Summary
GABAergic inhibitory interneurons are embedded in almost all central neuronal networks, where they act to influence cell excitability, spike timing, synchrony, and oscillatory activity, i.e. almost every physiologically relevant process in the mammalian central nervous system [1][2]. Consequently, presynaptic plasticity of the synaptic input onto, or the outputs from, a single inhibitory interneuron can have major ramifications for the activity of the often thousands of downstream target neurons. Here we discuss several recently described forms of presynaptic long-term potentiation (LTP) and long-term depression (LTD) occurring at synapses either made onto inhibitory interneurons, or at inhibitory synapses onto downstream targets in a number of central structures. As we will illustrate, the induction mechanisms underlying these disparate examples of presynaptic plasticity share few common features, however, their expression mechanisms converge on the presynaptic release machinery. We hypothesize that these varied forms of presynaptic plasticity can operate in a manner fundamentally distinct from most postsynaptic “point to point” forms of plasticity, to achieve powerful modification of the integration and output of large scale networks.
Synaptic plasticity of excitatory synaptic transmission onto and between glutamatergic principal cells is arguably one of the most heavily studied topics in the neurosciences. Much has been made of cortical NMDA receptor-dependent LTP as a mechanism underlying learning and memory, and the intense focus this form of plasticity has received has taught us a great deal about the role of glutamate receptors in the central nervous system [3]. The induction and expression of this form of plasticity are both generally accepted to be postsynaptic and synapse specific, that is, the locus of change remains largely within the appropriately activated individual synapse. It is easy to imagine how this “point to point” plasticity would strengthen individual connections between coincidently-active cells. However, if one considers that a single cortical pyramidal cell receives thousands of excitatory synapses onto as many postsynaptic spines and that each input delivers a relatively small voltage change, it is difficult to imagine how the potentiation of a single synapse could significantly shape or alter the output of the individual neuron or the network in which it is embedded. In contrast, presynaptic forms of plasticity have the potential to greatly influence all of the transmitter release sites within a given axon, such that changes in the output of one cell could modify the activity of thousands of its downstream targets [4]. A recent surge in the literature has documented a number of mechanistically distinct forms of presynaptic plasticity that regulate either the input onto, or the output of local circuit GABAergic inhibitory interneurons. Here we describe the cellular mechanisms identified in presynaptic plasticity involving GABAergic interneurons that are especially well-suited to control larger ensembles of target neurons.
I. Excitatory synapses onto GABAergic interneurons
i. Mossy Fiber-Stratum Lucidum Interneuron Long Term Potentiation
The best characterized form of presynaptic long term potentiation resides at the mossy fiber (MF) synapse between the principal neurons of the dentate gyrus (granule cells) and CA3 pyramidal cells of the hippocampus proper [5]. At this synapse, high frequency stimulation (HFS) of the presynaptic mossy fiber axon triggers an enduring elevation in presynaptic release probability (Pr) and a potentiation of the excitatory synaptic potential. This increase in release probability relies on presynaptic adenylyl cyclase formation, and a cAMP-PKA dependent alteration of the active zone protein RIM1α's function [6] [7].
The architecture of the presynaptic MF synapse is unique. MF synapses onto CA3 pyramidal cells are large (~5–10μm in diameter) with multiple independent release sites with low initial Pr [8] [9]. Numerous fine filopodia radiate from these MF boutons and their synaptic terminations target GABAergic interneurons within the hilus and CA3 stratum lucidum [10]. Filopodial synapses are small diameter (~1μm) and typically have a single release site with a very high Pr between 0.5–0.7 [11] [12]. At naïve MF-interneuron synapses the same HFS that triggers LTP at the MF-pyramidal cell synapse results in presynaptic long lasting depression of transmission. The mechanism underlying this LTD has been reviewed previously (for reviews see [13] [14]) and will not be described in depth here. To summarize, HFS of MF-interneuron synapses triggers glutamate release in concentrations sufficient to activate presynaptically-expressed mGluR7b, which triggers PKC formation and a down-regulation of Ca2+ influx through P/Q voltage-gated Ca2+ channels to reduce Pr [15,16] (Figure 1A). mGluR7b acts as a metaplastic switch such that on binding of agonist, mGluR7b is rapidly internalized and delivery of subsequent HFS triggers a de-depression or LTP of synaptic transmission. Thus, the presence or absence of mGluR7b on the presynaptic surface dictates whether the mossy fiber synapse onto interneurons weakens or strengthens in response to HFS.
