Abstract
The present studies sought to determine whether prenatal cocaine administration (15 mg/kg b.i.d. between gestational ages 1–20) had enduring effects on emotional behavior of rats. Rats prenatally treated with cocaine interacted less with other rats in the social interaction test of anxiety at both 30 and 120 days of age. However, there were no differences in the elevated plus maze test of anxiety. Rats prenatally treated with cocaine were significantly more immobile in the forced-swim test at 60 and 120 days of age. In addition, animals exposed to prenatal cocaine were more sensitive to the enhancing effect of phencyclidine (2.0 mg/kg) on startle responses to an acoustic stimulus. The cocaine-treated animals tested at 50 to 60 days of age showed higher levels of prepulse inhibition, in comparison to the saline group, after vehicle pretreatment, but not after phencyclidine. Although there were gender differences in the expression of some of these behavioral tasks, there were no gender differences in the effects of cocaine. These findings indicate that when emotional behavior is altered by prenatal cocaine administration, the effects are enduring.
Keywords: Prenatal cocaine treatment, Emotional behavior, Social interaction test, Plus maze test, Forced swim test, Development
1. Introduction
The effects of prenatal cocaine exposure have been widely studied in humans and rodents [5,21–23,25]. However, despite evidence that a change in emotional behavior in children is a common consequence of prenatal cocaine exposure [5,26,27,32], there have been relatively few studies in rodents that have studied emotional behavior. One study reported that infant rats exposed to cocaine were less able to compete for water, but this effect appeared to be transient, as adolescent and adult rats were able to compete as well [39]. However, these rats became more aggressive during the competition for water, suggesting some enduring effects on emotional behavior [23,39]. Other work has shown that early play behavior was altered in rats given prenatal cocaine treatment, such that they were more submissive and less likely to solicit play than normal animals [37,38]. Prenatal exposure to cocaine has also been demonstrated to have gender-specific effects on social or aggressive behavior. In one study, male rats exposed to 30 mg/kg of cocaine during gestation were less likely to approach other nonexposed conspecifics for social interaction and less likely to reciprocate contact when 90 days of age, whereas females prenatally exposed to the same dose exhibited no apparent effects on social behavior, other than a slight increase in mildly aggressive behavior towards nonexposed females [22]. Another report indicated that rats exposed to cocaine in utero exhibited a decreased startle response to an acoustic stimulus [20], but there was no evidence about whether these differences persisted over several ages. In contrast, female rats prenatally exposed to 30 mg/kg of cocaine vocalized faster and more often following an air puff stimulus than did control females or males (prenatally exposed or controls) when tested as adults, suggesting some long-term changes in sensitivity to stimuli [21]. Thus, previous studies indicate that prenatal cocaine exposure can induce postnatal behavioral changes of various types. It is unclear how long these endure, and whether they could predispose to the development of specific psychiatric disorders in humans, such as anxiety, mood, psychotic, and personality disorders.
The purpose of the present set of experiments was to determine whether prenatal cocaine administration would have enduring effects on emotional behavior in rats and to assess possible changes within the context of paradigms developed as models of psychiatric disorders. The social interaction and elevated plus maze tests were used to assess anxiety-related behaviors in the rats. These tests have been widely validated [7–11,31] but do not measure the same type of anxiety [11]. The forced-swim test, a test widely used to detect drugs with antidepressant activity [4], was used to provide an index of the depressive tendency of the rats. The acoustic-startle test was used to study the reactivity and sensory gating of the rats to external stimuli. Previous work has linked changes in acoustic startle responses to altered dopamine function in brain regions involved in sensorimotor processing [35,36], and the test in animals for sensory gating has been used to model some aspects of schizophrenia (for review, see [17]). Because the neuro-chemical changes associated with prenatal cocaine exposure can emerge and vary across development [19,23,33], subjects of different ages were included in each procedure.
2. Methods
2.1. Animals
Sprague–Dawley (Charles River, Raleigh, NC) rats were habituated to the animal quarters for a week. Rats were housed in a standard animal facility at 22°C, with 50% humidity and a 12:12 light:dark cycle (lights on, 0700-1900). Virgin females were placed with experienced males, and pregnancy was confirmed by the appearance of a vaginal plug. Pregnant females were randomly assigned to an experimental group that received twice-daily injections of cocaine hydrochloride (15 mg/kg, s.c.) or to a control group that received twice daily injections of isotonic saline. Injections were given from Day 1–20 of gestation; Day 1 was the day after the discovery of a sperm plug. Rat pups were immediately placed with surrogates upon birth and kept with the surrogate mothers until weaning at 21 days of age, when they were placed in same-sex group cages. One male and one female rat were chosen from each of 46 litters for behavioral testing at 30, 60, or 120 days of age or, for the acoustic startle procedure, at either 50–60 or 120 days of age. Thus, each age group consisted of a different set of animals. Rats tested in the social task were also used for the plus maze and swim tasks, in that order. The elevated plus maze and forced-swim tests were not conducted in the 30-day old rats because of their small size. Rats tested for acoustic startle were only used for the one task, with different rats used at the different test ages.
