Abstract
Cough and breathing are generated by a common muscular system. However, these two behaviors differ significantly in their mechanical features and regulation. The current conceptualization of the neurogenic mechanism for these behaviors holds that the multifunctional respiratory pattern generator undergoes reconfiguration to produce cough. Our previous results indicate the presence of a functional cough gate mechanism that controls the excitability of this airway defensive behavior, but is not involved in the regulation of breathing. We propose that the neurogenesis of cough, breathing, and other nonbreathing behaviors is controlled by a larger network, of which the respiratory pattern generator is part. This network we term a holarchical system. This system is governed by functional control elements known as holons, which confer unique regulatory features to each behavior. The cough gate is an example of such a holon. Neurons that participate in a cough holon may include behavior selective elements. That is, neurons that are either specifically recruited during cough and/or tonically-active neurons with little or no modulation during breathing but with significant alterations in discharge during coughing. We also propose that the holarchical system is responsible for the orderly expression of different airway defensive behaviors such that each motor task is executed in a temporally and mechanically discrete manner. We further propose that a holon controlling one airway defensive behavior can regulate the excitability of, and cooperate with, holons unique to other behaviors. As such, co-expression of multiple rhythmic behaviors such as cough and swallow can occur without compromising airway defense.
Keywords: Cough, Control of breathing, Rhythm generation, Brainstem, Inspiratory, Expiratory, Reflexes, Abdominal, Diaphragm, Respiratory neuron, Swallow, Larynx
1. Introduction
The cough reflex is a patterned and rhythmic behavior. Furthermore, the expiratory bursting during coughs can result in expulsive airflows that reach 12 L/s and peak in as little as 30 ms after the end of the compression phase (Knudson et al., 1974). These characteristics are consistent with the concept that cough, unlike breathing, can be considered a ballistic behavior. While this behavior can occur as a single event, repetitive airway stimulation can elicit repeated coughs, which can outlast the stimulus (Fig. 1). As such, any model of the neurogenesis of cough should account for rhythmicity of the behavior.
Fig. 1.
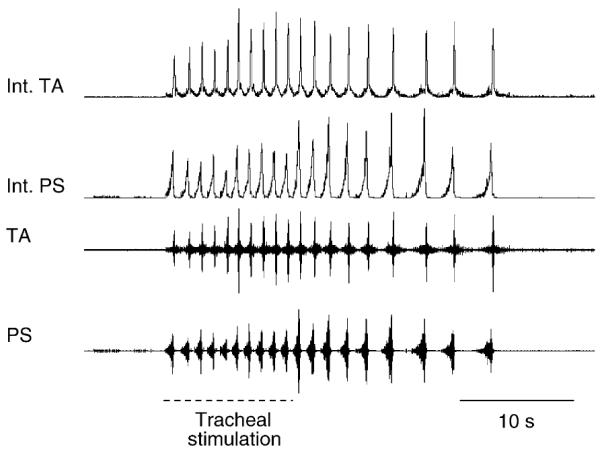
Example illustrating that repetitive coughing can outlast the inducing stimulus. Electromyographic (EMG) recordings from the parasternal (PS) and the transversus abdominis (TA) muscles in an anesthetized cat. Tracheal stimulation indicates that a flexible cannula was introduced into the intrathoracic airway and rotated at approximately 2 Hz for a duration of 10 s. The cannula was immediately removed at the end of the 10 s stimulation period. Note that repetitive coughing continues for approximately 15 s after cessation of the stimulus. Int. PS and TA traces represent moving averages of their respective EMG recordings with a 100 ms time constant.
Cough and breathing are not the same behavior. As noted above, the mechanics of the two behaviors are different. Furthermore, the regulation of cough differs greatly from that of breathing. The well-known relationship between volume and phase durations is not present during cough (Bolser and Davenport, 2000a). Indeed, pulmonary slowly adapting receptor (SAR) feedback is not inspiratory-inhibitory during cough as it is during breathing. Instead, SAR afferent input is permissive for the production of cough induced from the trachea and facilitatory for laryngeal cough (Hanacek et al., 1984; Sant’Ambrogio et al., 1984). The effects of chemoreceptive feedback also are different for cough and breathing. Mild to moderate poikilocapnic hypoxia inhibits tracheobronchial cough (Tatar et al., 1986) and moderate to severe hypercapnia has a slight suppressive effect on this behavior (Nishino et al., 1989). These stimuli elicit well-known increases in ventilation. In essence, the three most studied regulatory afferent mechanisms on breathing, SAR feedback, hypercapnia, and hypoxia all have very different effects of the two behaviors. These regulatory differences suggest that the neurogenic mechanism for cough differs significantly from that for breathing.
The motor pattern and regulatory differences between cough and breathing could be consistent with very different neurogenic mechanisms controlling the two behaviors. However, many of the same elements that participate in the neurogenesis of breathing also contribute to the production of cough (Shannon et al., 2000; Baekey et al., 2001, 2004). The fact that the same elements can participate in the neurogenesis of two very different behaviors is explained by a process known as reconfiguration as proposed by Shannon and coworkers (Shannon et al., 2000; Baekey et al., 2001, 2004; Lindsey et al., 1992). In essence, the concept of reconfiguration holds that a network of functionally connected neurons can produce more than one behavior by undergoing rearrangements of their discharge patterns as well as the manner in which they functionally interact to modify the motor output of the system. In this context, reconfiguration indicates that the system has undergone a process of reorganization so that it can produce a different behavior. As such, the respiratory network is proposed to be multifunctional. That is, the current conceptualization of this network is deemed sufficient to generate multiple behaviors. However, this hypothesis and the network model proposed by Shannon and coworkers makes it difficult to account for differences in the regulation of the two behaviors (as stated above) or the presence of a functionally identified gating mechanism that controls the excitability of cough (Bolser et al., 1999, 2003; Bolser and Davenport, 2002).