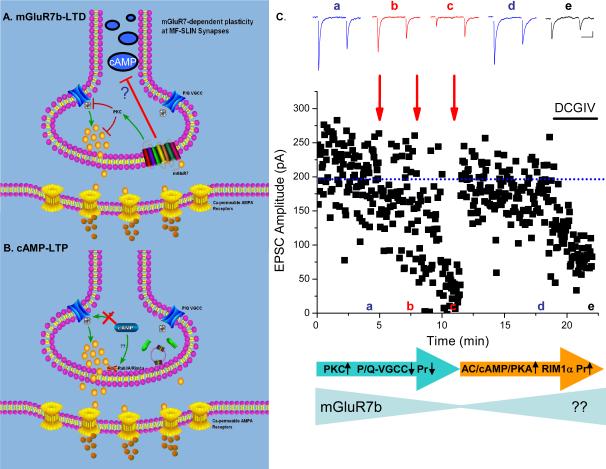
Figure 1. State-dependent plasticity at hippocampal mossy fiber-interneuron synapses.
A. mGluR7b LTD: At naïve synapses with mGluR7b surface expressed on the presynaptic terminal, a high frequency stimulus induction paradigm (typically 100Hz for 1 sec) liberates glutamate in concentrations sufficient to activate presynaptic mGluR7b. mGluR7b, via a PKC-dependent cascade, triggers a reduction in the synaptic Ca transient arising through P/Q voltage-gated calcium channels. mGluR7b activation may also prevent or downregulate cAMP formation. The reduction in P/Q channel function precipitates a reduction in the transmitter release probability and long lasting depression of synaptic transmission. B. cAMP-LTP: Following HFS-induced LTD, mGluR7b is internalized. A subsequent round of high frequency stimuli now triggers activation of the adenylyl cyclase/cAMP/PKA pathway, that through the presynaptic scaffold protein RIM1α induces an increase in transmitter release probability and long term potentiation of synaptic transmission. C. Repeated rounds of high frequency stimulation of MF synapses onto stratum lucidum interneurons first triggers LTD of synaptic transmission. After two rounds of HFS, LTD is saturated and a subsequent round of the same HFS triggers dedepression/LTP of synaptic transmission. Traces above show individual records of synaptic events triggered by paired pulses of stimuli. Note the reduction in the paired pulse ratio upon LTD and the restoration of the paired pulse ratio on de-depression. Schematic below follows the same model as shown in panels A and B to illustrate that LTD formation requires PKC dependent down regulation of P/Q channel function. Internalization of mGluR7b proceeds as LTD is saturated. De-depression requires activation of adenylyl cyclase (AC)/cAMP/PKA dependent cascades which require RIM1α. Whether mGluR7b is trafficked back to the presynaptic surface is an open question. Adapted from and used with permission [17].
Although possessing a presynaptic locus of expression, the de-depression of MF-interneuron synaptic transmission is not simply the molecular reversal of the LTD triggered at naïve synapses, i.e. a HFS-induced increase in the P/Q Ca2+ transient and restoration of the Pr (Figure 1B). Two-photon Ca2+ imaging of the presynaptic filopodial terminals revealed that Ca2+ transients remained unchanged after HFS-induced LTP, consistent with electrophysiological experiments showing a lack of P/Q Ca2+ channel involvement in establishing this LTP [17]. As described above, HFS-induced LTP at MF-pyramidal cell synapses involves adenylyl cyclase-cAMP and PKA formation. At naïve MF-interneuron synapses however, basal synaptic transmission is insensitive to adenylyl cyclase activation by forskolin [18] [17]. Following agonist activation and internalization of mGluR7b, forskolin triggers robust synaptic potentiation that is not accompanied by changes in the presynaptic Ca2+ transient. This potentiation is prevented by inhibitors of both adenylyl cyclase and PKA formation and shares all of the features of LTP at the MF-pyramidal cell synapse. Consistent with this mechanism, de-potentiation/LTP at MF-interneuron synapses is absent in the RIM1α knockout mouse. This suggests that surface expressed mGluR7b acts either to downregulate cAMP formation, or alternatively, to sequester putative PKA-targets on RIM1α (and/or its partners) responsible for LTP. Consistent with this latter hypothesis, mGluR7b was observed to exist in a macromolecular complex with RIM1α that could be coimmunoprecipitated only when mGluR7b was expressed on the presynaptic surface [17]. Following mGluR7b internalization, the efficiency of RIM1α coprecipitation is diminished. This loss of interaction presumably frees the RIM1α substrate, priming MF-interneuron terminals to become LTP competent. An aspect of these studies worthy of emphasis is the observation that although both PKC- and PKA-dependent cascades are available in MF-interneuron synaptic terminals, they appear to be operational only under certain conditions (i.e. the presence or absence of surface mGluR7b).