2.2. Social interaction test
Social interactions were recorded on videotape in a test arena (wooden box 60 × 60 cm, with 35-cm high walls) lit by low light (30 lux). The floor was marked off into sixteen 15 × 15 cm squares. A camera with an automatic iris was mounted vertically above the arena, and the rats were observed on a monitor by an observer who was blind to the drug treatment and test condition. The time spent in social interaction (sniffing, following, and grooming the partner; boxing and wrestling) provided the index of anxiety, with lower scores reflecting a more anxious animal. Locomotor activity was scored by counting the number of squares entered during the 5-min test session. This test was originally developed and extensively validated in File's laboratory, where it has been used to assess the anxiolytic impact of lesions, drugs, peptides, and naturalistic anxiogenic stimuli, such as predator odor [7–10,13,14]. Recently, File and her colleagues have applied these tasks to understand more fully the role of serotonergic mechanisms in animal anxiety [2,12,18].
2.3. Elevated plus maze test
The plus maze was made of wood and consisted of two opposite open arms of 50 × 10 cm and two opposite arms enclosed by 40-cm-high walls. The arms were connected by a central 10 × 10 cm square, and the maze therefore formed a “plus” shape. The maze was elevated 50 cm from the floor and lit by dim light. The percentage of time spent on the open arms of the maze provided the major measure of anxiety, whereas the number of closed-arm entries provided the best measure of locomotor activity in this test [7,8,31].
2.4. Forced-swim test
The forced-swim test involved placing the rat in a cylinder (18-cm diameter, 40-cm tall) with enough 25°C water so the rat could not touch bottom with its hindpaws. Immobility, the absence of movement in three of the four paws, was recorded during a single 5-min session. This test was included because there are differences between rat strains selectively bred for differences in serotonergic and cholinergic function [29,30], and there is some evidence that prenatal cocaine exposure can alter serotonergic function [19,23,28].
2.5. Sensory gating test
For each test, rats were set in a small plastic cage, placed in a sound-attenuating chamber, with startle amplitude measured using a strain gauge transducer routed through a Model 7 polygraph recorder (Grass Instruments, Quincy, MA). Readings of dB levels were taken by a Precision Sound Level Meter and Analyzer (GenRad, Westford, MA). Background noise (65 dB) was provided by a fan attached to the chamber. The acoustic stimulus (a 100-dB burst of noise) was emitted through a microphone in the test chamber. Scores were derived for startle amplitude to the stimulus presentation alone and for startle amplitude when a low-intensity, nonstartling stimulus (a 75-dB burst of noise) preceded the startle stimulus by 100 ms. The percentage inhibition observed with the prepulse stimulus was derived by the following formula:
The first stage of testing provided vehicle data for each subject. In a second stage, the same subjects were used to assess whether prenatal exposure to cocaine can result in supersensitivity to the disruptive impact of a moderate dose of phencyclidine (PCP) on prepulse inhibition. At least 5 days following the first test for the acoustic startle response, each animal received a second test following treatment with PCP (2.0 mg/kg). On the basis of previous work [24,34] and given the parameters of the acoustic startle procedure, we predicted that this drug dose would significantly reduce, but not fully prevent, inhibition of the startle response by the prepulse stimulus.
For each session, animals were given an injection of either vehicle (saline) or PCP and then placed in the testing cage for a 10-min habituation period. In all, subjects were given, in a semirandomized order, 20 trials with the acoustic stimulus alone and 20 trials with the prepulse stimulus, for a total of 40 trials per session. Prepulse inhibition of acoustic startle was employed because changes in dopaminergic function may alter this behavioral task [25] and because there is some evidence that prenatal cocaine exposure can alter dopaminergic function later in life [19,33]
2.6. Procedure
Separate groups of rats were used for the sensory gating test and the other three tasks. Previous unpublished findings have revealed that prior experience of a rat with the social interaction test did not alter behavioral responses in the elevated plus maze or swim tests. Therefore, the following sequence was employed: at about 30, 60, or 120 days of age, pairs of rats with the same treatment and similar body weights were placed in the open-field arena for a 5-min sample of social interaction behavior. Time spent in social interaction and the number of squares entered were scored for each individual animal. For the 60-day and 120-day groups, 2 or 3 days after the completion of the social interaction test, the rats were placed in the elevated plus maze for a 5-min session, followed by a 5-min forced-swim test.
2.7. Statistical analysis
Initial analyses of the behavioral data were carried out by three-way ANOVAs, with gender, age, and cocaine treatment as the major factors. Gestational variables were compared by two-way ANOVAs. If the main or interaction effects were significant, follow-up analyses were carried out by Tukey's test or one-way ANOVAs. Significance was set at p ≤ 0.05.