To reconcile the concepts outlined above, we hypothesize that breathing and a multiplicity of other behaviors with radically different motor patterns can be generated by a brainstem network consisting of interconnected subsystems. The system concept differs from the current hypothesis of a multifunctional network in that it incorporates the role of elements that are functionally connected with the core respiratory network but are only active or modulated during specific behaviors. This system has at its core the network responsible for the neurogenesis of breathing. This hypothesis is an extension of the reconfiguration hypothesis proposed by Shannon and coworkers in which the neurogenesis of cough is explained by reconfiguration of a core network responsible for breathing into a network that produces cough. However, this hypothesis recognizes a higher organizational framework than the respiratory network alone. This framework is holarchical (Koestler, 1967). A holarchical system is made up of subsystems known as holons (after the Greek word holos or “whole”) that act as control elements (Koestler, 1967). A feature of holons is that not only do they control lower level subsystems but they are also subservient to higher-level holons. An important characteristic of holarchical systems is that when the subsystems merge, new characteristics appear that were not predicted by the behavior of the subsystems (Koestler, 1967). The substantial differences in the regulation of cough and breathing can be explained by this emergence. We have inferred the presence of a unique holon in the neurogensis of cough. This holon has been termed a gating mechanism (Bolser and Davenport, 2002) and confers unique regulatory features to cough relative to breathing. The gating mechanism is a functional entity that, when active, enables or permits single or repetitive coughs to occur. Nonbreathing behaviors involving respiratory muscles, such as cough, would be produced by subsystems (assemblies, Lindsey et al., 2000) of neurons that include parts of the respiratory network, as well as behavior-selective elements. The term behavior-selective indicates neurons that participate in the production of a limited number of the range of motor tasks involving respiratory muscles. In the current context, it is meant to highlight neurons that are not spontaneously active during breathing but are recruited during other behaviors. Furthermore, we include in this category neurons that have little or no respiratory modulation, but undergo significant modulation of their discharge patterns during other behaviors, such as cough (Baekey et al., 2003). Key features of the holarchical system hypothesis include: (a) retasking of some elements that are breathing-modulated; (b) spontaneously active and recruited elements that are behavior selective; (c) assemblies of retasked and recruited elements that, in concert with respiratory modulated components, confer unique characteristics to the network such that spatiotemporal features of the pattern and regulation of the resultant behavior bear little similarity to that of breathing.
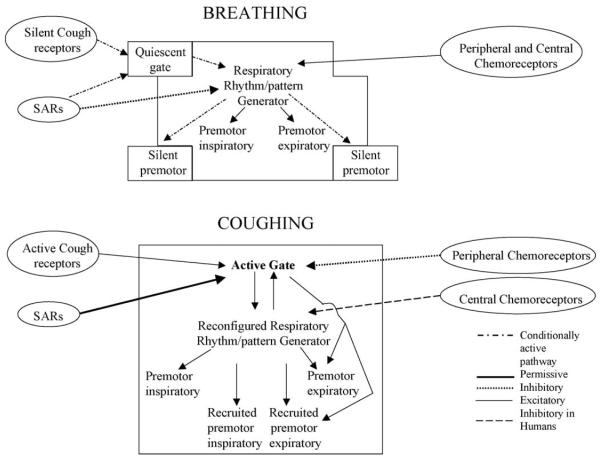
2. The gating mechanism
The evidence supporting the presence of a gating mechanism was obtained from studies of the effects of antitussive drugs on the cough and breathing patterns (May and Widdicombe, 1954; Bolser et al., 1999). This hypothesis accounts for the fact that antitussive drugs do not suppress breathing at doses that inhibit cough (May and Widdicombe, 1954; Bolser et al., 1999), suggesting the presence of an element important for cough that does not participate in the neurogenesis of breathing. Indeed, antitussive drugs do not inhibit tracheobronchial cough by a generalized suppression of the entire central cough pattern generator, rather they have very specific effects on various components of this system. For example, central administration of antitussive drugs does not alter inspiratory or expiratory phase durations or inspiratory burst amplitude during tracheobronchial cough, yet they decrease cough number (the number of coughs elicited per stimulus trial) and expiratory muscle EMG burst amplitude (Bolser et al., 1999). Our model (Bolser et al., 1999, 2003; Bolser and Davenport, 2002) reflects this observation in that the cough pattern generator and inspiratory premotor activity are relatively insensitive to the effects of antitussive drugs. The reduction in tracheobronchial cough number elicited by antitussive drugs is not due to prolongation of the duration of individual cough cycles. Therefore, antitussive drugs must inhibit tracheobronchial cough number by an action on elements that are not responsible for regulating phase durations. Given that the current proposed role of the core respiratory network involves regulation of phase durations for both breathing and cough (Shannon et al., 1996, 1998, 2000, 2004b; Baekey et al., 2001; Bolser et al., 2003), we propose that this network represents a subsystem that is itself controlled by the gating mechanism (Fig. 2). As such, the presence of an antitussive-sensitive gating mechanism accounts for the differential effects of antitussive drugs on the tracheobronchial cough and breathing patterns. In essence, antitussive drugs have selective effects on cough relative to breathing because they act on a control element (holon) of the system that is functional only when afferents that induce cough are stimulated. The influence of the holon is initiated when sensory afferents that elicit cough bring it to threshold and thus enable it to regulate the behavior of the reconfigured respiratory pattern generation system (Fig. 2). The designation of the gate as “active” in Fig. 2 is meant to represent a functional influence of the gate on the reconfigured respiratory pattern generator. While this organizational framework implies that the gate actively excites the reconfigured respiratory pattern generator, the details of the gating mechanism at the synaptic level are unknown and may include complex effects including disinhibition.