What function might such a complex mechanism of differentially targeted, state-dependent, presynaptic plasticity provide the mossy fiber system? Under naïve conditions, high frequency firing of granule cells would strengthen transmission at MF-pyramidal cell synapses while weakening transmission at neighboring MF-interneuron synapses. Strengthening MF-pyramidal cell transmission, in the face of reduced feedforward inhibition, would enhance drive within the auto-associational CA3 network. One can imagine however, that repeated high frequency activity in this pathway would saturate both the LTP and the LTD processes, resulting in a synaptic stalemate. We would suggest that the internalization of mGluR7b and the consequent emergence of the PKA-dependent dedepression of MF-interneuron transmission allows this temporal mismatch in synaptic strength to be countered, ensuring restoration of the feedforward inhibitory control of the CA3 network and a consequent attenuation of excitatory drive within the associative network (Figure 1C). Given that mossy fibers target approximately an order of magnitude more interneurons than pyramidal cells this underscores that a presynaptic distribution of synaptic plasticity will offer profound and rapid state-dependent control of feedforward inhibition within the networks.
ii. TRPV1 channel-dependent LTD
The first study directly linking TRPV1 channels with long-term depression was described at excitatory synapses in the hippocampus [19]. Whereas HFS of CA3 pyramidal cell axons elicits LTP at synapses on CA1 pyramidal cells, stimulation of the same afferents triggers LTD at synapses on neighboring interneurons (Figure 2). Group I metabotropic glutamate receptors are activated by released glutamate, and in turn generate arachidonic acid in the interneuron; this intermediate is rapidly converted to 12-(S)-HPETE by 12-lipoxygenase. 12-(S)-HPETE is a membrane-permeable lipid with a short half-life, and evidence suggests that it travels retrogradely across the synapse, where it activates TRPV1 channels on presynaptic glutamatergic nerve terminals. TRPV1, a member of a large family of non-selective cation channels, was first described in the peripheral nervous system as a multimodally-gated channel activated both by heat and by capsaicin, the spicy component in chili peppers [20]. Its role in the CNS is as yet poorly defined [21]. At excitatory synapses on hippocampal interneurons, capsaicin or the endogenous agonist, 12-(S)-HPETE, depressed synapses without high-frequency afferent stimulation, and antagonists of TRPV1 blocked synaptically-induced LTD. Trpv1 knockout mice lacked LTD and excitatory synapses on interneurons from these animals were not depressed in response to either capsaicin or 12-(S)-HPETE.
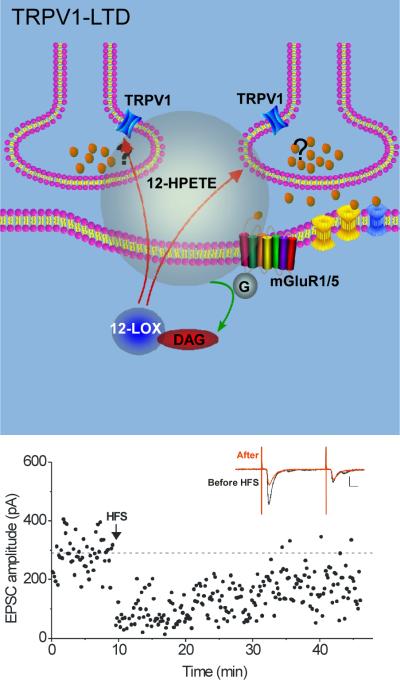
Figure 2. TRPV1-LTD at excitatory synapses on CA1 interneurons requires retrograde signaling.
Top panel, High frequency stimulus (100Hz for 1 sec) liberates glutamate that activates group I mGluRs. These are coupled to phospholipase C, which generates diacylglycerol (DAG). Cleavage of DAG produces arachidonic acid, a substrate for 12-lipoxygenase (12-LOX). Activation of 12-LOX produces 12-(S)-HPETE, a short-lived lipophilic molecule thought to pass through the postsynaptic plasma membrane. 12-(S)-HPETE activates TRPV1 channels present on the presynaptic terminals of CA3 pyramidal cells, and their activation elicits long-term depression of glutamate release. Bottom panel, example experiment; at the arrow, HFS was delivered, triggering TRPV1-LTD. Inset shows EPSCs before (black) and after (red) HFS, and the large accompanying decrease in paired-pulse facilitation characteristic of presynaptic LTD. Adapted from [19].