3. Results
3.1. Gestational variables
Maternal weight gain, number of pups per litter, and litter weight were not different between saline and cocaine dams for the litters used in the acoustic startle test. The cocaine-exposed mothers for the pups tested at 30 days of age showed significantly less weight gain (106.6 ± 6.7 g) across gestation in comparison to the saline-treated dams [133 ± 6.7 g; F(1, 18) = 8.01, p < 0.01]. A similar result was found for the mothers of pups tested at 60 and 120 days of age, with cocaine treatment leading to significantly less weight gain (102.2 ± 6.4 vs. 127.1 ± 6.1 g) during pregnancy [F(1, 17) = 8.01, p < 0.01). The reduced weight gain associated with cocaine treatment in these groups did not lead to differences in number of pups per litter or litter weight.
3.2. Social interaction
The results for time spent in social interaction are illustrated in Fig. 1. These findings were analyzed by three-way ANOVA. This analysis confirmed the visual inspection of Fig. 1: the main effect of cocaine treatment was highly significant [F(1, 102) = 34.49, p < 0.0001], but there was no significant effect of age [F(2, 102) = 1.86, NS] and only a small effect of gender [F(1, 102) = 4.31, p = 0.04], with females being more active. There was also a significant interaction between age and cocaine treatment [F(2, 102) = 4.75, p < 0.01], a reflection of the fact that the cocaine-exposed rats exhibited less social interaction at 30 and 120 days of age, but not at 60 (see Fig. 1).
Fig. 1.
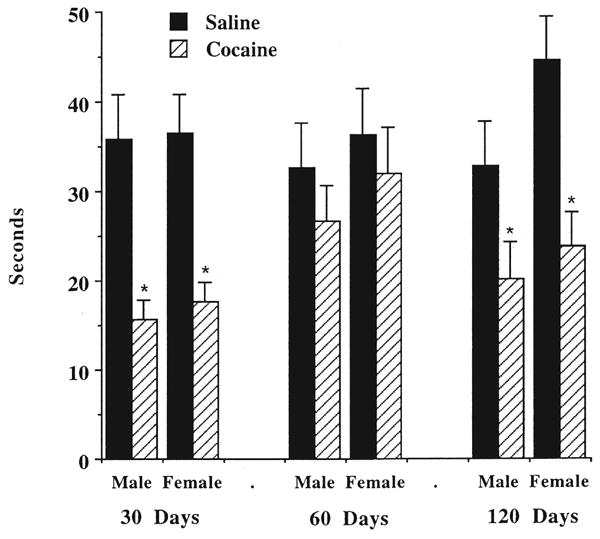
The effects of prenatal cocaine exposure on social interaction in 30-, 60- and 120-day-old rats. Rats were treated b.i.d. with 15 mg/kg cocaine hydrochloride from gestation days 1–20. They were weaned at 21 days of age and housed in same-sex groups of up to five until the time of testing. Pairs of rats with similar weights were used in the open-field apparatus for 5 min. Data are mean second of social interaction for 6–10 rats. *Significantly different, p < 0.05, from saline-treated controls, according to Tukey's test.
Locomotor activity of the rats in the social interaction test is illustrated in Fig. 2. This figure presents a very different pattern from the results displayed in Fig. 1. There do not appear to be any major differences in the saline- and cocaine-exposed groups, and the 3-way ANOVA confirmed this lack of effect [F(1, 102) = 3.00, NS]. In contrast, there were highly significant age [F(2, 102) = 26.71, p < 0.0001] and gender [F(1, 102) = 15.82, p < 0.0001] effects; generally, older animals and female rats are more active (see Fig. 2). Thus, prenatal administration of cocaine has a significant effect only on the behavioral measure that is reflective of the anxiety state of the rat.
Fig. 2.
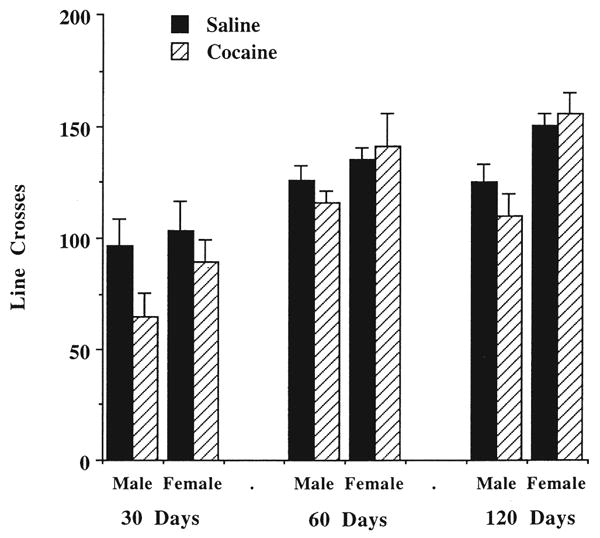
Effects of prenatal cocaine exposure on locomotor activity of 30-, 60-, and 120-day old rats in the social interaction test. See legend to Fig. 1 for treatment conditions. Data are the mean number of lines crossed for 6–10 rats. ANOVA indicated that cocaine treatment did not significantly alter locomotor activity, but there were significant age and gender effects.
