 small villages of Oddar Meanchey Province, Cambodia as determined by the Kato-Katz fecal examination in May 2007 and November 2009
aEchinostoma ilocanum specimens were recovered from 2 of these patients.
bDefined as trematode eggs of 20-32 µm in length (considered mostly to be Opisthorchis viverrini and/or Haplorchis spp.).
cInclude eggs of Trichuris trichiura, Trichostrongylus orientalis, and Taenia saginata.
In order to recover the adult echinostomes, 2 (males aged 16 and 36 years) among the symptomatic patients were selected for anthelmintic treatment and purging. After obtaining informed consent, they were treated with a single oral dose of 10-15 mg/kg praziquantel (Shinpoong Pharm. Co., Seoul, Korea), and purged with 30-40 g MgSO4 an hour later. The diarrheic feces were collected and processed as previously described [4]. Worms were fixed with 10% formalin, stained with acetocarmine, and morphologically identified. Fecal examination and anthelmintic treatment of villagers were officially approved by the Ministry of Health, Cambodia, under the agreement of the Korea-Cambodia International Collaboration on Intestinal Parasite Control in Cambodia (2006-2011).
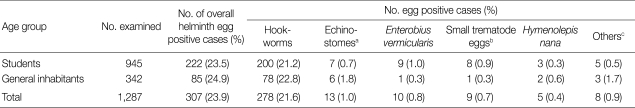
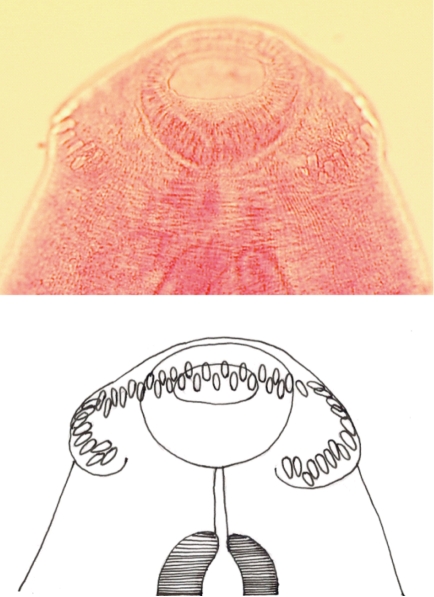
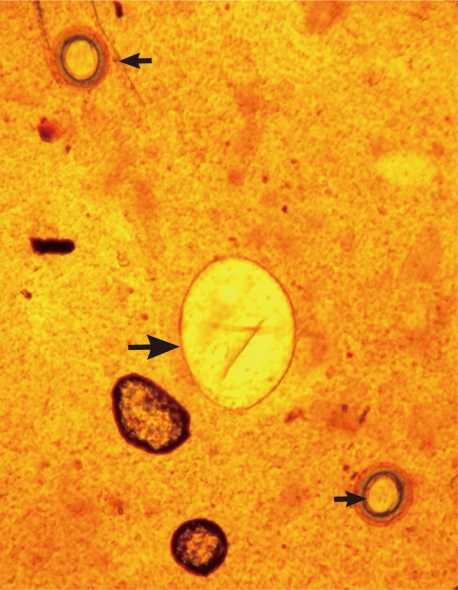
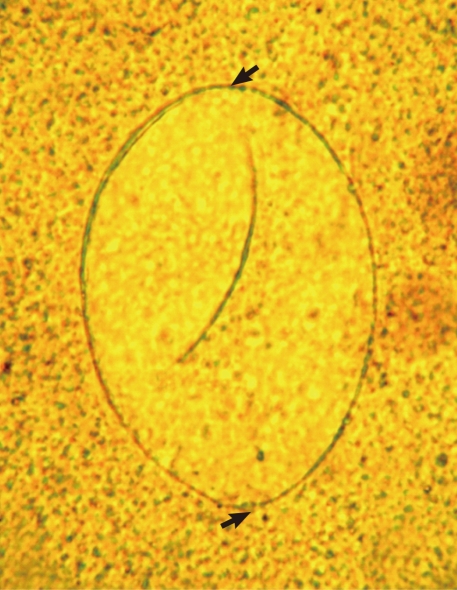
A total of 14 Echinostoma ilocanum adult flukes were collected from the 2 cases (1 and 13 worms, respectively). No other trematode species were recovered. The worms were elongated (Fig. 1), 6.7 mm (5.2-8.0 mm) long, and 1.0 mm (0.8-1.2 mm) wide (n=10), with 49-51 collar spines on the head collar (Fig. 2) and two round or slightly lobated testes. The eggs (Figs. 3, 4) were 94 µm long (89-99 µm) and 55 µm wide (52-58 µm) (n=10), and had a relatively wide, somewhat inconspicuous operculum, a thin and refractile shell, and abopercular wrinkles terminally. In Kato Katz fecal smears, the operculum and abopercular wrinkles of the eggs are less apparently seen (Fig. 4).