TRPV1 channels modulate transmitter release in the spinal cord, substantia nigra, hypothalamus and other regions [21]. In most cases, glutamate release is increased; however, TRPV1 channel activation at hippocampal synapses on interneurons results in long-term depression of glutamate release. We speculate that Ca2+ entry through the TRPV1 channel activates a Ca2+-dependent second messenger cascade responsible for the LTD. The involvement of presynaptic Ca2+ channels or presynaptic proteins is as yet unknown. At least a proportion of the same synapses have also been reported to exhibit classic NMDAR-dependent LTP. This mechanism would allow the synapses to recover their original potency, although by utilizing distinct cellular mechanisms the potentiation is not a simple reversal of the depression [22].
Why is robust LTP instead of TRPV1-LTD observed at synapses on pyramidal cells if 12-(S)-HPETE depresses glutamate release from the CA3 glutamatergic nerve terminals following HFS? In another example of target-specific plasticity, no evidence was found that TRPV1 channels on these terminals cause significant synaptic depression; capsaicin or 12-(S)-HPETE at concentrations that potently depressed synapses on interneurons had no effect on those on pyramidal cells, even though the pyramidal cells express TRPV1 channels [19]. As with the mossy fiber synapses, it is clear that the presynaptic synapses on interneurons are different. Either TRPV1 channels are segregated to presynaptic terminals innervating GABAergic interneurons, or the as yet undefined signaling cascade downstream from TRPV1 channel activation is operational only in these nerve terminals. In the initial report of TRPV1-LTD it was observed that after HFS that triggered LTD, neighboring unstimulated synapses on the same interneuron were also depressed [23]. Although a more direct test of this idea has not yet been made, it appears that 12-(S)-HPETE may travel along the interneuron dendrite or through the extracellular volume to depress nearby glutamatergic synapses.
It is intriguing that an unidentified TRP channel is also implicated in HFS-induced LTP at nearby synapses between CA1 pyramidal cells and oriens-alveus interneurons, although this channel may be located on the postsynaptic neuron [24]. mGluR1 and mGluR5 appear to play a role in raising intracellular Ca2+ in the O-A interneuron, and subsequently a chain of postsynaptic kinases are needed to trigger the LTD. Potentiation of the same synapses, perhaps utilizing the same underlying mechanisms, has also been reported using a different stimulus protocol. Activation of Ca2+-permeable AMPARs triggers LTP when the neuron is sufficiently hyperpolarized, presumably to provide substantial driving force for Ca2+, as the potentiation was blocked when the neuron was depolarized [25,26]. The potentiation of the synapse, however, appears to result from increased presynaptic glutamate release, as with both stimulus protocols the coefficient of variance and paired pulse ratio change after LTP induction [27] [24] as well as other changes characteristic of presynaptically maintained LTP [26]; the required retrograde messenger remains to be identified.
II. Plasticity of GABAergic synapses
As mentioned above, plasticity of excitatory synapses on GABAergic neurons has the potential to influence large groups of principle neurons innervated by a single GABAergic cell, by ratcheting up or down the excitability of the interneuron and the consequent likelihood of GABA release at the many target cells inhibited by these neurons. In contrast, the modulation of small groups of GABAergic synapses may instead provide local control of a region of the postsynaptic cell dendrite, for example, rather than more global control of clusters of postsynaptic neurons. As with excitatory synapses, both pre- and postsynaptic modifications can change the strength of GABAergic synapses. Here we will focus on two examples in which retrograde signaling triggers long-term changes in presynaptic transmitter release.