3.3. Elevated plus maze
As illustrated in Fig. 3, prenatal cocaine administration did not significantly influence the time spent in the open arms of the elevated plus maze, the primary measure that reflects anxiety in this task [F(1, 65) = 0.23, NS]. Age [F(1, 65) = 3.73, p = 0.06] or gender [F(1, 65) = 0.23] also did not significantly influence time spent in the open arms (Fig. 3). There were also no differences for the other measures in this task as a consequence of cocaine administration (data not shown).
Fig. 3.
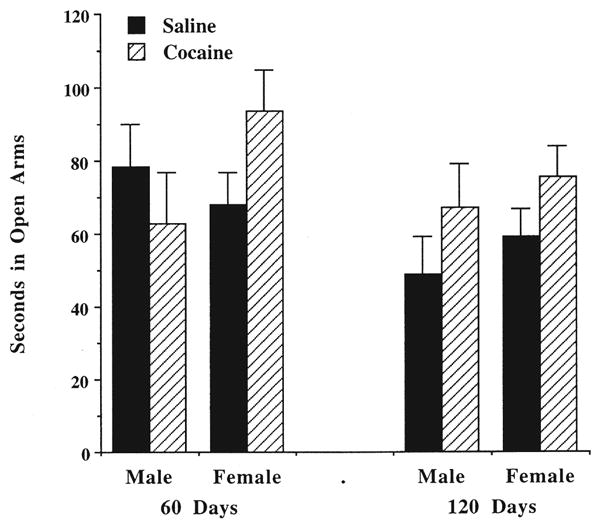
Effects of prenatal cocaine exposure on the time in open arms of the elevated plus maze. See legend to Fig. 1 for treatment conditions. Data are mean seconds for 6–10 rats. ANOVA indicated that there were no significant main effects. Thus, prenatal cocaine exposure did not significantly affect measures in the plus maze.
3.4. Forced-swim test
Rats that were prenatally exposed to cocaine were more immobile in the forced swim test, as illustrated in Fig. 4 [F(1, 65) = 48.52, p < 0.0001]. At both ages tested, the cocaine-exposed rats were more immobile. These data also illustrate that the female rats tend to be more active in the swim test than do male rats [F(1, 65) = 13.45, p = 0.001], but there is no difference between the two age groups [F(1, 65) = 0.13, NS]. The male–female difference in immobility was seen only in the 60-day age group (Fig. 4), resulting in a significant gender × age interaction [F(1,65) = 4.09, p < 0.05].
Fig. 4.
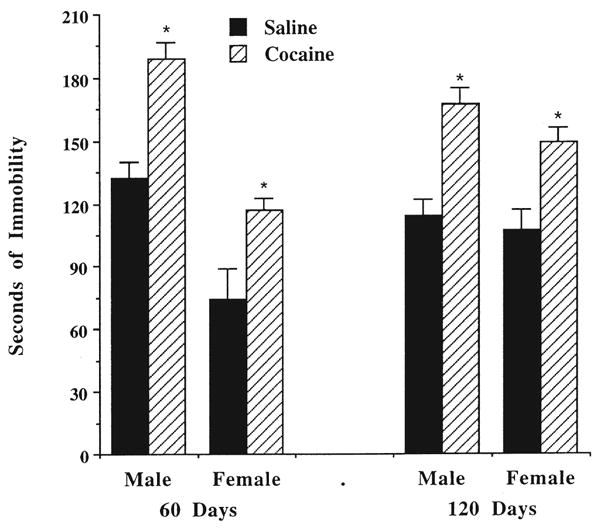
Effects of prenatal cocaine exposure on immobility in the forced-swim test. See legend to Fig. 1 for treatment conditions. Data are the mean seconds of immobility for 6–10 rats. Cocaine-exposed rats were more immobile than their age-matched, saline-treated counterparts. *Significantly different, p < 0.05, from saline-treated controls, according to Tukey's test.
3.5. Acoustic startle test
Figure 5 shows the effects of prenatal cocaine exposure on startle amplitude measures in the test for sensory gating. Initial results from an overall three-way ANOVA indicated that gender did not have a significant effect on startle amplitude, nor did any significant interactions occur between group (prenatal treatment) and age. The animals given pretreatment with cocaine and the animals tested at an older age had, in general, greater startle responses, reflected in the significant main effects of group and age for two experimental conditions: vehicle pretreatment, stimulus alone [group, F(1, 107) = 4.91, p < 0.05; age, F(1, 107) = 13.58, p < 0.0005], and PCP pretreatment, prepulse [group, F(1, 107) = 5.84, p < 0.05; age, F(1,107) = 4.89, p < 0.05]. Only age had a significant impact on startle amplitude for prepulse trials after vehicle pretreatment [age, F(1, 107) = 10.71, p < 0.005], whereas only prenatal cocaine exposure had a significant effect on responses to the acoustic stimulus alone after PCP [group, F(1,107) = 13.95, p < 0.001].
Fig. 5.