Fig. 2.
Schematic representation of holarchical organization of respiratory motor control system during breathing and cough. The figure highlights the presence of conditionally active elements and the concept that regulation of each behavior by well-known sensory inputs is different. Furthermore, the reconfigured respiratory rhythm/pattern generator functions as a subsystem that is subservient to the gating mechanism during cough. The gating mechanism is an example of a holon that, when active, is part of a larger system. The designations of “quiescent” and “active” when applied to the gate represent functional influences on the rest of the system. This organizational framework can account for regulatory differences between laryngeal and tracheobronchial cough even though the motor patterns of these behaviors are very similar. We have proposed two different holons (gating mechanisms) for laryngeal and tracheobronchial cough (Bolser and Davenport, 2002). However, both holons make use of the same reconfigured respiratory rhythm/pattern generator to control the spatiotemporal features of coughing.
Cough can commonly be produced by stimulation of either tracheobronchial or laryngeal sensory receptors (Korpas and Tomori, 1979). These two types of cough differ in their sensitivity to antitussive agents (Korpas and Tomori, 1979) as well as the suppressive effects of poikilocapnic hypoxia (Tatar et al., 1986). We therefore have developed the hypothesis that separate holons control laryngeal and tracheobronchial cough. This hypothesis is represented as separate gating mechanisms for laryngeal and tracheobronchial cough (Bolser and Davenport, 2002). These gating elements also integrate afferent feedback from SARs through their interneurons (pump cells). Unlike their integral role in the production of tracheobronchial cough, SARs only have a facilitatory effect on laryngeal cough (Hanacek et al., 1984; Sant’Ambrogio et al., 1984). Each holon utilizes the reconfigured core respiratory network to regulate the spatiotemporal features of the cough motor pattern. This concept is entirely consistent with previous work indicating that the core respiratory network participates in the production of both laryngeal and tracheobronchial cough (Shannon et al., 1996, 1998, 2000, 2004b).
3. What is the role of putative second order relay neurons in the gating mechanism?
Afferent input to the cough neurogenic system is proposed to be transmitted by second order rapidly adapting receptor (RAR) interneurons and pump cells (which are responsible for the permissive effect of SARs on tracheobronchial cough) through a gating mechanism. However, recent work (Canning et al., 2004) suggests that the traditional view that RARs mediate the production of cough may need to be amended. These investigators showed that another population of tracheal sensory afferents mediates the production of cough in guinea pigs. They term this population cough receptors. The implications of this finding extend into the central neurogenesis of cough. What is the identity of the second order interneurons to which this novel population of sensory afferents projects? Relatively little information exists on the properties of RAR second order interneurons (Lipski et al., 1991). However, given that the properties of cough receptors differ significantly from RARs (Canning et al., 2004), the extent to which this previous information on RAR relay neurons will be useful in predicting the behavior of cough receptor second order interneurons is unknown. Based on horseradish peroxidase studies of projections of tracheal afferents (Kalia and Mesulam, 1980), it is likely that cough receptor second order interneurons are located near to or intermingled with RAR relay neurons.
Cough receptor relay neurons are proposed to provide excitatory input to virtually all elements of the system, including neurons that participate in the control of cough phase durations (Shannon et al., 1996, 1998, 2000). Decreased excitability of this group of neurons would be expected to result in prolongation of cough phase durations. Indeed, unilateral vagotomy elicits exactly this effect on the cough reflex induced by ammonia in rabbits (Hanacek et al., 1984). This intervention decreased the frequency of coughing without altering the number of coughs per trial, indicating that cough phase durations were prolonged. Antitussive drugs do not alter cough (or respiratory) phase durations at doses sufficient to decrease cough number. These drugs also selectively decrease expiratory motor activation during cough at doses that do not reduce inspiratory motor activation, which is not consistent with suppression of elements (cough receptor relay neurons) that increase excitability of both inspiratory and expiratory premotor neurons. Collectively, these findings do not support an action of antitussive drugs on cough receptor relay neurons. Suppression of pump cell activity by antitussive drugs is unlikely to be responsible for our observations because in our study these drugs had no effect on eupneic respiratory phase durations or diaphragm EMG amplitude (Bolser et al., 1999). SAR afferent input does have an integral role in the production of tracheobronchial cough, and it is a permissive one (Hanacek et al., 1984; Sant’Ambrogio et al., 1984). Indeed, in a recent study, we found no evidence for a direct role of SAR afferent input on phase durations during tracheobronchial cough (Bolser and Davenport, 2000a). We propose that pump cells and cough receptor relay neurons are relatively insensitive to antitussive drugs and do not participate in the gating mechanism. An alternative hypothesis is that subpopulations of cough receptor relay neurons or pump cells exist that mediate different functions. One or more of these subpopulations could participate in the gating mechanism.