i. Endocannabinoid LTD
The best characterized form of GABAergic synapse plasticity is endocannabinoid-mediated LTD (eCB-LTD), first observed in the basolateral amygdala and hippocampus [28] [29], but now widely reported at excitatory and inhibitory synapses throughout the nervous system [30]. Like TRPV1-LTD described above, eCB-LTD can be activated by postsynaptic mGluR1/5 receptors, requires a retrograde lipid signal, and is maintained by a persistent decrease in presynaptic glutamate release (Figure 3a). Endocannabinoids (anandamide or 2-arachidonylglycerol) can be produced in the postsynaptic cell following glutamate released onto mGluR1/5 receptors, or can instead be generated by a poorly understood mechanism following a simple rise in intracellular Ca2+. Endocannabinoids are highly lipophilic compounds that may move passively from the postsynaptic cell, but more likely are transported out of the cell where they act as retrograde messengers. They then bind to presynaptic cannabinoid 1 (CB1) receptors, among the most prevalent G-protein coupled receptors in the mammalian brain. A short-term synaptic depression lasting several seconds follows CB1 receptor activation, by depressing voltage-gated Ca2+ currents and perhaps increasing voltage-gated K+ currents [30]. At GABAergic synapses, this process is termed DSI (depolarization-induced suppression of inhibition) [31] [32]. However, longer duration activation of CB1 receptors over a period of minutes elicits LTD through cellular mechanisms distinct from those of DSI. eCB-LTD requires inhibition of adenylate cyclase and subsequent reduction in the activity of cAMP-dependent protein kinase (PKA) [30][33]. Moreover, in the hippocampus, the active zone protein, RIM1α is required for eCB-LTD, although this does not appear to require dephosphorylation at a key PKA site [30]. As described above RIM1α is required for several examples of presynaptically-maintained LTP at excitatory synapses as well, and thus may be a shared element used to regulate synaptic strength in many forms of presynaptic plasticity.
Figure 3. Presynaptic forms of plasticity at GABAergic synapses utilize retrograde signaling.
A. eCB-LTD. Group I mGluRs at excitatory synapses (via DAG generated by phospholipase C) and/or elevation of postsynaptic Ca2+ lead to activation of diacylglycerol lipase (DAG lipase). This produces 2-arachidonylglycerol (2-AG) that is transported out of the postsynaptic cell and crosses the synapse retrogradely to activate presynaptic CB1 receptors (CB1Rs) on neighboring GABAergic synapses. Persistent activation of CB1 receptors over a period of minutes triggers eCB-LTD by a RIM1α-dependent mechanism. B. LTPGABA at synapses on VTA dopamine neurons is initiated by a rise in intracellular Ca2+, which can come through NMDAR channels. Nitric oxide synthase (NOS) is a Ca2+/calmodulin (CaM)-dependent enzyme required to synthesize nitric oxide (NO), a membrane-permeant gas with a short half-life. Upon its release from the postsynaptic cell, NO travels retrogradely to activate soluble guanylate cyclase (GC) in nearby presynaptic GABAergic terminals. GC synthesizes cGMP, which in turn activates cGMP-dependent protein kinase (PKG). PKG is required for long-term potentiation of GABA release by an unknown mechanism. Morphine and other opioids bind to presynaptic receptors and interfere with LTPGABA between the release of NO and generation of cGMP. Adapted from [37,38].
The requirement for minutes-long endocannabinoid binding suggests that this process will not result from a simple activation of a few synapses. Specific patterns of synaptic activity may modulate the degradative enzymes or endocannabinoid transporters to regulate the persistence of the endocannabinoid signal and thus set the threshold for eCB-LTD [30]. Functionally, eCB-LTD can be quite powerful, entirely silencing the output of some interneurons [34] and reducing firing rate in others that happen to be near the site of release [35]. Intriguingly, a mechanism for reversal of eCB-LTD, or for potentiation of these synapses, has not yet been reported, leaving open the question of how the circuit handles a long-term reduction of inhibition.