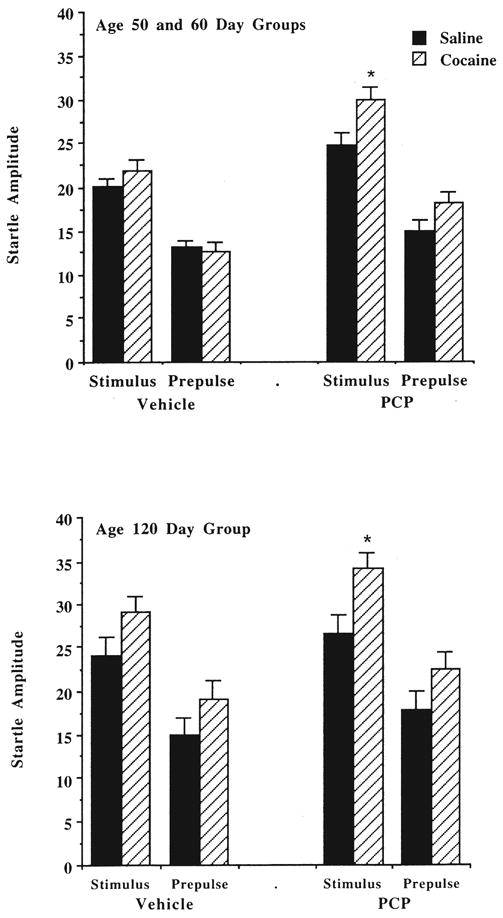
Effects of prenatal cocaine exposure on acoustic startle amplitude. See legend to Fig. 1 for treatment conditions. Data are mean (+SEM) amplitude measures in mm (from polygraph recordings), following either vehicle (saline) or PCP (2.0 mg/kg) pretreatment. *Significantly different, p < 0.05, in comparison to adjacent saline-treated group.
Because the primary goal of the startle test was to determine changes attributable to prenatal drug exposure, the data were collapsed across gender and reanalyzed for each age group by a repeated measures ANOVA. For animals tested at age 50 to 60 days, there was a significant interaction between group and startle condition [F(3, 222) = 3.72, p < 0.05], and a significant effect of the repeated measure [F(3, 222) = 105.45, p < 0.0001]. Group means for each experimental condition were then tested using one-way ANOVAs, and the results indicated that the cocaine-treated animals had a significantly higher startle response only after PCP administration, and only to the acoustic stimulus alone [F(1, 74) = 6.54, p < 0.05]. For the animals tested at 120 days, there was a significant main effect of group [F(1, 37) = 4.64, p < 0.05], and a significant effect of the repeated measure [F(3, 111) = 52.77, p < 0.0001]. As observed with the younger animals, cocaine pretreatment led to an enhanced startle response under only one experimental condition: PCP treatment and testing with the acoustic stimulus alone [F(1, 37) = 7.22, p < 0.05].
Figure 6 presents the percentage prepulse inhibition (PPI) for the vehicle and PCP test sessions. As markedly apparent from the graph, prenatal cocaine exposure did not confer an enhanced sensitivity to PCP effects on PPI. Overall, PCP did not disrupt sensory gating in any of the groups tested. However, differences in the treatment groups were observed after vehicle administration to the animals tested between 50 and 60 days of age. A one-way ANOVA on the group means indicated that the rats given exposure to cocaine had a higher PPI (or greater inhibition of the startle response), in comparison to the saline-treated subjects [F(1, 74) = 4.01, p < 0.05]. This effect was not observed in the animals tested at 120 days of age.
Fig. 6.
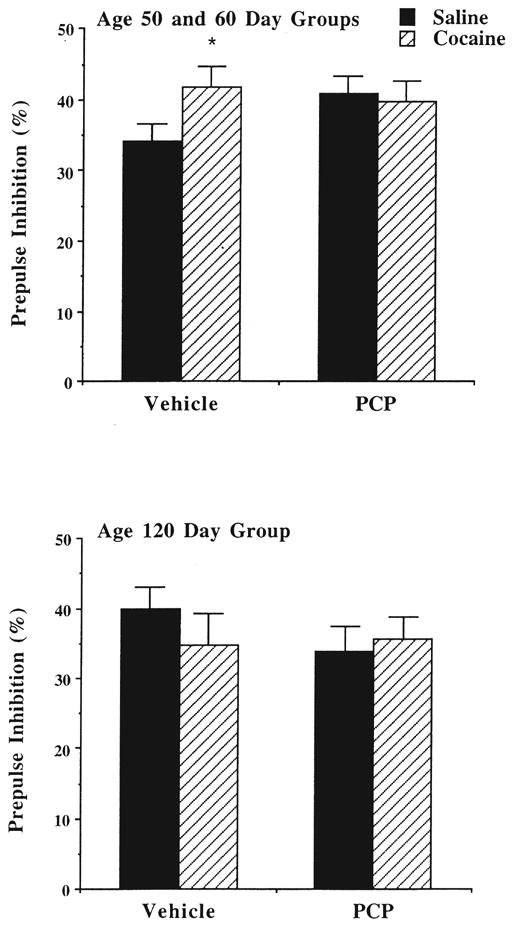
Effects of prenatal cocaine exposure on percentage prepulse inhibition. See legend to Fig. 1 for treatment conditions. Data are mean (+SEM) percentage, after either vehicle (saline) or PCP (2.0 mg/kg) pretreatment. *Significantly different, p < 0.05, in comparison to adjacent saline-treated group.
