Abstract
The urge-to-cough is a respiratory sensation that precedes the cough motor response. Since affective state modulates the perception of respiratory sensations such as dyspnoea, we wanted to test whether nicotine, an anxiolytic, would modulate the urge-to-cough and hence, the cough motor response. We hypothesized that withdrawal from and administration of nicotine in smoking subjects would modulate their anxiety levels, urge-to-cough and cough motor response to capsaicin stimulation. Twenty smoking (SM) adults (8F, 12M; 22 ± 3 years; 2.9 ± 2.0 pack years) and matched non-smoking (NS) controls (22 ± 2 years) were presented with randomized concentrations of capsaicin (0–200 µM) before and after nicotine (SM only) gum and/or placebo (SM and NS) gum. Subjects rated their urge-to-cough using a Borg scale at the end of each capsaicin presentation. Cough number and cough motor pattern were determined from airflow tracings. Subjects completed State-Trait Anxiety Inventory (STAI) questionnaires before and after gum administration. SM subjects that withdrew from cigarette smoking for 12 h exhibited an increase in anxiety scores, a greater number of coughs and higher urge-to-cough ratings compared to NS subjects. Administration of nicotine gum reduced anxiety scores, cough number and urge-to-cough ratings to match the NS subjects. There was no effect of placebo gum on any of the measured parameters in the SM and NS groups. The results from this study suggest that modulation of the central neural state with nicotine withdrawal and administration in young smoking adults is associated with a change in anxiety levels which in turn modulates the perceptual and motor response to irritant cough stimulants.
Keywords: Urge-to-cough, Affective sensation, Gating, Anxiety, Capsaicin, Respiratory sensation
1. Introduction
We previously published that the urge-to-cough is a respiratory sensation that is a component of the cough cognitive motivational system [1,2]. We proposed that the sensory processes related to initiating a cough project to the brainstem respiratory control centre and to higher brain centres, in particular the somatosensory area in the cerebral cortex and affective areas in the cortex and subcortex [2]. Cognitive awareness of an urge-to-cough precedes the cough motor response when subjects are asked to attend to the cough stimulus [1,3,4]. It has also been reported that once conscious detection of an urge-to-cough occurs, the magnitude of the urge-to-cough can be scaled using psychophysical measures [3]. There is a direct relationship between cough stimulus intensity and the perceptual scaling of an urge-to-cough [3]. It has been hypothesized that the urge-to-cough, similar to other respiratory sensations [5], is gated into the discriminative (somatosensory) and affective neural systems [2]. Respiratory sensory gating has been demonstrated using airway obstruction in a paired stimulus paradigm in normal subjects [6]. It has also been suggested that the central cognitive neural gating systems can be modulated by changes in affect, attention and experience [5]. We reasoned that if gating respiratory sensations can be modulated by neural affective mechanisms, then increasing or decreasing a subject’s affective state will change the perception of an urge-to-cough.
Reflex cough is initiated by sensory stimulation from peripheral afferents [7–9]. These afferents are known to project to the cerebral cortex, affective and cognitive centres [5,10]. Stimulation of these afferents is the first step in eliciting the cough sensation that precedes the motor action of cough [7,8,11,12]. Afferents eliciting cough probably have dual projections to the brainstem for eliciting motor reflex cough action and to higher brain centres for affective and cognitive awareness of cough [2,5,13]. Cognitive awareness of an impending cough, an urge-to-cough, means that these afferents activated neurocognitive pathways which have properties of detection (cognitive awareness), stimulus evaluation and behavioural responses. The magnitude of the sensory stimulation is directly related to the stimulus evaluation (magnitude estimation), the number of coughs and the cough intensity [4,14]. Negative affect has been shown to increase the perception of other respiratory sensations, such as dyspnoea in normal subjects [15–18]. Anxiety is also associated with an increased awareness of respiratory sensation [15,18,19]. Thus, we hypothesize that changing the affective state such as increasing anxiety would increase the cognitive sense of an urge-to-cough and may increase the cough motor response.
Cigarette smoke and nicotine are known to modulate both peripheral afferent activity and central neural afferent activity [20–24]. Nicotine delivered to the airways stimulates lung afferents [22]. However nicotine also acts on central neural mechanisms as an anxiolytic [25,26]. Withdrawal from smoking is accompanied by increased measures of anxiety [27]. Non-smokers, when exposed to cigarette smoke, cough vigorously; however individuals who have a history of smoking seldom cough when similarly inhaling cigarette smoke [28]. If nicotine is a stimulant of airway afferents, then the question arises as to why inhalation of nicotine in cigarette smoke fails to stimulate cough or potentiate cough in individuals with chronic exposure to cigarette smoke. One possibility is that nicotine acts on higher brain central neural systems to decrease cough responsiveness and a sense of urge-to-cough in smokers. We hypothesize that withdrawal from smoking for 12 h in individuals with a history of one pack per day or less of cigarette smoking would increase their anxiety state, enhance their cough response to capsaicin and increase their sense of an urge-to-cough. We further hypothesize that acute systemic (oral) administration of nicotine would inhibit the capsaicin-induced reflex cough response and decrease their sensation of an urge-to-cough.
2. Methods
2.1. Subjects
Twenty healthy non-smoking (NS) subjects (8F, 12M; age: 22 ± 2 years) and 20 healthy smoking (SM) subjects (8F, 12M; age: 22 ± 3 years) with no history of chronic respiratory or neurological disease participated in this study. SM subjects reported smoking for 2.9 ± 2.0 pack years and were not actively taking nicotine replacements or medications to quit smoking. All subjects consented to participating as per the regulations set forth by the Institutional Review Board at the University of Florida.
2.2. Procedure
2.2.1. Cardiopulmonary measurements
Before each experimental day, subjects performed pulmonary tests (forced expiration and impulse oscillometry) to ensure they met the inclusion criteria. An FEV1/FVC greater than 80% of predicted and a blood pressure less than 140/90 mmHg were required for the subjects to remain in the study. Only one subject was excluded based on these criteria.
2.2.2. Capsaicin administration
After the subject was instrumented, the subject was seated comfortably in a chair and a facemask was placed over their nose and mouth. The nebulizers containing the capsaicin solutions were kept in the airflow fume hood to prevent premature exposure to capsaicin. For capsaicin delivery, the nebulizers were inserted into the inlet on the facemask, and the subjects were coached by the experimenter to inspire deeply through their facemask which triggered aerosolization of the capsaicin solution.