4. The core respiratory network, reconfiguration, and the gating mechanism
Respiratory neurons undergo changes in discharge during cough that support reconfiguration of elements that are spontaneously active during breathing into a network that participates in the production cough. Functionally identified synaptic influences between components of the model support the network arrangement shown in previous reports (Shannon et al., 1998, 2000; Baekey et al., 2001, 2004).
We have proposed that the gating mechanism controls the excitability of the whole system and is sensitive to suppression by antitussive drugs (Bolser et al., 1999; Bolser and Davenport, 2002). In this context, our hypothesis incorporates both reconfiguration of the respiratory pattern generator and the gating mechanism. However, the exact synaptic relationship between the gating mechanism and specific elements of the network model are currently unknown.
Shannon and coworkers (Shannon et al., 1996, 1998, 2000, 2004b; Baekey et al., 2001, 2004; Bolser et al., 2003) have constructed a model of the cough pattern generator that incorporates most of the different groups of dorsal respiratory group and ventral respiratory group/Botzinger complex (VRG/Bot) neurons that also participate in breathing. According to the model, different classes of these neurons interact with one another to control inspiratory and expiratory phase durations during cough, the magnitude of motor drive to spinal motoneurons, and the activation of laryngeal muscle motoneurons that determine caliber of the larynx. As stated above, sensory input to this cough pattern generator is mediated by cough receptor relay neurons, pump cells, and laryngeal relay neurons (Shannon et al., 1996, 1998, 2000, 2004b; Baekey et al., 2001, 2004; Bolser et al., 2003). It should be noted that most of the information on which this model is based has been generated from experiments in which single fictive coughs were produced. The extent to which this synaptic model fully accounts for motor patterns during repetitive coughing is not clear. Indeed, some respiratory neuron groups undergo significant variations in discharge patterns during successive fictive coughs within a series (Wang et al., 2005).
A feature of the model is that, for the most part, respiratory neurons undergo alterations in discharge pattern during cough that are consistent with their participation in the neurogenesis of this behavior. Furthermore, functional interactions between neuron groups incorporated into the model are supported by the results of electrophysiological methods, such as cross correlation analysis (Shannon et al., 1998, 2000; Baekey et al., 2001).
Neurons that are identified by their discharge patterns during breathing as primarily inspiratory or expiratory do not change their major phase of discharge during cough. That is, inspiratory neurons do not become expiratory neurons during cough and vice versa. However, the variability of discharge pattern of some neuron populations within a phase can be very high. For example, expiratory augmenting (E-Aug) neurons of the rostral and caudal VRG can undergo a shift in their discharge patterns to decrementing during cough (Oku et al., 1994; Shannon et al., 1998, 2000). For the premotor E-Aug neurons in the caudal VRG, this represents a change in discharge that mirrors the motor bursting in expiratory muscles (Shannon et al., 1998, 2000). However, one group has reported that activities of these neurons do not always mirror abdominal motor bursting during fictive cough (Oku et al., 1994). Rostral VRG/Bot E-Aug neurons also exhibit this change and in the model have been identified as E-Aug-early to acknowledge this fact. Furthermore, Bongianni and coworkers (Bongianni et al., 1998) have shown that rostral VRG/Bot E-Aug neurons that discharge in this manner during cough have peak discharge rates that are directly correlated with the amplitude of peak abdominal neurogram activity during cough. Some rostral VRG/Bot E-Aug neurons do not exhibit this change (they remain E-Aug) during cough whereas others undergo decreases in discharge rate (Shannon et al., 1996, 1998, 2000, 2004b; Bongianni et al., 1998; Baekey et al., 2001, 2004). Rostral VRG/Bot expiratory decrementing (E-Dec) neurons appear to have discharge patterns that remain decrementing during cough (Shannon et al., 1996, 1998, 2000, 2004b; Bongianni et al., 1998; Baekey et al., 2001, 2004).
Shannon and coworkers (Shannon et al., 1996, 1998, 2000, 2004b) have proposed that E-Aug-early neurons provide excitatory input to caudal medullary expiratory bulbospinal neurons during cough. Furthermore, these investigators have proposed that the duration of discharge of E-Aug early neurons and caudal expiratory bulbospinal neurons is limited by inhibition from a subpopulation of Botzinger expiratory augmenting neurons (E-Aug late) that discharge in the latter portion of the phase during cough. E-Aug late neurons have limited activity in the early expiratory phase because of inhibition by expiratory decrementing neurons (E-Dec).