4. Discussion
Rats that were prenatally exposed to cocaine were both more immobile in the forced swim test and interacted less in the social interaction test. Thus, they appear to exhibit elements of both depression and anxiety. Replicating previous work with male, but not female, rats [22,23], no differences were seen in measures obtained in elevated plus maze, demonstrating that not all forms of anxiety were changed by prenatal cocaine treatment. The differences in immobility and social interaction were enduring because they were observed in the 120-day-old rats as well as the younger rats. However, because there were no differences in the 60-day-old groups, these results support previous findings that prenatal exposure to cocaine in rats can be associated with a developmental pattern of abnormalities, in which behavioral changes that are apparent at younger ages seem to be masked at or near puberty, only to re-emerge after puberty and through adulthood [21–23]. The findings on immobility contrast with a previous report [3] showing reduced immobility in animals prenatally exposed to cocaine and tested by swim stress. However, these investigators used a much higher dose (40 mg/kg b.i.d.) and a different strain of rats. Overall, the behavioral changes observed in the present studies might be analogous to those emotional changes that have been reported in children who have been exposed to cocaine prenatally [26,27,32].
The pattern of behavioral differences between the saline- and cocaine-exposed rats is reminiscent of that seen in rats that have been selectively bred for differences in serotonin1A (5-HT1A) receptor function [30]. The line of rat that was selectively bred to have increased sensitivity to the hypothermic effects of the selective 5-HT1A receptor agonist, 8-OH-DPAT, were more immobile in the forced-swim test and interacted less in the social interaction test [29], just like the cocaine-exposed rats (Figs. 1 and 4). Interestingly, neither the 8-OH-DPAT-selected rats [29] nor the rats prenatally exposed to cocaine (Fig. 3) exhibit differences in the elevated plus maze. This parallel suggests that an enduring alteration in serotonergic function might mediate the behavioral consequences of prenatal cocaine administration. Indeed, there is evidence for alterations in serotonergic function in rats exposed to cocaine prenatally [19,23,28]. Studies are in progress to characterize which 5-HT receptor subtypes are altered in cocaine-exposed rats.
The results from the acoustic startle test showed that the prenatal-cocaine rats were more sensitive to the enhancing effect of PCP on startle responses following the single acoustic stimulus presentation. This change appeared to be relatively enduring, because it was observed in both of the age groups. Differences in startle amplitude were not found between the saline- and cocaine-treatment groups after vehicle injections, but the animals exposed to prenatal cocaine and tested at age 50 to 60 days showed a small (but significant) tendency toward higher PPI. In contrast to previous findings [24,34], PCP did not disrupt the observed prepulse inhibition in either the cocaine or saline groups. Because only a single dose of PCP was tested, it is possible that changes in PPI might have been found at higher drug doses. Nevertheless, this dose of PCP did enhance the startle response in the rats prenatally exposed to cocaine.
Past work has provided conflicting accounts of changes in startle responses after exposure to cocaine early in development. Studies of human infants and children that were exposed to cocaine in utero have demonstrated enhanced [1] or partially attenuated [27] eyeblink responses to, respectively, glabellar tap or acoustic stimuli. Similarly, in animal models of cocaine abuse in pregnancy, some researchers have reported no significant impact of prenatal [15,16] or postnatal [6] cocaine treatment in tests of acoustic startle, whereas other workers have observed a decrease in startle amplitude after prenatal cocaine [20]. Differences in response sensitivity to air-puff stimuli have also been found in rats prenatally exposed to cocaine, particularly in adult females [21]. A number of factors could contribute to these differential results, including dose and timing of the cocaine exposure and, in the case of the animal work, strain of rat and method of drug administration [20].
In this study, the most prominent differences between the drug treatment groups emerged following testing with PCP. Dow–Edwards [6] also found no effect of early cocaine treatment on baseline startle responses but did find a greater sensitivity to the amplitude-enhancing impact of 8-OH-DPAT. Other researchers have failed to show a similar sensitization to the effects of cocaine [16] or d-amphetamine [20] after prenatal cocaine treatment, suggesting that changes in startle responses might be mediated by altered neurotransmission of glutamate and serotonin, rather than dopamine, receptors. Indeed, as indicated above, there is documented evidence for alterations in serotonergic function in rats exposed to cocaine prenatally [19,23,28]. The present findings suggest that there is a need for further investigation of possible alterations in glutamatergic function in rats prenatally exposed to cocaine.
Overall, prenatal administration of cocaine leads to selective but enduring changes in emotional behavior as reflected by reduced contacts in the social interaction test and increased immobility in the forced swim test. It has been suggested that prenatal cocaine exposure may result in a pattern of developmental abnormalities that occur over time and differ depending on sex of the subject, age, task, and level of stress the subject is under [22]. The alterations seen suggest abnormal responses to stress and stimuli such that the animal over-reacts or responds as if threatened in a non-threatening situation. This pattern suggests that in utero changes in development as a result of cocaine exposure may later appear in the form of behavioral and neuroanatomical abnormalities. Experimental studies of factors that can ameliorate these changes may provide clues to both strategies that might be used to improve the status of infants who are prenatally exposed to cocaine and the underlying molecular changes that accompany prenatal cocaine exposure. Overall, findings from this line of research could have implications for our understanding of the pathogenesis of specific psychotic disorders.