Seven capsaicin concentrations (5, 10, 25, 50, 100, 150 and 200 µM capsaicin in 80% physiological saline, 10% Tween 20, and 10% ethanol) plus one placebo (0 µM capsaicin) were presented to the subject in a blinded-randomized order. Each capsaicin presentation was separated by approximately 1 min.
2.2.3. Nicotine administration
One piece of nicotine gum (Nicorette, 4 mg nicotine) was administered to the smoking subjects only. Both smoking and non-smoking subjects received placebo gum (generic mint gum). Smoking subjects were blinded to the gum they received.
2.2.4. Psychophysical measurements
2.2.4.1. Urge-to-cough ratings
At the end of each capsaicin presentation, subjects were asked to rate their urge-to-cough [3]. This sensation was described to the subjects as the intensity of their need to cough, regardless of whether a cough was produced or not. Scores ranged from 1 (no urge-to-cough) to 10 (maximum urge-to-cough).
2.2.4.2. State-Trait Anxiety Inventory (STAI)
A subset of SM (4F; 6M) and NS (5F; 6M) subjects were asked to complete an STAI questionnaire assessing the level of state and trait anxiety before and after nicotine and/or placebo gum. Data were analyzed following the STAI test manual [29].
2.3. Protocol
One trial consisted of three presentations of each capsaicin and placebo solution in a randomized order, for a total of 24 presentations per trial. NS subjects completed 2 experimental days, whereas SM subjects completed 3 experimental days. On day 1, both NS and SM subjects completed only one trial to help them get accustomed to the experimental apparatus and procedure, and to establish their perceptual rating scale. On day 2, the NS subjects completed one trial (pre-trial), chewed placebo gum for 30 min, then completed a second trial (post-trial). SM subjects followed the same protocol as the NS subjects with the exception that they were blinded to the gum they received (i.e. placebo vs nicotine gum). On day 3, SM subjects followed the same protocol as day 2, except that they were given the gum that they did not receive on day 2. Smoking subjects were asked to refrain from cigarettes for at least 12 h before the experiment.
2.4. Data analysis
2.4.1. Cough number
The number of expulsive events was counted for 30 s following the presentation of each capsaicin and placebo solution. C2 and C5 indices of cough sensitivity were determined for the NS and SM subjects during the pre- and post-trials using the mean capsaicin concentration that generated two and five expulsive events respectively.
The average of the number of total expulsive events was plotted over capsaicin concentration for the NS and SM subjects during the pre- and post-trials. Cough threshold was determined as the minimum capsaicin concentration that generated one or more expulsive events. Data points were fitted with a two-compartment polynomial curve.
2.4.2. Cough type
Airflow recordings were used to differentiate between the two major airway reflexes studied: cough reaccelerations (CR; inspiration followed by one or more expulsive events) and expiration reflexes (ER; one or more expulsive events without a preceding inspiration). Examples of a CR and ER airway reflex are illustrated in previous papers [4,14,30]. The CRs and ERs were categorized based on the number of expulsive events (i.e. CR1, CR2, CR3, CR4 and ER1, ER2, ER3, etc.) and the total number of each airway reflex was plotted over the capsaicin concentration for NS and SM subjects during the pre- and post-trials.