ii. Nitric oxide-guanylate cyclase dependent LTP
Nitric oxide (NO) is a key second messenger in smooth muscle and for many years has been reported to influence the strength of central synapses [36]. Recently it was found that NO acts as a retrograde signal to potentiate GABAergic synapses on the dopaminergic principal neurons of the ventral tegmental area (VTA) (Figure 3B). Like eCB-LTD in the hippocampus, NO-triggered LTP can be initiated by postsynaptic glutamate receptor activation, requires a retrograde messenger and is maintained by a long-lasting change in neurotransmitter release. LTPGABA was elicited using 100 Hz stimulation of afferents with AMPARs blocked [37]. Postsynaptically delivered BAPTA (a Ca2+ chelator) entirely blocked synaptically triggered LTPGABA, but the paired pulse ratio and coefficient of variation were both altered, suggesting a long-lasting increase in presynaptic GABA release. These results suggested that LTPGABA was initiated by a postsynaptic rise in Ca2+ but required a retrograde signal to potentiate the synapses. Considerable evidence suggested that NO is the signaling molecule. Inhibition of nitric oxide synthase, or application of NO scavengers such as hemoglobin, entirely blocked LTPGABA, while application of an NO donor potentiated GABAA-mediated synaptic transmission. The most common target of NO is the heme group on soluble guanylate cyclase (sGC), responsible for synthesis of cGMP. Consistent with this mode of action, inhibition of guanylate cyclase or cGMP-dependent protein kinase (PKG) also blocked LTPGABA, while application of a cGMP analogue potentiated GABAA synapses [37,38]. The mechanism by which cGMP elevation persistently increases GABA release is unknown as yet, but recent work has shown that these synapses are similarly potentiated by cAMP/PKA, and both cyclic nucleotides appear to work on a common mechanism to increase transmitter release [38]. By analogy with other cAMP-potentiated synapses, it is possible that LTPGABA may also be RIM1α-dependent. Only GABAA synapses, and not GABAB synapses onto the same postsynaptic neuron, are potentiated in response to NO [38]. This provides target-specificity, and suggests that the NO-sGC cascade (or downstream signaling elements) are absent or unavailable in GABAergic terminals apposed to GABAB receptors in this brain region. Furthermore, although HFS might be expected to release quantities of NO from dopamine cells throughout the local region, thus depressing the majority of GABAergic synapses, LTPGABA could be prevented by chelation of intracellular Ca2+ in the postsynaptic dopamine neuron. Similar results were observed with endocannabinoid modulation (Gerdeman et al., 2002; Chevaleyre and Castillo, 2003), and argue that the retrograde factors released from neighboring active neurons do not spread very far from the site of release at concentrations effective to modulate neurotransmitter release.
The VTA is a brain region necessary for processing reward and essential for drug addiction. A recent hypothesis suggests that synaptic plasticity represents an important cellular mechanism targeted by drugs of abuse, modifying normal responses for long periods [39,40]. Opioid receptors are most often found on GABAergic neurons and nerve terminals throughout the brain, and therefore, effects of opioids on LTPGABA were tested. Surprisingly, a single exposure to morphine in vivo caused a complete loss of LTPGABA in VTA slices 24 hours later. Morphine only remains present in the brain for a few hours, so these findings indicate a long-term adaptation in the circuit. Moreover, potentiation of the synapses by NO was also lost 24 hours after in vivo morphine, while cGMP was fully able to potentiate these synapses. These observations, taken together, indicate that after a single exposure to morphine in vivo, guanylate cyclase at this synapse does not function normally. Either the enzyme has become less sensitive to NO, or guanylate cyclase levels have been reduced so substantially that insufficient cGMP can be made to potentiate the GABAergic synapses [37].
These data identify an alteration in an addiction-related brain area following a single morphine exposure. The loss of LTPGABA is like removing a normal brake on the system, and is likely to increase the firing rate of dopamine neurons, a common feature of addictive drugs [41]. This change in the reward system may represent an early neuroadaptation increasing the vulnerability of the brain to subsequent morphine exposure. The loss of LTPGABA was seen in the majority of recorded cells, making it likely that overall inhibition to the VTA is seriously compromised after even a single drug treatment.
While HFS was used to trigger LTPGABA in these experiments on VTA neurons, a recent study found that similar LTPGABA could be elicited using much milder stimuli [42]. In thalamic relay neurons, either brief trains of postsynaptic action potentials or rebound excitation following hyperpolarization caused LTP of spontaneous GABAA-mediated IPSCs. NO scavengers or chelation of intracellular Ca2+ in the thalamic neuron blocked LTPGABA, which resulted from increased GABA release. Rebound excitation and action potential firing following a hyperpolarization is characteristic of these cells, and the ensuing postsynaptic Ca2+ entry is sufficient to trigger the LTPGABA. It is interesting that several different forms of postsynaptically-maintained LTP of inhibitory synapses have also been reported following rebound excitation or specific patterns of postsynaptic firing [43–45].
Retrograde messengers and signal integrity
One theoretical objection to the existence of plasticity requiring a retrograde messenger is that spread of a retrograde factor will indiscriminately potentiate or depress multiple synapses carrying unrelated information, a mechanism that could degrade information processing. Rather than modifying relevant, active synapses, the spread of a lipophilic messenger through the tissue volume would instead modify synapses in physical proximity to the released signal without regard to synapse origin. In the years since the first examples of retrograde signaling have appeared, experiments have found repeatedly that retrograde signaling preserves a level of specificity. As we collect more examples of synaptic plasticity in regions with more than one major postsynaptic target cell, it is becoming clear that close neighbor cells with different functions (such as a hippocampal pyramidal cell vs. a GABAergic interneuron) may form synapses with presynaptic terminals that express differential signaling cascades. This target cell-specificity, seen with TRPV1-LTD and some examples of eCB-LTD, provides a mechanism to limit the spread of plasticity, at least to one class of synapses in a given region.