Fig. 1.
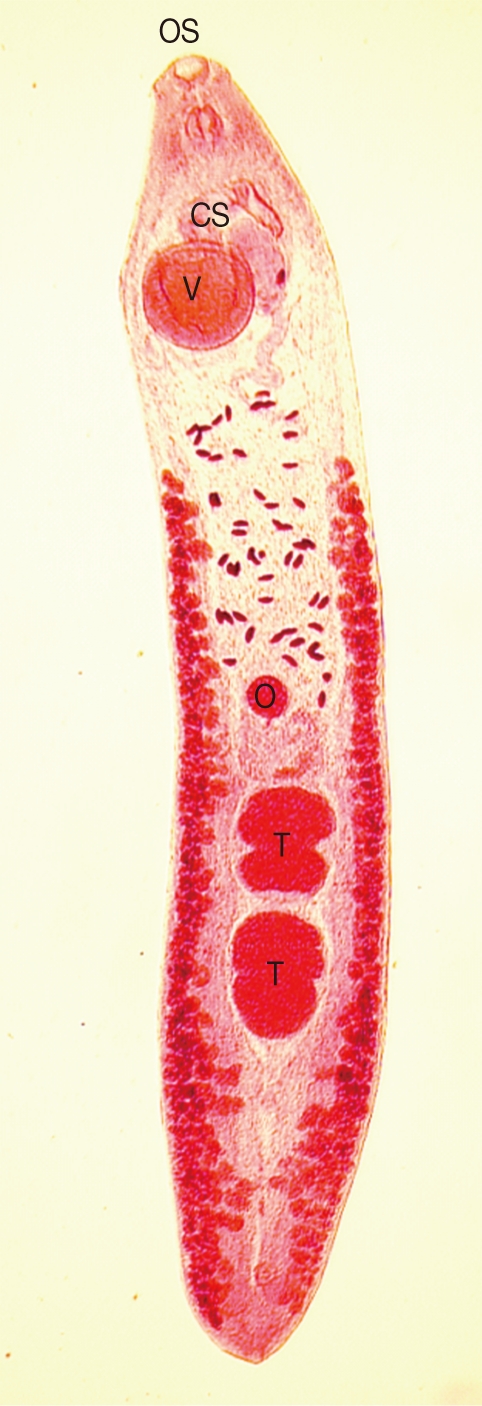
An adult Echinostoma ilocanum specimen (7.5 mm long) recovered from a villager from Oddar Meanchey Province, Cambodia, after chemotherapy and purgation. It shows the characteristic morphologies of its head collar, oral sucker (OS), ventral sucker (V), cirrus sac (CS), uterus, ovary (O), and two lobated testes (T). ×20.
Fig. 2.
A close up view (up) and line drawing (down) of the head collar and 49 collar spines of E. ilocanum adult. ×100.
Fig. 3.
An Echinostoma ilocanum egg (99×56 µm; larger arrow) and 2 Taenia sp. eggs (small arrows) detected in a Kato-Katz fecal smear of a patient. ×200.
Fig. 4.
Another E. ilocanum egg (102×58 µm) found in a Kato-Katz fecal smear of a patient. Note the thin and inconspicuous operculum (arrow; up) and a tiny abopercular knob at its terminal end (arrow; down). ×400.