2.4.3. Psychophysical measurements
The average of the urge-to-cough ratings was first plotted over capsaicin concentration for NS and SM subjects during the pre- and post-trials to determine the minimum capsaicin concentration that elicited an urge-to-cough sensation greater than 1 (i.e. threshold). Ratings were then plotted against capsaicin concentration on a log–log scale and a linear regression was used to fit the data. The slope of the line was used as a measure of the sensitivity of the urge-to-cough to capsaicin, and compared between trials (pre vs post), subjects (NS vs SM) and treatment (placebo vs nicotine).
The average state and trait anxiety scores before and after nicotine and/or placebo gum administration were compared for within and between group differences.
2.4.4. Statistics
The results were compared using a paired-t test, repeated measures ANOVA and post-hoc between group analyses performed with Student–Newman–Keuls method for paired multiple comparisons where appropriate. The significance criterion was set at p < 0.05.
3. Results
3.1. Subjects
The physical parameters (age, gender, and BMI) for the smoking (SM) subjects and their matched non-smoking (NS) controls, and the baseline cardiopulmonary measurements (FEV1, FVC, MAP) taken prior to each experimental day are listed in Table 1.
Table 1.
Physical and baseline cardiorespiratory parameters for smoking (SM) and non-smoking (NS) subjects.
| SM subjects | NS subjects | |
|---|---|---|
| Age (years) | 22 ± 2 | 22 ± 3 |
| Gender (F, M) | 8, 12 | 8, 12 |
| BMI (kg/m2) | 27.2 ± 5.5 | 24.2 ± 3.7 |
| FEV1/FVC (% predicted) | 93 ± 12 | 100 ± 8 |
| Systolic (mmHg) | 117 ± 8 | 115 ± 10 |
| Diastolic (mmHg) | 70 ± 6 | 69 ± 7 |
| HR (bpm) | 78 ± 11 | 77 ± 9 |
| Pack years | 2.9 ± 2.0 | – |
3.2. Cough response
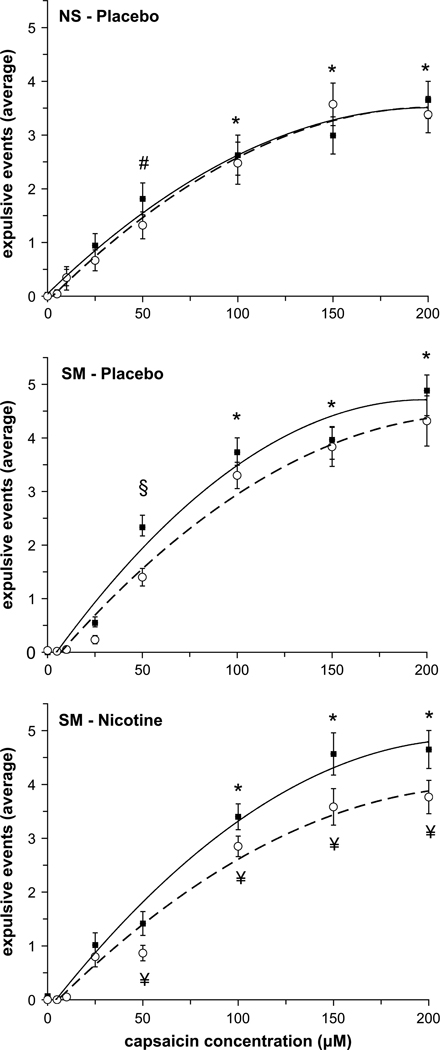
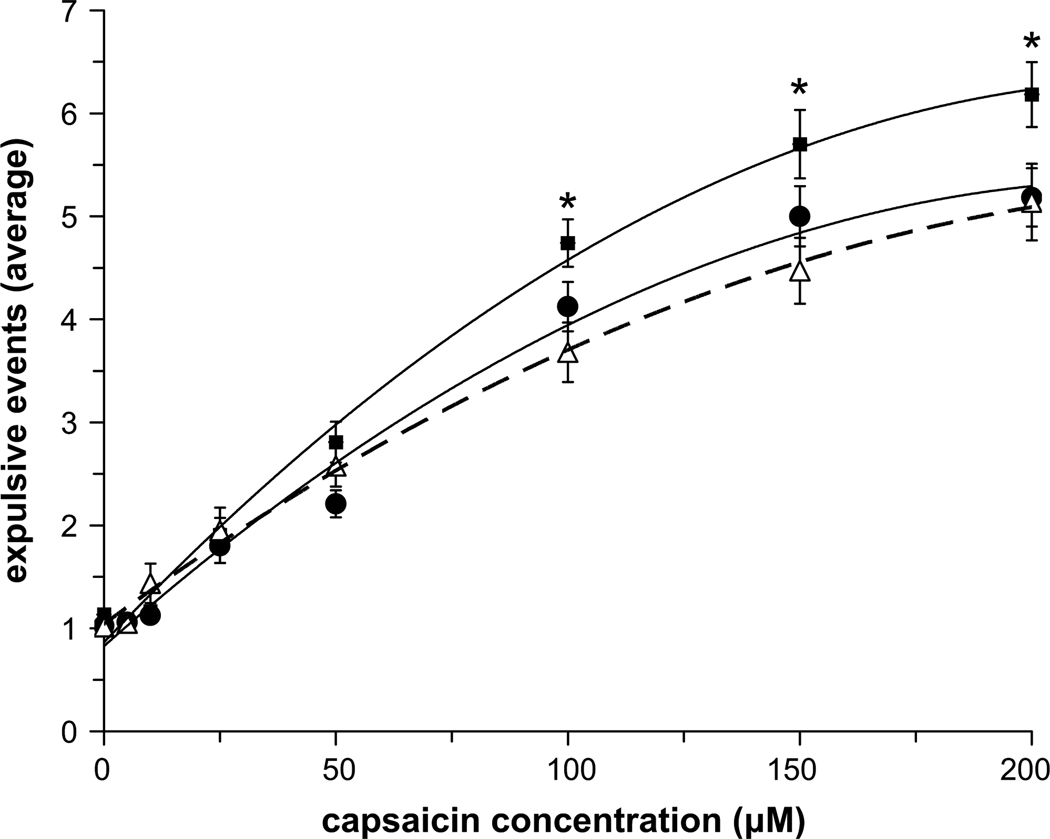
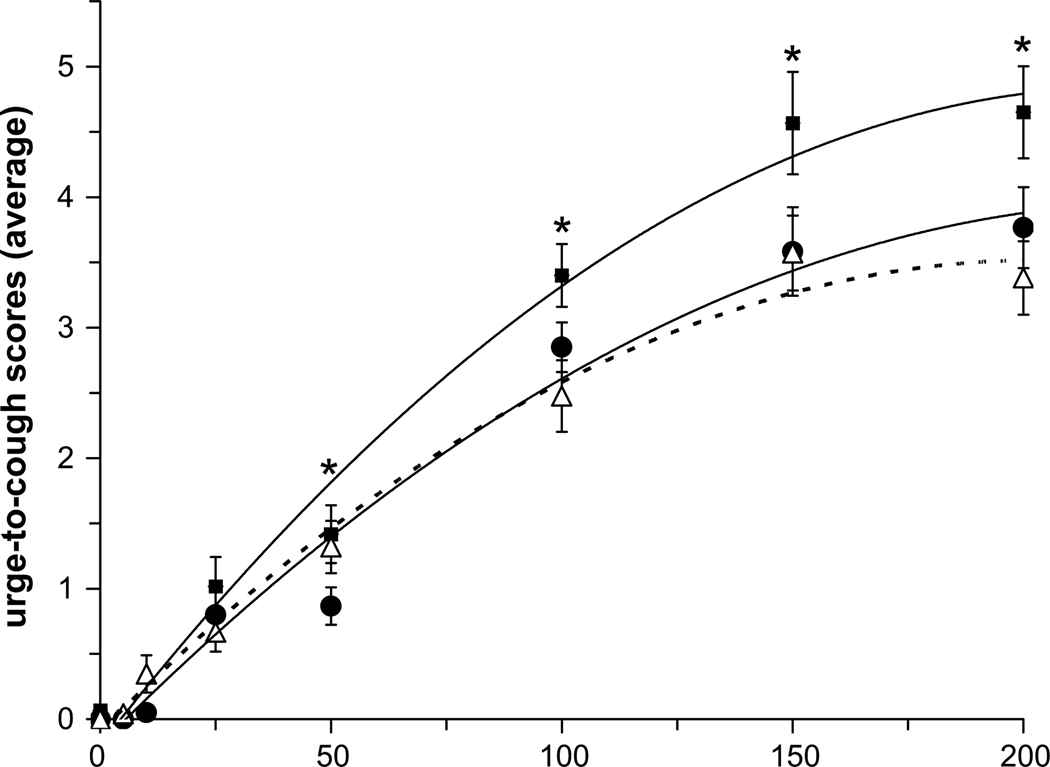
The average number of expulsive events generated for each capsaicin concentration before (pre-trial) and after (post-trial) treatment (placebo and/or nicotine gum) for the NS and SM subjects was fitted with a second order polynomial equation (Fig. 1). There was no effect of placebo (i.e. mint) gum on the number of expulsive events generated in the NS and SM group (Fig. 1, top and middle panels); however there was a significant decrease in the average number of expulsive events generated in the SM group following nicotine gum administration at capsaicin concentrations above 100 µM (Fig. 1, bottom panel). When post-trials for the NS and SM groups were compared, SM subjects who withdrew from cigarette smoking for 12 h generated more expulsive events at a given capsaicin concentration compared to NS subjects; however, following nicotine administration (i.e. Nicorette gum) the number of expulsive events generated was reduced to equal the NS cough response (Fig. 2).
Fig. 1.