Another way to limit the targets of a retrograde signal would be to require presynaptic terminals to be active in order for the synapse to undergo plasticity, the simple presence of a retrograde messenger being insufficient. By definition, a retrograde messenger is not synapse-specific unless it is degraded within the narrow confines of the synapse. However, the requirement for presynaptic activity necessarily limits the spread of information. In eCB-LTD, this requirement has been observed at hippocampal inhibitory synapses as well as at several excitatory synapses [46] [47] [48] [30,49]. Where it has been tested, a key signal is an activity-dependent rise in presynaptic Ca2+, which must occur coincident with the endocannabinoid signal. This coincidence requirement is somewhat analogous to the way Hebbian NMDAR-dependent LTP limits the spread of potentiation selectively to neighboring active synapses, those experiencing postsynaptic depolarization sufficient to unblock the NMDAR channel. While NMDAR-dependent LTP or LTD will spread to neighboring active synapses on a length of depolarized dendrite, however, retrograde signal-dependent plasticity could in theory spread to neighboring active presynaptic terminals on different postsynaptic target cells.
Conclusions
There is still much to be learned about the underlying mechanisms of all of these forms of synaptic plasticity. In each case, under what physiological conditions is each form active? What is the functional distance over which they operate, and what are the precise circuit consequences? What molecular players in presynaptic terminals are important for the long-term reduction of increase in neurotransmitter release? At this point, it appears that synaptic plasticity mechanisms that involve GABAergic interneurons either pre- or postsynaptically are widely diverse when compared with synaptic plasticity mechanisms involving principle neurons; is this apparent difference real or have we simply not yet described the range of synaptic mechanisms utilized in excitatory networks? What is clear is that presynaptic plasticity of the GABAergic system can operate over a wide continuum as a potent mechanism to regulate both large scale neuronal networks and single GABAergic terminals. How these forms of plasticity dovetail with other conventional forms of postsynaptic long term plasticity at principal neuron glutamatergic synapses is a challenge that remains for a thorough understanding of the dynamic and long term regulation of synaptic transmission within the mammalian central nervous system.
References
- 1.McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- 2.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Comprehensive review of the roles played by inhibitory interneurons within cortical circuits
- 3.Sudhof TC, Malenka RC. Understanding synapses: past, present, and future. Neuron. 2008;60:469–476. doi: 10.1016/j.neuron.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Lucid commentary on the state of the field of synaptic glutamate receptors and their associated mechanisms of plasticity.
- 4.Toth K, McBain CJ. Target-specific expression of pre- and postsynaptic mechanisms. J Physiol. 2000;525(Pt 1):41–51. doi: 10.1111/j.1469-7793.2000.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- 6.Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1α is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- 7.Kaeser PS, Kwon HB, Blundell J, Chevaleyre V, Morishita W, Malenka RC, Powell CM, Castillo PE, Sudhof TC. RIM1αphosphorylation at serine-413 by protein kinase A is not required for presynaptic long-term plasticity or learning. Proc Natl Acad Sci U S A. 2008;105:14680–14685. doi: 10.1073/pnas.0806679105. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Suggests that the posited role of PKA phosphorylation of RIM1αat serine 413 as the mechanism for two forms of presynaptic LTD may not be the case.
- 8.Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation--feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Bischofberger J, Engel D, Li L, Geiger JR, Jonas P. Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat Protoc. 2006;1:2075–2081. doi: 10.1038/nprot.2006.312. [DOI] [PubMed] [Google Scholar]
- 10.Acsady L, Kamondi A, Sik A, Freund T, Buzsaki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei S, McBain CJ. Two loci of expression for long-term depression at hippocampal mossy fiber-interneuron synapses. J Neurosci. 2004;24:2112–2121. doi: 10.1523/JNEUROSCI.4645-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence JJ, Grinspan ZM, McBain CJ. Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. J Physiol. 2004;554:175–193. doi: 10.1113/jphysiol.2003.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McBain CJ. Differential mechanisms of transmission and plasticity at mossy fiber synapses. Prog Brain Res. 2008;169:225–240. doi: 10.1016/S0079-6123(07)00013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelkey KA, McBain CJ. Target-cell-dependent plasticity within the mossy fibre-CA3 circuit reveals compartmentalized regulation of presynaptic function at divergent release sites. J Physiol. 2008;586:1495–1502. doi: 10.1113/jphysiol.2007.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron. 2005;46:89–102. doi: 10.1016/j.neuron.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- 17.Pelkey KA, Topolnik L, Yuan XQ, Lacaille JC, McBain CJ. State-dependent cAMP sensitivity of presynaptic function underlies metaplasticity in a hippocampal feedforward inhibitory circuit. Neuron. 2008;60:980–987. doi: 10.1016/j.neuron.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Reveals that de-depression of MF-interneuron transmission utilizes a nascent cAMP dependent pathway unavailable at naïve synapses.