Suppression of the discharge of E-Aug-early neurons (for example, by codeine) would result in disfacilitation of caudal expiratory bulbospinal neurons and result in reduced expiratory motor drive during cough. However, this action would also result in prolongation of the discharge of E-Dec and thus caudal E-Aug bulbospinal neurons by disinhibition. Ultimately, these synaptic effects would be manifest as a prolongation of abdominal expiratory activity and the cough expiratory phase duration by codeine. Our previous findings (Bolser et al., 1999 and unpublished observations) indicate that codeine has no such action on expiratory phase durations or motor burst durations. As such, elements of the reconfigured respiratory network that participate in phase timing are unlikely to directly participate in the function of the gating mechanism.
Other known elements of the system could have a role in the gating mechanism. Caudal medullary expiratory premotor neurons and a subset of rostral E-Aug-early neurons could potentially participate in the gating mechanism and be sensitive to antitussive drugs. This participation presumes that at least some of these neurons control the excitability of the network during cough.
5. Recruited and tonically-active elements
Medullary neurons that are normally silent but are recruited during cough (behavior selective neurons) have been observed by several laboratories (Jakus et al., 1985; Shannon et al., 1998). The prevalence of these behavior selective neurons may be under-appreciated. For example, recruited neurons were observed during cough in 20/20 electrode penetrations into the VRG in the region of the obex (Jakus, unpublished observations). The contribution of behavior selective elements may be responsible for regulating the function of the core pattern generation network to allow it to mediate multiple tasks. Such tasks may require motor patterns and regulation that are beyond the capabilities of the original configuration of the pattern generation network. The most basic of these tasks may include amplification of motor drive to spinal motoneuron pools. For example, expiratory motor drive during cough can be an order of magnitude greater than that produced during breathing-related behaviors, such as expiratory thresh-old loading (Bolser et al., 2000b). This amplification of motor drive may be important in the recruitment of significant numbers of respiratory motoneurons during airway defensive behaviors. A large proportion of the phrenic motoneuron pool is not activated by maximal chemical stimuli, but is recruited during expulsive behaviors (Sieck and Fournier, 1989). However, behavior selective elements may have more important roles in the network, such as modulation of the temporal components of the pattern, controlling the regulation of the behavior, and/or the excitability of cough. These behavior selective elements may also include neurons with little respiratory modulation that undergo significant changes in pattern during cough. Tonically-active neurons in the raphe nuclei and the pons have been identified that exhibit profound alterations in discharge during cough, while having little or no modulation in their discharge patterns during breathing (Baekey et al., 2003; Shannon et al., 2004a). The extent to which these tonically-active and cough modulated neurons interact with elements of the core respiratory network is unknown. However, Shannon (Shannon et al., 2004b) has indicated preliminary evidence exists supporting functional interactions between these neurons and cough-modulated neurons of the VRG/Bot. Furthermore, it is clear that neurons with little respiratory modulation can influence phasic respiratory neurons in complex ways (Li et al., 1999; Lindsey et al., 2000).
6. The holarchical system and the expression of multiple behaviors
A variety of different behaviors are produced by the respiratory muscles. These behaviors include cough, sneeze, gasp, vomiting, augmented breaths, expiration reflex, aspiration reflex, breathing, swallowing, and the asphyxic response. Each of these behaviors is produced by unique changes in the mechanics of the respiratory system. While the mechanics and neurogenesis of most of these behaviors have been studied individually, the control mechanisms responsible for regulating the expression of each behavior in relation to another are unknown. That is, what is the makeup of the control system that ensures that multiple behaviors will not be expressed simultaneously; resulting in mechanically inappropriate motor acts? For example, in awake animals behaviors that include laryngeal abduction do not occur during vomiting under normal conditions (Lang et al., 2002). The identity of the regulatory mechanism preventing laryngeal abduction or the occurrence of behaviors that include dilation of the larynx during vomiting is unknown, but it is very important to prevent aspiration.
Airway defensive behaviors that have very different mechanical features can occur in rapid sequence. How is this “behavioral switching” controlled? That is, what is the resultant motor response when afferent input reaches the system that is sufficient to produce two or more of these behaviors simultaneously? In this scenario, the control mechanisms for more than one behavior are competing for a common motor system. We propose that there are interactive holons within the system that control the emergence of a given behavior in relation to others. Such a system would ensure an orderly expression of multiple behaviors that have different mechanical characteristics. An example of this orderly expression can be seen in Fig. 3. Continuous mechanical stimulation of the larynx and pharyngeal mucosa over the period of approximately 20 s produces several different nonbreathing behaviors, including expiration reflex, aspiration reflex and laryngeal cough. Expiration reflex is an expulsive behavior that has no inspiratory component and consists of a sudden and short burst of expiratory muscle activity (Korpas, 1972b; Korpas and Tomori, 1979; Korpas and Jakus, 2000). Aspiration reflex is a gasp-like behavior with no expiratory component that is elicited by mechanical stimulation of the pharyngeal airway (Korpas and Tomori, 1979). In the figure, these three behaviors occur in an orderly fashion such that each is expressed without temporal “interference” with the other. As such, a sequence of patterned and functionally meaningful behaviors is produced. Moreover, hybrid behaviors (those expressing the motor characteristics of more than one airway defensive behavior simultaneously) are not expressed. In the absence of a control system that regulates the expression of different behaviors, the risk of producing motor bursts that represent such hybrid behaviors is high. Hybrid behaviors are likely to be mechanically inappropriate for effective airway defense. Further evidence that the system behaves in this manner can be found from observations of the sequence of expulsive behaviors that occurs immediately after single mechanical stimuli to the larynx. In this situation, expiration reflex frequently precedes laryngeal cough (Korpas, 1972a; Korpas and Tomori, 1979; Baekey et al., 2004). This sequence is critically important for airway defense because the laryngeal airway must be cleared by the sudden expulsive airflow generated by expiration reflex before the inspiratory component of laryngeal cough begins. Otherwise, the risk of aspiration of foreign material during the inspiratory phase of laryngeal cough is high.