Average number of expulsive events in the NS (top) and SM (middle) groups before (closed squares) and after (open circles) placebo gum administration, and following nicotine gum administration in the SM group (bottom). Symbols indicate significant differences: € significantly different from 0, 5, 15, 25, 50, 100 µM capsaicin (pre- and post-trials); * significantly different from 0, 5, 10, 25, 50 µM capsaicin (pre- and post-trials); # significantly different from 0, 5, 10, 25 µM capsaicin (pre- and post-trials); & significantly different from 0, 5, 10 µM capsaicin (pre- and post-trials); § significantly different from 0, 5, 10, 25 µM capsaicin (pre-trial only); ¥ significantly different from the corresponding pre-trial value.
Fig. 2.
Comparison of post-trial expulsive events following placebo gum administration in NS (open triangles) and SM (closed squares) groups, and in the SM group following nicotine gum administration (closed circles). * indicates significant differences from the corresponding post-trial nicotine (SM) and post-trial placebo (NS) values. There were no differences between post-trial nicotine (SM) and post-trial placebo (NS) values.
The median capsaicin concentrations that generated two and five expulsive events (i.e. C2 and C5, respectively) for all groups and conditions are listed in Table 2. There was no difference in the C2 values between subjects or conditions. C5 was the same between NS and SM subjects during pre- and post-placebo conditions; however C5 increased in SM subjects following nicotine administration.
Table 2.
Median capsaicin values eliciting two and five expulsive events (C2 and C5, respectively) in smoking (SM) and non-smoking (NS) groups before and after gum administration (nicotine and/or placebo).
| C2 | C5 | ||||
|---|---|---|---|---|---|
| Pre-trial | Post-trial | Pre-trial | Post-trial | ||
| NS | Placebo gum | 50 | 50 | 100 | 100 |
| SM | Placebo gum | 50 | 50 | 100 | 100 |
| SM | Nicotine gum | 50 | 100 | 100 | 150a |
Significantly greater than placebo gum (p < 0.05).
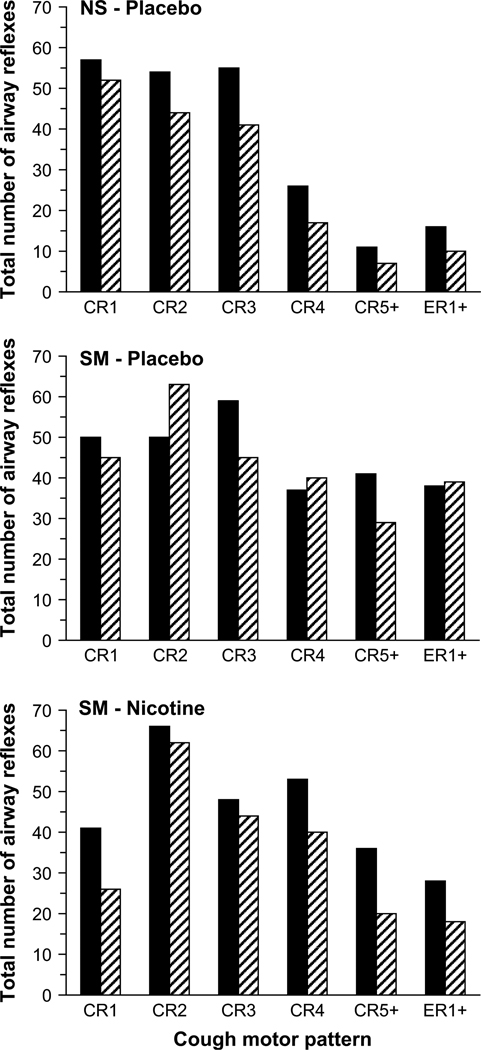
Airway reflexes for all groups and conditions were categorized as cough reaccelerations, CR1, CR2, CR3, CR4 and CR5+ (containing five or more expulsive events), or expiratory reflexes, ER1+ (containing one or more expulsive events). The total numbers of airway reflexes for each CR and ER category at 100, 150 and 200 µM capsaicin were summed for each trial since the difference in the total number of airway reflexes generated did not vary much between the three highest concentrations of capsaicin. Fig. 3 illustrates the total number of airway reflexes generated by the NS and SM groups before (pre-trial) and after (post-trial) nicotine and/or placebo gum administration. The most common airway reflex in all groups was CR2. Placebo gum did not have a consistent effect on the type of airway reflex generated. Following nicotine administration, the number of CRs and ERs decreased, with more prominent decreases in CRs with a greater number of expulsive events (i.e. CR4 and CR5+) and more subtle decreases in CR2.
Fig. 3.
Cough motor patterns before (solid) and after (hatched) placebo gum administration in NS subjects (top), SM subjects (middle) and after nicotine gum administration in SM subjects (bottom). The total number of cough reaccelerations (CR) and expiratory reflexes (ER) at 100, 150 and 200 µM of capsaicin was summed for each group and condition.
3.3. Urge-to-cough
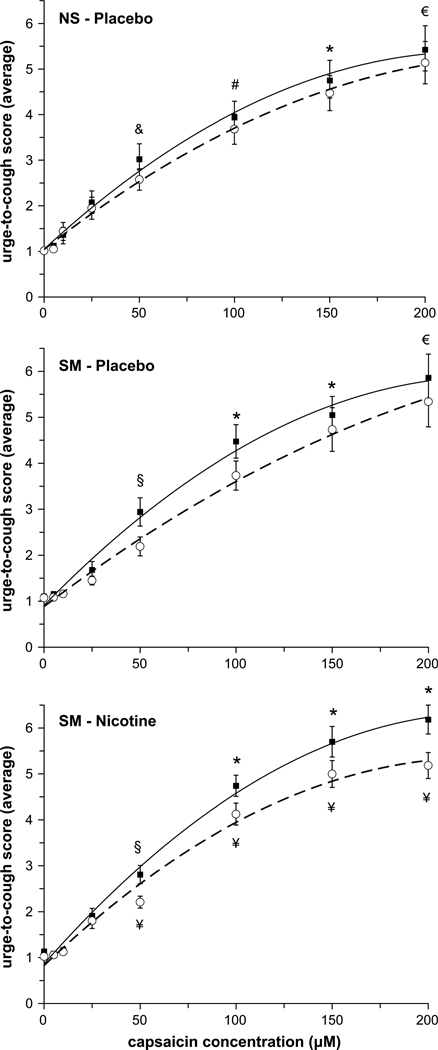
The average urge-to-cough scores at each capsaicin concentration before (pre-trial) and after (post-trial) treatment (nicotine and/or placebo gum) for the NS and SM subjects were fitted with a linear regression equation and plotted on a log–log plot to determine the urge-to-cough sensitivity (i.e. slope). There was no effect of placebo gum on the sensitivity of the urge-to-cough response in either the NS group (pre vs post: 2.4 and 2.1 au/100 µM capsaicin, r2 = 0.96 and 0.97, respectively) or the SM group (pre vs post: 2.6 and 2.3 au/100 µM capsaicin, r2 = 0.96 and 0.98). Following nicotine administration in the SM group, there was no difference in the urge-to-cough sensitivity (pre vs post: 2.8 and 2.3 au/100 µM capsaicin, r2 = 0.95 and 0.98). The curve that best fit the urge-to-cough scores (i.e. r2 = 0.99 for all curve fits) was a second order polynomial equation (Fig. 4). There was no significant effect of placebo gum on the urge-to-cough ratings in the NS and SM groups (Fig. 4, top and middle panels); however, after nicotine administration the urge-to-cough scores were significantly lower at higher capsaicin concentrations in the SM group (Fig. 4, bottom panel). When post-trials for the NS and SM groups were compared, SM subjects who withdrew from cigarette smoking for 12 h reported higher urge-to-cough scores for a given capsaicin concentration compared with NS subjects; however, following nicotine administration the scores decreased to equal the NS response (Fig. 5).