Acknowledgments
This work was partially supported by the National Institutes on Drug Abuse (R29-DA08456 to J.M.J.) and the Mental Health and Neuroscience Clinical Research Center (MH33127), Department of Psychiatry, UNC.
References
- 1.Anday EK, Cohen ME, Kelley NE, Leitner DS. Effect of in utero cocaine exposure on startle and its modification. Dev Pharmacol Ther. 1989;12:137–45. [PubMed] [Google Scholar]
- 2.Andrews N, File SE, Fernandes C, Gonzalez LE, Barnes NM. Evidence that the median raphe nucleus-dorsal hippocampal pathway mediates diazepam withdrawal-induced anxiety. Psychopharmacology. 1997;130:228–34. doi: 10.1007/s002130050233. [DOI] [PubMed] [Google Scholar]
- 3.Bilitzke PJ, Church MW. Prenatal cocaine and alcohol exposures affect rat behavior in a stress test (the Porsolt swim test) Neurotoxicol Teratol. 1992;14:359–64. doi: 10.1016/0892-0362(92)90043-a. [DOI] [PubMed] [Google Scholar]
- 4.Borsini F, Meli A. Is the forced swimming test a valid predictor of antidepressant action? Psychopharmacology. 1988;94:147–60. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 5.Delaney–Black V, Covington C, Templin T, Ager J, Martier S, Sokol R. Prenatal cocaine exposure and child behavior. Pediatrics. 1998;102:945–50. doi: 10.1542/peds.102.4.945. [DOI] [PubMed] [Google Scholar]
- 6.Dow–Edwards DL. Modification of acoustic startle reactivity by cocaine administration during the postnatal period: comparison with a specific serotonin reuptake inhibitor. Neurotoxicol Teratol. 1996;18:289–96. doi: 10.1016/s0892-0362(96)90029-x. [DOI] [PubMed] [Google Scholar]
- 7.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Meth. 1980;2:219–38. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 8.File SE. How good is social interaction as a test of anxiety? In: Simon P, Soubrie P, Widlocher D, editors. Animal Models of Psychiatric Disorders. Volume 1: Selected Models of Anxiety Depression and Psychosis. Basel, Switzerland: Karger; 1988. pp. 151–66. [Google Scholar]
- 9.File SE. Behavioral detection of anxiolytic action. In: Elliott JM, Heal DJ, Marsden CA, editors. Experimental Approaches to Anxiety and Depression. London, UK: John Wiley; 1992. pp. 25–44. [Google Scholar]
- 10.File SE. The social interaction test of anxiety. Neurosci Protocols. 1993;10(1):1–7. [Google Scholar]
- 11.File SE. Animal measures of anxiety. In: Crawley J, Gerfen C, McKay R, Rogawski M, Sibley D, Skolnick P, editors. Current Protocols in Neuroscience. Chapter 8.3. New York: John Wiley; 1998. pp. 1–5. [Google Scholar]
- 12.File SE, Gonzalez LE, Andrews N. Comparative study of pre and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J Neurosci. 1996;16:4810–5. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.File SE, Hyde JRG. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.File SE, Hyde JRG. A test of anxiety that distinguishes between the actions of benzodiazepines and those of other minor tranquillisers and of stimulants. Pharmacol Biochem Behav. 1979;11:65–9. doi: 10.1016/0091-3057(79)90298-3. [DOI] [PubMed] [Google Scholar]
- 15.Foss JA, Riley EP. Elicitation and modification of the acoustic startle reflex in animals prenatally exposed to cocaine. Neurotoxicol Teratol. 1991;13:541–6. doi: 10.1016/0892-0362(91)90063-3. [DOI] [PubMed] [Google Scholar]
- 16.Foss JA, Riley EP. Failure of acute cocaine administration to differentially affect acoustic startle and activity in rats prenatally exposed to cocaine. Neurotoxicol Teratol. 1991;13:547–51. doi: 10.1016/0892-0362(91)90064-4. [DOI] [PubMed] [Google Scholar]
- 17.Geyer MA, Swerdlow NR, Mansbach RS, Braff DL. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull. 1990;25:485–98. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez LE, Andrews N, File SE. 5-HT1A and benzodiazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test but not in the elevated plus-maze. Brain Res. 1996;732:145–53. doi: 10.1016/0006-8993(96)00517-3. [DOI] [PubMed] [Google Scholar]
- 19.Henderson MG, McMillen BA. Changes in dopamine serotonin and their metabolites in discrete brain areas of rat offspring after in utero exposure to cocaine or related drugs. Teratology. 1993;48:421–30. doi: 10.1002/tera.1420480506. [DOI] [PubMed] [Google Scholar]