Fig. 3.
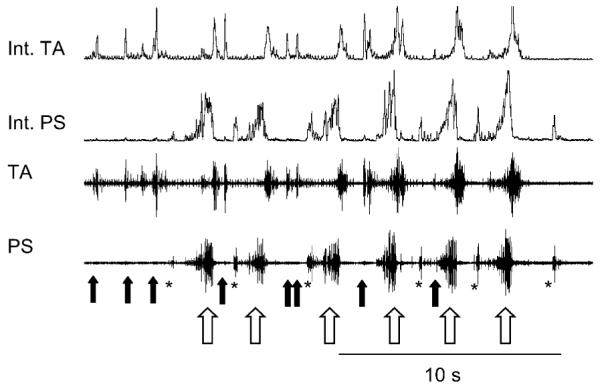
Example of the coordinated occurrence of multiple airway defensive behaviors during mechanical stimulation of the pharyngeolaryngeal airway. The solid arrows mark the occurrence of expiration reflexes, the open arrows indicate individual laryngeal coughs in a repetitive series, and the asterisks demark aspiration reflexes. Note what appears to be a relatively disordered motor pattern can be resolved into the repetitive occurrence of three separate behaviors. The expiration and aspiration reflexes occur during the cough expiratory interval and are brief enough to be fully expressed before the next cough begins. Abbreviations as in Fig. 1.
Rhythmic behaviors, such as locomotion (McCrea, 2001), breathing (Rybak et al., 2004), and swallowing (Jean, 2001) are controlled by central pattern generators that represent complex neural networks capable of producing the same behavior in a repeated fashion. Other behaviors, such as reflexes, can appear to be rhythmic if an inducing afferent stimulus is applied in a periodic manner. However, in the absence of periodic afferent stimulation, the reflex will not become rhythmic. In contrast, the periodicity of a rhythmic behavior is determined centrally and is not produced solely by oscillations of afferent feedback. However, the role of afferent feedback in regulating the behavior of a central pattern generator can be complex, involving frank excitation to the system as well as allowing the system to respond to external perturbations (McCrea, 2001).
According to these criteria, neither expiration reflex nor aspiration reflex are rhythmic behaviors. They are highly patterned behaviors and can be produced multiple times with sequential mechanical stimulation, but their frequency of occurrence is determined by the frequency of mechanical stimulation and they do not become rhythmic after the stimulus ceases (Korpas and Tomori, 1979). These observations conform to the definition of a reflex (Levy et al., 2006). The example in Fig. 3 shows that reflex (nonrhythmic) airway defensive behaviors can occur in conjunction with rhythmic airway defensive behaviors such as coughing, although the timing of their occurrence is controlled in an orderly fashion.
It should be noted that aspiration reflex and expiration reflex are behaviors that are initiated and completed in a very short time frame (approximately 100–200 ms) relative to the cycle duration of repetitive laryngeal cough. These reflexes occur in the cough expiratory interval (Fig. 3), which is long enough to allow for completion of these behaviors before the next cough cycle begins. We have observed that the duration of the laryngeal cough expiratory phase is not altered by the occurrence of an expiration reflex in that interval (unpublished observations), indicating that the cough rhythm is not perturbed by this behavior. This observation suggests that the neural elements required for the production of expiration reflex do not interfere with the function of the network controlling cough expiratory duration. However, other investigators have reported perturbations of the expiratory interval during breathing after the occurrence of expiration reflex (Korpas and Tomori, 1979; Baekey et al., 2004), although these effects were not consistent.
These observations suggest that the holarchical control system that regulates the expression of multiple behaviors is “nested” in a manner that allows the sequential occurrence of a rhythmic behavior such as cough and airway defensive reflexes that are nonrhythmic (do not require a central pattern generator for their expression). In essence, holons controlling both rhythmic behaviors and reflexes can be active in an overlapping fashion in this organizational framework.
The extent to which the holarchical system will allow for simultaneous expression of multiple rhythmic nonbreathing behaviors is not well understood. However, it is known that rhythmic swallow can alternate between repetitive laryngeal coughs (Gestreau et al., 2000). This observation indicates that the system will allow alternating expression of two rhythmic nonbreathing behaviors. To be sure, there must be significant temporal correlation between the two behaviors for each to be executed in an appropriate fashion. Indeed, in an example shown in one study (Gestreau et al., 2000) swallows were initiated and completed in the cough expiratory interval. This overlapping expression of two rhythmic behaviors is entirely consistent with the concept that the oscillators for swallow and cough are functionally separate (Jean, 2001; Saito et al., 2003). However, each oscillator must generate an appropriate behavior without interfering with the expression or rhythmicity of the other. In terms of how the holarchical system functions, holons controlling the expression of each behavior must both cooperate and enable their respective rhythm generating holons to function. In essence, the excitability of both behaviors must be controlled while allowing for synchronization of each oscillator to occur. In the absence of such regulation, effective airway protection during swallow is not possible. Swallowing is typically considered to be an ingestive behavior, however, this behavior can have an airway protective function in that it removes ejected material from the pharyngeal airway (Ludlow, 2005).