Fig. 4.
Average urge-to-cough ratings before (closed squares) and after (open circles) placebo gum administration in the NS (top) and SM (middle) groups, and following nicotine gum administration in the SM group (bottom). Symbols indicate significant differences: € significantly different from 0, 5, 15, 25, 50, 100 µM capsaicin (pre- and post-trials); * significantly different from 0, 5, 10, 25, 50 µM capsaicin (pre- and post-trials); # significantly different from 0, 5, 10, 25 µM capsaicin (pre- and post-trials); & significantly different from 0, 5, 10 µM capsaicin (pre- and post-trials); § significantly different from 0, 5, 10, 25 µM capsaicin (pre-trial only); ¥ significantly different from the corresponding pre-trial value.
Fig. 5.
Comparison of post-trial expulsive events following placebo gum administration in the NS (open triangles) and SM (closed squares) groups, and in the SM group following nicotine gum administration (closed circles). * indicates significant differences from the corresponding post-trial nicotine (SM) and post-trial placebo (NS) values. There were no differences between post-trial nicotine (SM) and post-trial placebo (NS) values.
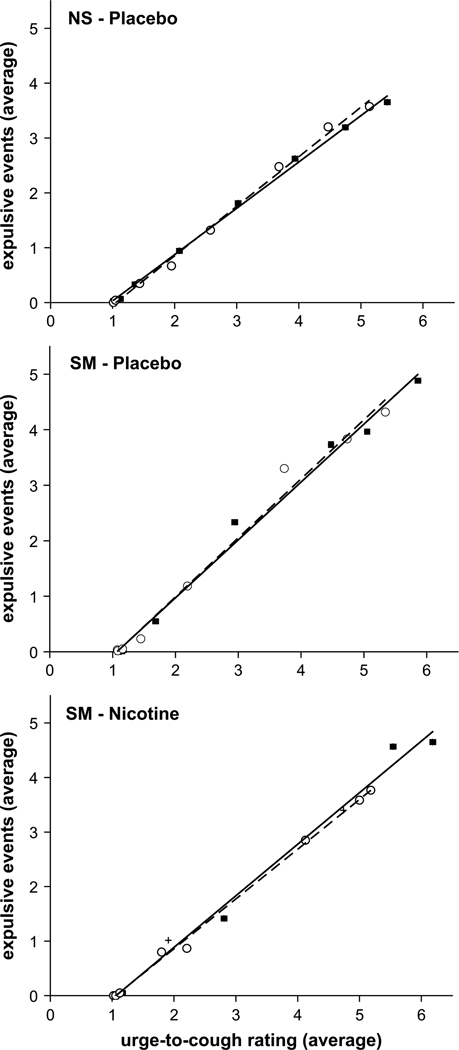
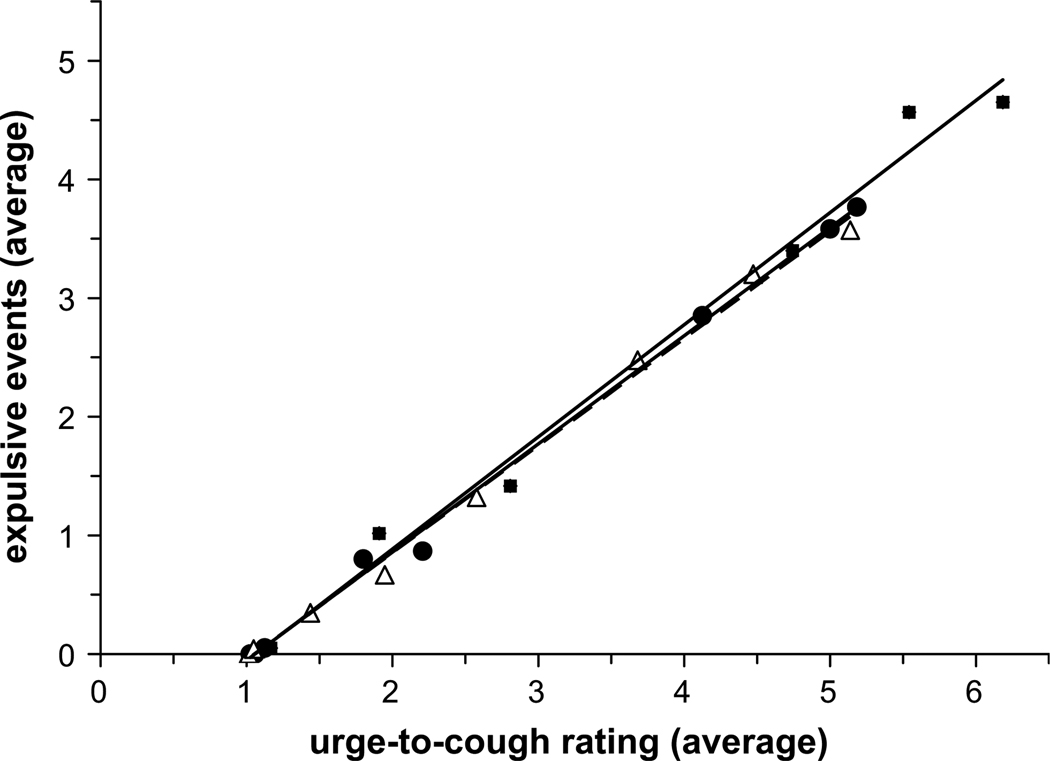
The relationship between the average number of expulsive events and the urge-to-cough scores demonstrates a strong linear relationship (r2 = 0.99 for all curves) (Fig. 6). The urge-to-cough scores increased by one increment every one to two expulsive events for both groups. Comparing the post-trial results for the NS and SM groups, nicotine did not affect this relationship; however it decreased the magnitude of this relationship at a given capsaicin concentration (Fig. 7).