- 18.Maccaferri G, Toth K, McBain CJ. Target-specific expression of presynaptic mossy fiber plasticity. Science. 1998;279:1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- 19.Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–759. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; **First demonstration of TRP channel-dependent long-term depression and description of 12-(S)-HPETE as a retrograde messenger.
- 20.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 21.Kauer JA, Gibson HE. Hot flash: TRPV channels in the brain. Trends in Neurosciences. 2009 doi: 10.1016/j.tins.2008.12.006. in press. [DOI] [PubMed] [Google Scholar]
- 22.Lamsa K, Heeroma JH, Kullmann DM. Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci. 2005;8:916–924. doi: 10.1038/nn1486. [DOI] [PubMed] [Google Scholar]
- 23.McMahon LL, Kauer JA. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron. 1997;18:295–305. doi: 10.1016/s0896-6273(00)80269-x. [DOI] [PubMed] [Google Scholar]
- 24.Topolnik L, Azzi M, Morin F, Kougioumoutzakis A, Lacaille JC. mGluR1/5 subtype-specific calcium signalling and induction of long-term potentiation in rat hippocampal oriens/alveus interneurones. J Physiol. 2006;575:115–131. doi: 10.1113/jphysiol.2006.112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- 26.Lamsa KP, Heeroma JH, Somogyi P, Rusakov DA, Kullmann DM. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007;315:1262–1266. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez Y, Morin F, Lacaille JC. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci U S A. 2001;98:9401–9406. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 29.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 30.Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annual Review of Physiology. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Excellent review of endocannabinoid long-term depression at excitatory and inhibitory synapses..
- 31.Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- 32.Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 34.Losonczy A, Biro AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci U S A. 2004;101:1362–1367. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002;34:787–796. doi: 10.1016/s0896-6273(02)00695-5. [DOI] [PubMed] [Google Scholar]
- 36.Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 37.Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]; **First demonstration that drugs of abuse target synaptic plasticity at GABAergic synapses. Delineation of the NO/sGC cascade required for LTPGABA.
- 38.Nugent FS, Niehaus JL, Kauer JA. PKG and PKA Signaling in LTP at GABAergic Synapses. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Progress in Neurobiology. 1998;54:1–42. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 40.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 41.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. PNAS. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bright DP, Brickley SG. Acting locally but sensing globally: impact of GABAergic synaptic plasticity on phasic and tonic inhibition in the thalamus. J Physiol. 2008;586:5091–5099. doi: 10.1113/jphysiol.2008.158576. [DOI] [PMC free article] [PubMed] [Google Scholar]; *First description of LTP of GABAergic synapses in thalamus utilizing nitric oxide.
- 43.Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21:827–835. doi: 10.1016/s0896-6273(00)80598-x. [DOI] [PubMed] [Google Scholar]
- 44.Ouardouz M, Sastry BR. Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. J Neurophysiol. 2000;84:1414–1421. doi: 10.1152/jn.2000.84.3.1414. [DOI] [PubMed] [Google Scholar]
- 45.Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- 46.Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 47.Singla S, Kreitzer AC, Malenka RC. Mechanisms for synapse specificity during striatal long-term depression. J Neurosci. 2007;27:5260–5264. doi: 10.1523/JNEUROSCI.0018-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heifets BD, Chevaleyre V, Castillo PE. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc Natl Acad Sci U S A. 2008;105:10250–10255. doi: 10.1073/pnas.0711880105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lachamp PM, Liu Y, Liu SJ. Glutamatergic modulation of cerebellar interneuron activity is mediated by an enhancement of GABA release and requires protein kinase A/RIM1alpha signaling. J Neurosci. 2009;29:381–392. doi: 10.1523/JNEUROSCI.2354-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]