Abstract
Antibody-binding fragments (Fab) are generated from whole antibodies by treatment with papain and can be separated from the Fc component using Protein-A affinity chromatography. Commercial kits are available, which facilitate the production and purification of Fab fragments; however, the manufacturer fails to report that this method is inefficient for antibodies with VH3 domains as a result of the intrinsic variable region affinity for Protein-A. A commercially available, modified Protein-A resin (MabSelect SuRe) has been engineered for greater stability. Here, we report that an additional consequence of the modified resin is the ability to purify VH3 family Fab fragments, which cannot be separated effectively from other components of the papain digest by traditional Protein-A resin. This improvement of a commonly used procedure is of significance, as increasingly, therapeutic antibodies are being derived from human origin, where VH3 is the most abundantly used variable region family.
Keywords: antibodies, immunoglobulin, purification, Staphylococcal, variable region
INTRODUCTION
Treatment of whole antibody molecules with the proteolytic enzyme papain (EC 3.4.22.2) digests the antibody into two identical antibody-binding fragments (Fab) and one Fc fragment. The Fab fragment retains the binding specificity of the parent antibody, and its separation from the Fc fragment is often desired for analytical, diagnostic, or therapeutic purposes. For analytical purposes, monovalent Fab fragments simplify the study of binding properties compared with the divalent whole antibody,1 and Fab fragments are more easily crystallized than whole antibodies for structure determination.2 The smaller Fab fragment (∼50 kDa compared with the 150-kDa parent antibody) has greater penetration into tissues and a shorter half-life, making it a useful in vivo reagent for diagnostic imaging or delivery of cytotoxic conjugates.3
Protein-A from Staphylococcus aureus is commonly used to purify antibodies as a result of its strong affinity toward the Fc fragment of all human and two of three mouse isotypes. Protein-A contains five highly similar domains (from the N-terminus: E, D, A, B, and C), each with specificity for Fc.4 It is thus used to facilitate the removal of Fc after papain digestion5 and is used in commercially available kits for Fab preparation. However, a less commonly recognized feature of Protein-A is the binding specificity of its D- and E-domains toward the variable region of antibodies from the VH3 subfamily.6,7 This feature makes separation of Fab and Fc by Protein-A impractical for VH3 family antibodies; this limitation is not reported in the product literature for Fab preparation kits.
With the emergence of transgenic mice and phage display technologies, therapeutic antibodies are increasingly derived from human sequences,8 where VH3 is an abundantly used VH domain.9,10 Human phage display libraries contain mostly VH3,11 and some libraries have an intentional bias for VH3-containing antibodies, as a result of functional prescreening for Protein-A binding,12 including commercially available Tomlinson I & J single-chain Fv (scFv) libraries composed entirely of VH3 sequences (Geneservice, Cambridge, UK). This highlights the need for a robust purification process for VH3 family Fab fragments.
A recombinant synthetic version of Protein-A consisting of repeated, modified B-domains has been expressed and is shown to lack VH3 domain-binding specificity.7,13 This synthetic Protein-A has been modified to make the new “Z-domain” resistant to harsh alkaline conditions and is thus ideal for use in large-scale biopharmaceutical purifications, as the resin can withstand rigorous regeneration procedures.14 The lack of variable region binding also allows elution of antibodies at a milder and more consistent pH range, protecting the product quality and allowing for a standardized purification process across a range of antibodies.15 This Z-domain resin is available commercially through GE Healthcare Australia (Rydalmere, NSW, Australia) under the tradename MabSelect SuRe. We have shown that MabSelect SuRe can be used to separate Fab reproducibly after papain digestion of therapeutic antibodies, including those from VH3 families.
MATERIALS AND METHODS
Commercial therapeutic antibodies Herceptin (trastuzumab), MabThera (rituximab), and Avastin (bevacizumab) were obtained from Roche Products (Dee Why, NSW, Australia), and Campath (alemtuzumab) was obtained from Genzyme Australia (North Ryde, NSW, Australia). These antibodies contain V-regions of subfamilies VH3, VH1, VH7, and VH4, respectively.16 An in-houseVH3 (IH3) fully human antibody, isolated by phage display and reformatted to IgG1, was expressed in CHO cells and purified on a 1-mL Protein-A HiTrap column (GE Healthcare Australia). The DNA sequence of the IH3 heavy-chain variable region was entered into the IMGT/V-Quest bioinformatics tool,17 which revealed IH3 to be of the VH3 subfamily.