Fig. 6.
Relationship between the average number of expulsive events and the average urge-to-cough ratings before (closed squares) and after (open circles) placebo gum administration in the NS (top) and SM (middle) groups, and following nicotine gum administration in the SM group (bottom). For every incremental increase in the urge-to-cough ratings, approximately one expulsive event was produced. There were no differences in this relationship (i.e. slope) between groups (SM vs NS) or treatment (nicotine vs placebo).
Fig. 7.
Relationship between the average number of expulsive events and the average urge-to-cough ratings following placebo gum administration in the NS (open triangles) and SM (closed squares) groups, and in the SM group following nicotine gum administration (closed circles). Nicotine withdrawal or administration did not change the sensitivity (i.e. slope) of the cough response to urge-to-cough ratings. However, nicotine withdrawal increased the magnitude of this relationship such that SM subjects rated higher urge-to-cough sensations and produced a greater number of coughs at high capsaicin concentrations compared with NS subjects. Administration of nicotine reduced this effect so that the SM response was the same as that for the NS subjects.
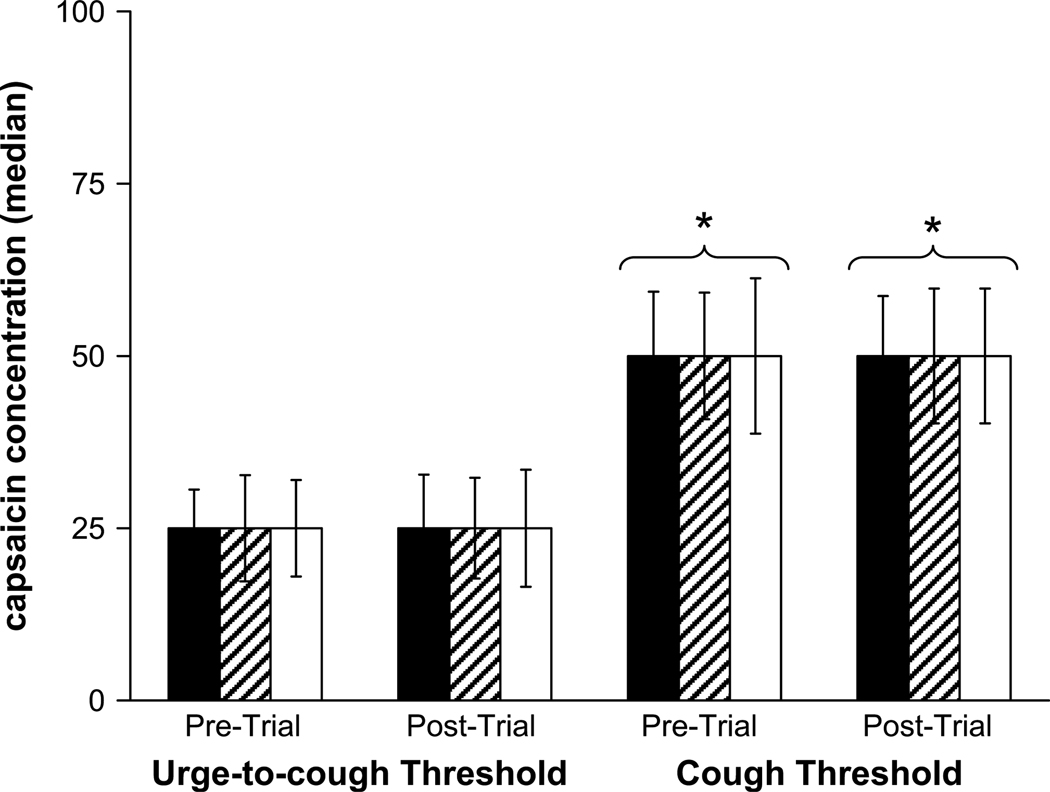
The minimum capsaicin concentration (i.e. threshold) required to elicit one or more expulsive event(s) or an urge-to-cough score of 1.5 or greater is illustrated in Fig. 8. There was no difference in threshold values between subjects or conditions; however, the urge-to-cough threshold was consistently lower than the cough threshold in all cases.
Fig. 8.
Capsaicin concentration at the urge-to-cough and the cough thresholds before and after placebo gum administration in the NS (solid bars) and SM (hatched bars) groups, and in the SM group before and after nicotine gum administration (open bars). The urge-to-cough threshold occurred at a lower capsaicin concentration compared with the cough threshold. There were no differences between groups (NS vs SM), treatments (nicotine vs placebo) or trials (pre- vs post-trial). * indicates a significant difference from the corresponding urge-to-cough threshold.
3.4. State-trait anxiety
There were no differences in state anxiety scores before or after nicotine and/or placebo gum administration in the SM and NS groups, although the SM subjects tended to score higher than the NS subjects. Trait anxiety scores before and after placebo gum administration were not different in either the NS or SM groups; however, there was a significant effect of nicotine on the trait anxiety scores in the SM group. Nicotine gum decreased trait anxiety scores from 36.3 ± 3 to 34.3 ± 3 (p = 0.03). When the SM and NS groups were compared, SM subjects generated consistently higher trait anxiety scores than NS subjects before (37.0 ± 2.4 and 32.7 ± 2.2 for SM and NS, respectively, p <0.05) and after (37.7 ± 2.4 and 31.7 ± 2.2 for SM and NS, respectively, p <0.05) placebo gum administration.
4. Discussion
The results of the study support the hypothesis that withdrawal from smoking enhances the cough response to capsaicin. Smokers that withdrew from smoking for 12 h had a greater cough response in terms of both the urge-to-cough and the number of coughs elicited by capsaicin compared with the control subjects. In addition, the urge-to-cough and number of coughs in smokers after 12 h of nicotine withdrawal were reduced when the smokers were administered nicotine orally. Administration of oral nicotine also resulted in the urge-to-cough and cough motor response in smokers to equal non-smokers. The results of this study suggest that modulation of the central neural state by nicotine withdrawal, associated with increased anxiety, modulates the motor and perceptual response to irritant cough stimulants.