7. Implications and future directions
There are many implications derived from investigating holarchies. The presence of a higher organizational framework than represented by the currently accepted respiratory network suggests there are neuronal elements that interact with the respiratory rhythm/pattern generator about which little information exists. Indeed, the presence of elements that are normally silent in close proximity to spontaneously active respiratory neurons raises the possibility that some interventions that are intended to study the neurogenesis of breathing might instead be influencing components of the holarchical system that are only conditionally active. As such, the resultant motor pattern changes may be less related to alterations in the respiratory pattern generator than the result of the artificial induction of selected components of the holarchical system that are not active during breathing. Such a network might produce rhythmic inspiratory motor behaviors, but bear relatively little resemblance to the configuration of the network for breathing.
Another implication of this organizational hypothesis is that the system could be induced to generate coughing by holon(s) not located in the brainstem. That is, the cough gating holon may well be actuated by suprapontine elements related to the production of voluntary cough. As such, a voluntary cough that is spatiotemporally identical to a reflexive behavior could be produced in the absence of sensory afferent input simply by bringing an appropriate holon (the gating mechanism) to threshold. As stated earlier, holarchical systems are composed of holons that not only control subsystems, but are themselves subservient to higher order holons (Koestler, 1967). In this context, suprapontine element(s) that mediate voluntary cough could be considered as higher order holons in the system.
Further testing of these hypotheses is likely to be a challenging endeavor. These hypotheses presume a more complex network controlling behaviors involving respiratory muscles than has been previously proposed. Approaches that focus on analysis of networks, their membership, and functional interactions are likely to be of value in testing hypotheses surrounding the holarchical system. This information is most readily obtained from in vivo experimental models in which multiple airway defensive behaviors can be produced. There are a limited number of such experimental models (Shannon et al., 1998; Canning et al., 2004). Reduced preparations are less likely to be of value until more specific information is available from in vivo models.
Acknowledgements
Supported by NIH R01 HL70125 (DCB). The authors thank Dr. John Widdicombe for insightful comments on a draft of the manuscript. We also thank Dr. Kendal Morris, Cheng Wang, and Maria-Mercedes Panqueva for their incisive comments and suggestions during the genesis of the manuscript.
Footnotes
This paper is part of a special issue entitled “Cough and its Regulation”, guest-edited by John G. Widdicombe and Bradley J. Undem.
References
- Baekey DM, Morris KF, Gestreau C, Li Z, Lindsey BG, Shannon R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. J. Physiol. 2001;534:565–581. doi: 10.1111/j.1469-7793.2001.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R. Medullary raphe neuron activity is altered during fictive cough in the decerebrate cat. J. Appl. Physiol. 2003;94:93–100. doi: 10.1152/japplphysiol.00341.2002. [DOI] [PubMed] [Google Scholar]
- Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R. Ventrolateral medullary respiratory network participation in the expiration reflex in the cat. J. Appl. Physiol. 2004;96:2057–2072. doi: 10.1152/japplphysiol.00778.2003. [DOI] [PubMed] [Google Scholar]
- Bolser D, Davenport PW, Golder FJ, Baekey DM, Morris KF, Lindsey BG, Shannon R. Neurogenesis of Cough. Blackwell Publishing; Malden, MA: 2003. [Google Scholar]
- Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulmonol. Pharmacol. Ther. 2002;15:221–225. doi: 10.1006/pupt.2002.0361. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J. Appl. Physiol. 1999;86:1017–1024. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Davenport PW. Volume-timing relationships during cough and resistive loading in the cat. J. Appl. Physiol. 2000a;89:785–790. doi: 10.1152/jappl.2000.89.2.785. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Reier PJ, Davenport PW. Responses of the anterolateral abdominal muscles during cough and expiratory threshold loading in the cat. J. Appl. Physiol. 2000b;88:1207–1214. doi: 10.1152/jappl.2000.88.4.1207. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Fontana GA, Pantaleo T. Discharge patterns of Botzinger complex neurons during cough in the cat. Am. J. Physiol. 1998;274:R1015–R1024. doi: 10.1152/ajpregu.1998.274.4.R1015. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guineapigs. J. Physiol. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestreau C, Grelot L, Bianchi AL. Activity of respiratory laryngeal motoneurons during fictive coughing and swallowing. Exp. Brain Res. 2000;130:27–34. doi: 10.1007/s002210050003. [DOI] [PubMed] [Google Scholar]
- Hanacek J, Davies A, Widdicombe JG. Influence of lung stretch receptors on the cough reflex in rabbits. Respiration. 1984;45:161–168. doi: 10.1159/000194614. [DOI] [PubMed] [Google Scholar]