The IH3 antibody (1 mg) and the commercial antibodies (0.25 mg each) were digested, using the Pierce Fab preparation kit and the Pierce Fab micro preparation kit (ThermoFisher Scientific, Scoresby, VIC, Australia), respectively. These kits differ only in the quantity of antibody processed. The antibodies were buffer-exchanged into the kit-supplied digestion buffer containing cysteine, using desalting columns, and then added to 0.8 mL-capped spin columns containing immobilized papain. After 6 h incubation with mixing at 37°C, the digest was collected by centrifugation, divided in half, and applied to the kit-provided Protein-A columns or to similar columns prepared using MabSelect SuRe resin (GE Healthcare Australia), equilibrated in PBS. After 10 min incubation with mixing at room temperature, the flowthrough fraction was collected by centrifugation. Bound fragments were eluted using the kit-provided elution buffer at pH 2.8 and then neutralized using 1/10 vol 1 M phosphate, pH 9. Fractions collected during the papain digest and Fab purification were analyzed under reducing conditions by SDS-PAGE, using 4–12% Bis-Tris (for IH3) or 12% Bis-Tris (for the commercial antibodies) NuPAGE gels (Invitrogen, Carlsbad CA, USA) run in MOPS buffer. The PAGE gels were stained using Coomassie blue.
RESULTS
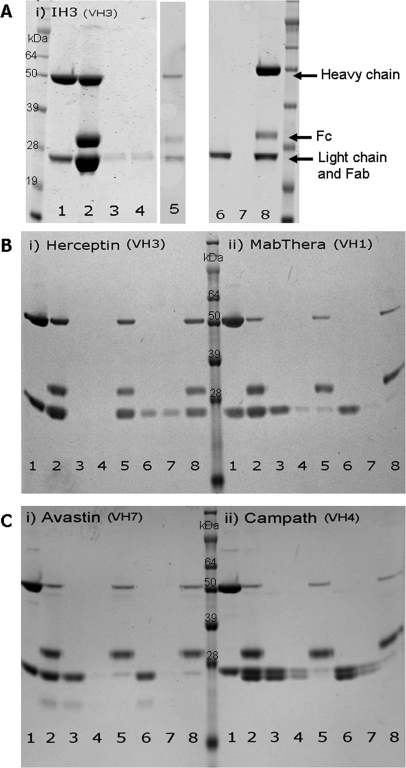
Early attempts in our laboratory to purify Fab fragments after papain digestion of IH3 with a commercial kit were highly inefficient, and <0.04 mg Fab was recovered from 1 mg digested antibody. As the antibody is of the VH3 subfamily, the Fab and the Fc fragments bind to the kit-provided Protein-A, and only minimal quantities of Fab remain in the flowthrough fraction. Comparatively, purification of the IH3 Fab using MabSelect SuRe (GE Healthcare Australia) enhanced the yield of the purified Fab fragment in the flowthrough fraction, and 0.2 mg was recovered from 1 mg digested antibody (Fig. 1A).
FIGURE 1.
Fab purification after papain digest of antibodies using Protein-A or MabSelect SuRe resins. SDS-PAGE, under reducing conditions, was performed on fractions collected during digestion and Fab purification of (A) IH3, (B,i) Herceptin and (B,ii) MabThera, and (C,i) Avastin and (C,ii) Campath. Each gel photo contains SeeBlue Plus2 protein ladder (Invitrogen), and the lanes contain whole antibody (1), papain-digested antibody (2), Protein-A flowthrough (3), Protein-A wash (4), Protein-A eluate (5), MabSelect SuRe flowthrough (6), MabSelect SuRe wash (7), and MabSelect SuRe eluate (8). In each gel, 12 μL of each fraction was loaded, except in gels B and C, where only 2 μL of the whole antibody and digested antibody was loaded. Undigested antibody under reducing conditions produces bands of ∼50 kDa (heavy chain) and 24 kDa (light chain). Papain digest generates bands at ∼30 kDa (Fc fragment) and 24 kDa (reduced Fab fragment and light chain originating from undigested antibody origin). MWs on the heavy chain are estimates only as a result of glycosylation on the Fc region.
Even more marked results were obtained with a papain digest of a commercial VH3 antibody, Herceptin (Roche Products), which also showed coelution of Fab and Fc on the kit-provided Protein-A but separation on MabSelect SuRe (Fig. 1B). For all other non-VH3 antibody subfamilies tested (i.e., VH1, VH7, and VH4), separation of Fab and Fc could be achieved effectively by Protein-A or MabSelect SuRe resins, as these families do not show variable region affinity toward Protein-A (Fig. 1B and C).