Inhalation of nicotine aerosol has been reported to sensitize airway afferents [20–22]. In addition, inhalation of smoke was reported to stimulate airway afferents [20–24] and to elicit cough [28]. Inhalation of smoke in naïve subjects appears to elicit a greater cough response than inhalation of smoke in chronic smokers suggesting that there is a cough response accommodation in smokers to smoke and nicotine stimuli in the airway. It has also been reported that smokers withdrawing from nicotine have increased cough response [31]. The fact that individuals with a history of smoking have an increased cough response when nicotine is withdrawn suggests a central modulation of brainstem cough motor output. In the present study, 12 h of nicotine withdrawal is approximately four to six half-life decreases in blood nicotine levels [32]. This means that the central neural nicotine levels are very low, decreasing the central effect of nicotine on central motor and cognitive affective mechanisms. When the smoking subjects in the present study were initially exposed to increasing concentrations of capsaicin, the motor cough response showed a significantly greater number of coughs than for the non-smokers (Fig. 2), particularly at capsaicin concentrations greater than 100 µM which elicited cough in all subjects, smokers and nonsmokers. When non-smokers were administered placebo gum there was no change in the cough motor response. Similarly when smokers were administered nicotine-free placebo gum, there was no change in the cough motor response that remained greater than the cough motor response in non-smokers. However, when nicotine gum was presented to the smokers there was a significant decrease in the cough motor response that reduced the number of coughs to equal non-smokers. The non-smoker post-placebo and smoker post-nicotine cough trials showed similar cough responses (Fig. 2). However if the smokers did not have oral nicotine the post-trial placebo smokers retained a significantly elevated cough motor response greater than their post-trial nicotine gum cough response. These results demonstrate that oral administration of nicotine modulates the cough motor response in smokers suggesting a central nicotine modulation of cough neural mechanisms.
We have previously reported that administration of graded concentrations of capsaicin elicits a corresponding increased cognitive sense of an urge-to-cough [3,4]. Similar to the cough motor response, when smokers withdrew for 12 h from smoking, administration of capsaicin elicited a significant increase in the sense of an urge-to-cough that was greater than with non-smokers. Administration of placebo gum did not change the sense of an urge-to-cough for either smokers or non-smokers; the post-trial placebo gum urge-to-cough response in smokers remained significantly greater than the post-trial placebo gum non-smoker urge-to-cough response. However, when oral nicotine gum was administered to the smokers there was a significant decrease in the urge-to-cough, similar to the decrease in the cough motor response at concentrations above 100 µM capsaicin. Again, the urge-to-cough response post-trial paralleled the cough motor response such that smokers after oral nicotine administration had similar urge-to-cough sensations elicited by capsaicin as non-smokers. However, smokers administered placebo gum had an increased post-trial urge-to-cough that was significantly greater than both non-smokers and nicotine treated smokers (Fig. 5). Thus, the effect of nicotine administered orally in smokers after nicotine withdrawal shows parallel effects on both the cough motor and cognitive urge-to-cough responses. These results support the hypothesis that nicotine is acting on central neural mechanisms to decrease both the cough motor response and the cognitive urge-to-cough. This conclusion is further supported when the motor and urge-to-cough responses are correlated (Figs. 6 and 7). There is a direct relationship, as we reported previously, between the cough motor response and the urge-to-cough [1–4]. Neither placebo gum nor oral nicotine changed the sensitivity (slope) of this relationship. The effect of nicotine withdrawal is to extend the relationship to a higher urge-to-cough and the corresponding cough motor response portion of the curve (Fig. 7). Thus, the effect of nicotine and smoking history is not on the sensitivity relationship between the cough neural response in the urge-to-cough sensitivity but on the magnitude of that response. This is further evidenced by the fact that there was no difference between 12 h smoking withdrawal, placebo gum, and nicotine administration on the threshold for eliciting either the urge-to-cough or the cough motor response (Fig. 8).
We previously proposed a model of the cough motor response and the urge-to-cough that suggests a central modulation of cough and sensory gating mediated by the affective nervous system [1,2]. In the present study we determine that trait anxiety was increased in smokers after 12 h smoking withdrawal. This suggests that the affective state changed such that after nicotine withdrawal, the smokers were more anxious, consistent with what has been reported previously for smoking withdrawal [25]. If, as we have previously proposed, the urge-to-cough is part of a motivation to action neural mechanisms for respiratory protection reflexes [1,2], and smoking withdrawal causes an increased anxious state mediated by the affective nervous system, then modulation of the affective state may be a gain-setting modulation of the gate for the sense of an urge-to-cough and the cough motor gate controlling the motor response. Increased affective drive, such as increased anxiety, decreases the gating out of stimuli into higher brain cognitive centres. This results in an increased sensation from the stimulus (capsaicin) due to a greater transmission through the gate into higher brain centres. Application of an anxiolytic substance such as nicotine increases the gating out of stimuli thus decreasing the cognitive sensation elicited by a cough stimulus such as capsaicin. The results of this study also suggest that affective state also modulates the cough motor gate. Increasing the affective state (in this study increased anxiety), decreases the gating out of the capsaicin stimulus increasing the cough motor response regulated by brainstem cough neural mechanisms. Oral administration of nicotine, similar to the cognitive response, decreases affective related anxiety and decreases the cough motor response. These results support the hypothesis that smoking withdrawal enhances the cough motor response by changing the gain of central neural mechanisms mediating both cognitive and motor cough behaviours. Administration of nicotine inhibits cognitive and motor cough responses in a parallel fashion that is consistent with the central neural anxiolytic effect of nicotine. It will be important to determine in future studies the relationships between ascending peripheral and descending central regulation of brainstem cough motor behaviour.