The results show the presence of human E. ilocanum fluke infection in Cambodia. E. ilocanum (syn. Euparyphium ilocanum) was first discovered in residents of Manila, the Philippines in 1907 [1]. Later, human infections with E. ilocanum were reported in Indonesia, China, Thailand, and India [4-7]. The main source of human infection is large freshwater snails, Pila luzonica (in the Philippines) and Viviparus javanicus (in Indonesia), and rats and dogs are animal reservoir hosts [1]. The principal mode of human infection is consumption of raw or undercooked snails. For example, kilawen or kinilaw is a preparation of raw mollusk, fish, or meat with salt, vinegar, lime juice, and spices in the Philippines [8,9]. In Cambodia, similar types of local food are available, and such dishes may have been the source of infection in our cases. However, life cycle studies should be performed in this area to determine the actual source of infection.
The clinical symptoms in our cases were relatively mild, and thus needed no urgent special medical care. The mildness of the symptoms may have been due to the relatively low worm burdens, i.e., 1 and 13 recovered worms per person. However, if the worm load is higher, significant clinical manifestations may occur [9]. In cases with heavy worm loads of the echinostome species, Artyfechinostomum malayanum, mortality due to intestinal perforation or marked malnutrition and anemia has been reported [1]. Severe damage of intestinal mucosa by echinostome flukes has been seen in human infections using gastroduodenal endoscopy and also has been in experimentally infected rats [1].
One of the epidemiologic and clinical problems in echinostomiasis is difficulty of diagnosis by fecal examination. The number of eggs produced per worm per day varies by species and is relatively small in certain species [1,2] compared with other helminth parasites, such as Ascaris lumbricoides. In addition, echinostome eggs are unfamiliar to laboratory personnel and clinicians and can be overlooked, particularly in Kato-Katz fecal smears. Recovery of adult flukes after anthelmintic treatment can help identify the echinostome fluke involved. However, this method is not feasible. To cope with this problem, training of microscopists to detect echinostome eggs is needed.
ACKNOWLEDGEMENTS
We thank the staff of National Centre for Parasitology, Entomology, and Malaria Control, Cambodia for their help in the Kato-Katz fecal examination.
References
- 1.Chai JY. Echinostomes in humans. In: Fried B, Toledo R, editors. The Biology of Echinostomes. New York, USA: Springer; 2009. pp. 147–183. [Google Scholar]
- 2.Chai JY, Shin EH, Lee SH, Rim HJ. Foodborne intestinal flukes in Southeast Asia. Korean J Parasitol. 2009;47(Suppl):S69–S102. doi: 10.3347/kjp.2009.47.S.S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohn WM, Chai JY, Yong TS, Eom KS, Yoon CH, Sinuon M, Socheat D, Lee SH. Echinostoma revolutum infection, Pursat Province, Cambodia. Emerg Infect Dis. 2011;17:117–119. doi: 10.3201/eid1701.100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonne C, Bras G, Lie KJ. Five echinostomes found in man in the Malayan Archipelago. Am J Dig Dis. 1953;20:12–16. doi: 10.1007/BF02881235. [DOI] [PubMed] [Google Scholar]
- 5.Yu SH, Mott KE. Epidemiology and morbidity od food-borne intestinal trematode infections. Trop Dis Bull. 1994;91:R125–R152. [Google Scholar]
- 6.Radomyos P, Radomyos B, Tungtrongchitr A. Multi-infection with helminthes in adults from northeast Thailand as determined by post-treatment fecal examination of adult worms. Trop Med Parasitol. 1994;45:133–135. [PubMed] [Google Scholar]
- 7.Grover M, Dutta R, Kumar R, Aneja S, Mehta G. Echinostoma ilocanum infection. Indian Pediatr. 1998;35:549–552. [PubMed] [Google Scholar]
- 8.Belizario VY, Geronilla GG, Anastacio MBM, de Leon WU, Saba-an AP, Sebastian AC, Bangs MJ. Echinostoma malayanum infection, the Philippines. Emerg Infect Dis. 2007;13:1130–1131. doi: 10.3201/eid1307.061486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graczyk TK, Fried B. Echinostomiasis: a common but forgotten food-borne disease. Am J Trop Med Hyg. 1998;58:501–504. doi: 10.4269/ajtmh.1998.58.501. [DOI] [PubMed] [Google Scholar]