DISCUSSION
The results presented here demonstrate that MabSelect SuRe (GE Healthcare Australia) is a more versatile alternative to Protein-A for the purification of Fab fragments after papain digestion of antibodies and may help researchers to troubleshoot their Fab preparations, as information regarding VH3 binding is notably absent from product information. Unlike the Protein-A included in commercial Fab preparation kits, MabSelect SuRe can be used to separate Fc from Fab of any V-region family, as the intrinsic ability of Protein-A to bind VH3 domains has been removed during the engineering of this affinity resin. The replacement of Protein-A with MabSelect SuRe is a significant improvement to the procedure for Fab purification, as many therapeutic mAb are of human origin, where VH3 domains are used in high abundance. This modification also provides the inherent advantages of using MAbSelect SuRe, including milder pH elution conditions for the Fc region and greater resistance to harsh regeneration procedures if the user wishes to scale up the procedure and re-use the resin.
ACKNOWLEDGMENTS
T.A.S. is a member of the Cooperative Research Centre for Biomarker Translation and receives funding from a Leukaemia Foundation Ph.D. Scholarship. The IH3 antibody was created from a scFv fragment isolated from a phage display library,11 kindly provided by Prof. James Marks (University of California, San Francisco, CA, USA).
Footnotes
The authors declare no competing interests.
REFERENCES
- 1. Mattes MJ. Binding parameters of antibodies reacting with multivalent antigens: functional affinity or pseudo-affinity. J Immunol Methods 1997;202:97–101 [DOI] [PubMed] [Google Scholar]
- 2. Kovari LC, Momany C, Rossmann MG. The use of antibody fragments for crystallization and structure determinations. Structure 1995;3:1291–1293 [DOI] [PubMed] [Google Scholar]
- 3. Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol 2005;23:1126–1136 [DOI] [PubMed] [Google Scholar]
- 4. Ljungberg UK, Jansson B, Niss U, Nilsson R, Sandberg BE, Nilsson B. The interaction between different domains of Staphylococcal Protein A and human polyclonal IgG, IgA, IgM and F(ab′)2: separation of affinity from specificity. Mol Immunol 1993;30:1279–1285 [DOI] [PubMed] [Google Scholar]
- 5. Zhao Y, Gutshall L, Jiang H, et al. Two routes for production and purification of Fab fragments in biopharmaceutical discovery research: papain digestion of mAb and transient expression in mammalian cells. Protein Expr Purif 2009;67:182–189 [DOI] [PubMed] [Google Scholar]
- 6. Hillson JL, Karr NS, Oppliger IR, Mannik M, Sasso EH. The structural basis of germline-encoded VH3 immunoglobulin binding to Staphylococcal Protein A. J Exp Med 1993;178:331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Starovasnik MA, O'Connell MP, Fairbrother WJ, Kelley RF. Antibody variable region binding by Staphylococcal Protein A: thermodynamic analysis and location of the Fv binding site on E-domain. Protein Sci 1999;8:1423–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 2010;9:767–774 [DOI] [PubMed] [Google Scholar]
- 9. Cook GP, Tomlinson IM. The human immunoglobulin VH repertoire. Immunol Today 1995;16:237–242 [DOI] [PubMed] [Google Scholar]
- 10. Davidkova G, Pettersson S, Holmberg D, Lundkvist I. Selective usage of VH genes in adult human B lymphocyte repertoires. Scand J Immunol 1997;45:62–73 [DOI] [PubMed] [Google Scholar]
- 11. Sheets MD, Amersdorfer P, Finnern R, et al. Efficient construction of a large nonimmune phage antibody library: the production of high-affinity human single-chain antibodies to protein antigens. Proc Natl Acad Sci USA 1998;95:6157–6162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jespers L, Schon O, Famm K, Winter G. Aggregation-resistant domain antibodies selected on phage by heat denaturation. Nat Biotechnol 2004;22:1161–1165 [DOI] [PubMed] [Google Scholar]
- 13. Nilsson B, Moks T, Jansson B, et al. A synthetic IgG-binding domain based on Staphylococcal Protein A. Protein Eng 1987;1:107–113 [DOI] [PubMed] [Google Scholar]
- 14. Linhult M, Gulich S, Graslund T, et al. Improving the tolerance of a Protein A analogue to repeated alkaline exposures using a bypass mutagenesis approach. Proteins 2004;55:407–416 [DOI] [PubMed] [Google Scholar]
- 15. Ghose S, Allen M, Hubbard B, Brooks C, Cramer SM. Antibody variable region interactions with Protein A: implications for the development of generic purification processes. Biotechnol Bioeng 2005;92:665–673 [DOI] [PubMed] [Google Scholar]
- 16. Magdelaine-Beuzelin C, Kaas Q, Wehbi V, et al. Structure-function relationships of the variable domains of monoclonal antibodies approved for cancer treatment. Crit Rev Oncol Hematol 2007;64:210–225 [DOI] [PubMed] [Google Scholar]
- 17. Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res 2008;36:W503–W508 [DOI] [PMC free article] [PubMed] [Google Scholar]