- Jakus J, Tomori Z, Stransky A. Activity of bulbar respiratory neurones during cough and other respiratory tract reflexes in cats. Physiol. Bohemoslov. 1985;34:127–136. [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol. Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat. II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J. Comp. Neurol. 1980;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Knudson RJ, Mead J, Knudson DE. Contribution of airway collapse to supramaximal expiratory flows. J. Appl. Physiol. 1974;36:653–667. doi: 10.1152/jappl.1974.36.6.653. [DOI] [PubMed] [Google Scholar]
- Koestler A. The Ghost in the Machine. The Macmillan Company; New York: 1967. [Google Scholar]
- Korpas J. Differentiation of the expiration and the cough reflex. Physiol. Bohemoslov. 1972a;21:677–680. [PubMed] [Google Scholar]
- Korpas J. Expiration reflex from the vocal folds. Physiol. Bohemoslov. 1972b;21:671–675. [PubMed] [Google Scholar]
- Korpas J, Jakus J. The expiration reflex from the vocal folds. Acta Physiol. Hung. 2000;87:201–215. doi: 10.1556/APhysiol.87.2000.3.1. [DOI] [PubMed] [Google Scholar]
- Korpas J, Tomori Z. Cough and other respiratory reflexes. S. Karger; Basel, New York: 1979. [Google Scholar]
- Lang IM, Dana N, Medda BK, Shaker R. Mechanisms of airway protection during retching, vomiting, and swallowing. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G529–G536. doi: 10.1152/ajpgi.00062.2002. [DOI] [PubMed] [Google Scholar]
- Levy MN, Berne RM, Koeppen BM, Stanton BA. Berne and Levy Principles of Physiology. Elsevier Mosby; St. Louis, MO: 2006. [Google Scholar]
- Li Z, Morris KF, Baekey DM, Shannon R, Lindsey BG. Multimodal medullary neurons and correlational linkages of the respiratory network. J. Neurophysiol. 1999;82:188–201. doi: 10.1152/jn.1999.82.1.188. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Hernandez YM, Morris KF, Shannon R, Gerstein GL. Dynamic reconfiguration of brain stem neural assemblies: respiratory phase-dependent synchrony versus modulation of firing rates. J. Neurophysiol. 1992;67:923–930. doi: 10.1152/jn.1992.67.4.923. [DOI] [PubMed] [Google Scholar]
- Lindsey BG, Morris KF, Segers LS, Shannon R. Respiratory neuronal assemblies. Respir. Physiol. 2000;122:183–196. doi: 10.1016/s0034-5687(00)00158-4. [DOI] [PubMed] [Google Scholar]
- Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J. Physiol. 1991;443:55–77. doi: 10.1113/jphysiol.1991.sp018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL. Central nervous system control of the laryngeal muscles in humans. Respir. Physiol. Neurobiol. 2005;147:205–222. doi: 10.1016/j.resp.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May AJ, Widdicombe JG. Depression of the cough reflex by pentobarbitone and some opium derivatives. Br. J. Pharmacol. Chemother. 1954;9:335–340. doi: 10.1111/j.1476-5381.1954.tb01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J. Physiol. 2001;533:41–50. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T, Hiraga K, Honda Y. Inhibitory effects of CO2 on airway defensive reflexes in enflurane-anesthetized humans. J. Appl. Physiol. 1989;66:2642–2646. doi: 10.1152/jappl.1989.66.6.2642. [DOI] [PubMed] [Google Scholar]
- Oku Y, Tanaka I, Ezure K. Activity of bulbar respiratory neurons during fictive coughing and swallowing in the decere-brate cat. J. Physiol. 1994;480(Pt 2):309–324. doi: 10.1113/jphysiol.1994.sp020361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Paton JF, Dick TE, St-John WM, Morschel M, Dutschmann M. Modeling the ponto-medullary respiratory network. Respir. Physiol. Neurobiol. 2004;143:307–319. doi: 10.1016/j.resp.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Saito Y, Ezure K, Tanaka I, Osawa M. Activity of neurons in ventrolateral respiratory groups during swallowing in decerebrate rats. Brain Dev. 2003;25:338–345. doi: 10.1016/s0387-7604(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Sant’Ambrogio G, Sant’Ambrogio FB, Davies A. Airway receptors in cough. Bull. Eur. Physiopathol. Respir. 1984;20:43–47. [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Li Z, Lindsey BG. Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat. J. Physiol. 2000;525(Pt 1):207–224. doi: 10.1111/j.1469-7793.2000.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Brainstem respiratory networks and cough. Pulmonol. Pharmacol. 1996;9:343–347. doi: 10.1006/pulp.1996.0045. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J. Appl. Physiol. 1998;84:2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Pontine respiratory group neuron discharge is altered during fictive cough in the decerebrate cat. Respir. Physiol. Neurobiol. 2004a;142:43–54. doi: 10.1016/j.resp.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG. Production of reflex cough by brainstem respiratory networks. Pulmonol. Pharmacol. Ther. 2004b;17:369–376. doi: 10.1016/j.pupt.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J. Appl. Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Tatar M, Korpas J, Polacek H, Zahradny V. Changes induced by severe hypoxia in respiratory defence reflexes in anaesthetized cats. Respiration. 1986;49:114–121. doi: 10.1159/000194868. [DOI] [PubMed] [Google Scholar]
- Wang C, Baekey DM, Morris KF, Nuding SC, Lindsey BG, Shannon R, Bolser DC. Discharge identity of medullary inspiratory neurons is altered during repetitive fictive cough. FASEB J. 2005;19:A655. doi: 10.3389/fphys.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]