References
- 1.Davenport PW. Urge-to-cough: what can it teach us about cough? Lung. 2008;186(Suppl. 1):S107–S111. doi: 10.1007/s00408-007-9045-7. [DOI] [PubMed] [Google Scholar]
- 2.Davenport PW. Clinical cough I: the urge-to-cough: a respiratory sensation. In: Chung KF, Widdicombe JG, editors. Handbook of experimental pharmacology: pharmacology and therapeutics of cough. Heidelberg: Springer; 2009. pp. 263–276. [DOI] [PubMed] [Google Scholar]
- 3.Davenport PW, Sapeinza CM, Bolser DC. Psychophysical assessment of the urge-to-cough. Eur Respir J. 2002;12:249–253. [Google Scholar]
- 4.Davenport PW, Bolser DC, Vickroy T, Berry RB, Martin AD, Hey JA, et al. The effect of codeine on the urge-to-cough response to inhaled capsaicin. Pulm Pharmacol Ther. 2007;20:338–346. doi: 10.1016/j.pupt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davenport PW, Vovk A. Cortical and subcortical central neural pathways in dyspnea. Respir Physiol Neurobiol. doi: 10.1016/j.resp.2008.10.001. in press. [DOI] [PubMed] [Google Scholar]
- 6.Chan PY, Davenport PW. Respiratory-related evoked potential measures of respiratory sensory gating. J Appl Physiol. 2008;105:1106–1113. doi: 10.1152/japplphysiol.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152:223–242. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Mazzone SB. Sensory regulation of the cough reflex. Pulm Pharmacol Ther. 2004;17:361–368. doi: 10.1016/j.pupt.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Mazzone SB. An overview of the sensory receptors regulating cough. Cough. 2005;1:2. doi: 10.1186/1745-9974-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzone SB, McGovern AE, Koo K, Farrell MJ. Mapping supramedullary pathways involved in cough using functional brain imaging: comparison with pain. Pulm Pharmacol Ther. 2009;22:90–96. doi: 10.1016/j.pupt.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulm Pharmacol Ther. 2002;15:221–225. doi: 10.1006/pupt.2002.0361. [DOI] [PubMed] [Google Scholar]
- 12.Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: a holarchical system? Respir Physiol Neurobiol. 2006;152:255–265. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazzone SB, McLennan L, McGovern AE, Egan GF, Farrell MJ. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med. 2007;176:327–332. doi: 10.1164/rccm.200612-1856OC. [DOI] [PubMed] [Google Scholar]
- 14.Vovk A, Bolser DC, Hey JA, Danzig M, Vickroy T, Berry R, et al. Capsaicin exposure elicits complex airway defensive motor patterns in normal humans in a concentration-dependent manner. Pulm Pharmacol Ther. 2007;20:423–432. doi: 10.1016/j.pupt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Peuter S, Lemaigre V, Van Diest I, Van den Bergh O. Illness-specific catastrophic thinking and overperception in asthma. Health Psychol. 2008;27:93–99. doi: 10.1037/0278-6133.27.1.93. [DOI] [PubMed] [Google Scholar]
- 16.Han JN, Zhu YJ, Luo DM, Li SW, Van Diest I, Van den Bergh O, et al. Fearful imagery induces hyperventilation and dyspnea in medically unexplained dyspnea. Chin Med J (Engl) 2008;121:56–62. [PubMed] [Google Scholar]
- 17.Van Diest I, De Peuter S, Eertmans A, Bogaerts K, Victoir A, Van den Bergh O. Negative affectivity and enhanced symptom reports: differentiating between symptoms in men and women. Soc Sci Med. 2005;61:1835–1845. doi: 10.1016/j.socscimed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Von Leupoldt A, Riedel F, Dahme B. The impact of emotions on the perception of dyspnea in pediatric asthma. Psychophysiology. 2006;43:641–644. doi: 10.1111/j.1469-8986.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 19.Van Diest I, Thayer JF, Vandeputte B, Van de Woestijne KP, Van den Bergh O. Anxiety and respiratory variability. Physiol Behav. 2006;89:189–195. doi: 10.1016/j.physbeh.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 20.Lee LY, Morton RF, Kou YR. Acute effects of cigarette smoke on breathing in rats: vagal and nonvagal mechanisms. J Appl Physiol. 1990;68:955–961. doi: 10.1152/jappl.1990.68.3.955. [DOI] [PubMed] [Google Scholar]
- 21.Kou YR, Lee LY. Stimulation of rapidly adapting receptors in canine lungs by a single breath of cigarette smoke. J Appl Physiol. 1990;68:1203–1210. doi: 10.1152/jappl.1990.68.3.1203. [DOI] [PubMed] [Google Scholar]
- 22.Kou YR, Frazier DT, Lee LY. The stimulatory effect of nicotine on vagal pulmonary C-fibers in dogs. Respir Physiol. 1989;76:347–356. doi: 10.1016/0034-5687(89)90075-3. [DOI] [PubMed] [Google Scholar]
- 23.Sellick H, Widdicombe JG. Activity of lung irritant receptors caused by cigarette smoke, dust and histamine aerosol. J Physiol. 1970;210:56P–7P. [PubMed] [Google Scholar]
- 24.Sellick H, Widdicombe JG. Stimulation of lung irritant receptors by cigarette smoke, carbon dust, and histamine aerosol. J Appl Physiol. 1971;31:15–19. doi: 10.1152/jappl.1971.31.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Buckley TC, Holohan DR, Mozley SL, Walsh K, Kassel J. The effect of nicotine and attention allocation on physiological and self-report measures of induced anxiety in PTSD: a double-blind placebo-controlled trial. Exp Clin Psychopharmacol. 2007;15:154–164. doi: 10.1037/1064-1297.15.2.154. [DOI] [PubMed] [Google Scholar]
- 26.Crawford HJ, McClain-Furmanski D, Castagnoli N, Jr, Castagnoli K. Enhancement of auditory sensory gating and stimulus-bound gamma band (40 Hz) oscillations in heavy tobacco smokers. Neurosci Lett. 2002;317:151–155. doi: 10.1016/s0304-3940(01)02454-5. [DOI] [PubMed] [Google Scholar]
- 27.Shiffman S. Effect of nicotine lozenges on affective smoking withdrawal symptoms: secondary analysis of a randomized, double-blind, placebo-controlled clinical trial. Clin Ther. 2008;30:1461–1475. doi: 10.1016/j.clinthera.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Gupta D, Aggarwal AN, Chaudhry K, Chhabra SK, D’Souza GA, Jindal SK, et al. Household environmental tobacco smoke exposure, respiratory symptoms and asthma in non-smoker adults: a multicentric population study from India. Indian J Chest Dis Allied Sci. 2006;48:31–36. [PubMed] [Google Scholar]
- 29.Spielberger CDG, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto: Consulting Psychologists Press; 1970. [Google Scholar]
- 30.Kim J, Davenport P, Sapienza C. Effect of expiratory muscle strength training on elderly cough function. Arch Gerontol Geriatr. 2008 May 3; doi: 10.1016/j.archger.2008.03.006. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31.Dicpinigaitis PV. Cough reflex sensitivity in cigarette smokers. Chest. 2003;123:685–688. doi: 10.1378/chest.123.3.685. [DOI] [PubMed] [Google Scholar]
- 32.Prignot J. Quantification and chemical markers of tobacco-exposure. Eur J Respir Dis. 1987;70:1–7. [PubMed] [Google Scholar]