- 20.Hughes HE, Donohue LM, Dow–Edwards DL. Prenatal cocaine exposure affects the acoustic startle response in adult rat. Behav Brain Res. 1996;75:83–90. doi: 10.1016/0166-4328(96)00175-1. [DOI] [PubMed] [Google Scholar]
- 21.Johns JM, Knapp DJ, Noonan LR. Prenatal exposure to cocaine treatment alters ultrasonic vocalizations to air puffs in adult female rat offspring. Soc Neurosci Abstr. 1994;20:250. [Google Scholar]
- 22.Johns JM, Noonan LR. Prenatal cocaine exposure affects social behavior in Sprague-Dawley rats. Neurotoxicol Teratol. 1995;17:569–76. doi: 10.1016/0892-0362(95)00017-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johns JM, Noonan LR, Simmerman LI, McMillen BA, Means LW, Walker C, Lubin DA, Meter KE, Nelson CJ, Pedersen CA, Mason GA, Lauder JM. Chronic cocaine treatment alters social/aggressive behavior in Sprague-Dawley rat dams and in their prenatally exposed offspring. Ann NY Acad Sci. 1998;846:399–404. [PMC free article] [PubMed] [Google Scholar]
- 24.Mansbach RS, Geyer MA. Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neuropsychopharmacology. 1989;2:299–308. doi: 10.1016/0893-133x(89)90035-3. [DOI] [PubMed] [Google Scholar]
- 25.Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94:507–14. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- 26.Mayes LC, Bornstein MH, Chawarska K, Granger RH. Information processing and developmental assessments in 3-month-old infants exposed prenatally to cocaine. Pediatrics. 1995;95:539–45. [PubMed] [Google Scholar]
- 27.Mayes LC, Grillon C, Granger R, Schottenfeld R. Regulation of arousal and attention in preschool children exposed to cocaine prenatally. Ann NY Acad Sci. 1998;846:126–43. doi: 10.1111/j.1749-6632.1998.tb09731.x. [DOI] [PubMed] [Google Scholar]
- 28.McReynolds AM, Meyer JS. Effects of prenatal cocaine exposure on serotonin and norepinephrine transporter density in the rat brain. Ann NY Acad Sci. 1998;846:412–4. [PubMed] [Google Scholar]
- 29.Overstreet DH, Daws LC, Schiller GD, Orbach J, Janowsky DS. Cholinergic/serotonergic interactions in hypothermia: implications for rat models of depression. Pharmacol Biochem Behav. 1998;59:777–85. doi: 10.1016/s0091-3057(97)00514-5. [DOI] [PubMed] [Google Scholar]
- 30.Overstreet DH, Rezvani AH, Knapp DJ, Crews FT, Janowsky DS. Further selection of rat lines differing in 5-HT1A receptor sensitivity: behavioral and functional correlates. Psychiatr Genet. 1996;6:107–17. doi: 10.1097/00041444-199623000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Pellow S, Chopin P, File SE, Briley M. Validation of open-closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Meth. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 32.Roberts J. Cocaine blamed for infants' emotional problems. Br Med J. 1996;312:1379. doi: 10.1136/bmj.312.7043.1379. [DOI] [PubMed] [Google Scholar]
- 33.Ronnekleiv OK, Fang Y, Choi W, Chai L. Changes in the midbrain-rostral forebrain dopamine circuitry in the cocaine-exposed primate fetal brain. Ann NY Acad Sci. 1991;846:165–81. [PubMed] [Google Scholar]
- 34.Swerdlow NR, Bakshi V, Geyer MA. Seroquel restores sensorimotor gating in phencyclidine-treated rats. J Pharmacol Exp Ther. 1996;279:1290–9. [PubMed] [Google Scholar]
- 35.Swerdlow NR, Braff DL, Geyer MA. GABAergic projection from nucleus accumbens to ventral pallidum mediates dopamine-induced sensorimotor gating deficits of acoustic startle in rats. Brain Res. 1990;532:146–50. doi: 10.1016/0006-8993(90)91754-5. [DOI] [PubMed] [Google Scholar]
- 36.Swerdlow NR, Caine SB, Geyer MA. Regionally selective effects of intracerebral dopamine infusion on sensorimotor gating of the startle reflex in rats. Psychopharmacology. 1992;108:189–95. doi: 10.1007/BF02245306. [DOI] [PubMed] [Google Scholar]
- 37.Wood RD, Bannoura MD, Johanson IB. Prenatal cocaine exposure: effects on play behavior in the juvenile rat. Neurotoxicol Teratol. 1994;16:139–44. doi: 10.1016/0892-0362(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 38.Wood RD, Molina VM, Wagner JM, Spear LP. Play behavior and stress responsivity in periadolescent rats exposed prenatally to cocaine. Pharmacol Biochem Behav. 1995;52:367–74. doi: 10.1016/0091-3057(95)00120-l. [DOI] [PubMed] [Google Scholar]
- 39.Wood RD, Spear LP. Prenatal cocaine alters social competition of infant and adult rats. Behav Neurosci. 1998;112:419–31. doi: 10.1037//0735-7044.112.2.419. [DOI] [PubMed] [Google Scholar]


