Abstract
Background
Clinical signs often fail to identify stroke patients who are at increased risk of aspiration. We hypothesized that objective measure of voluntary cough would improve the accuracy of the clinical evaluation of swallow to predict those patients who are at risk.
Methods
A comprehensive diagnostic evaluation was completed for 96 consecutive stroke patients that included cognitive testing, a bedside clinical swallow examination, aerodynamic and sound pressure level measures of voluntary cough, and “gold standard” instrumental swallowing studies (ie, videofluoroscopic evaluation of swallow [VSE] or fiberoptic endoscopic evaluation of swallow [FEES]). Stroke severity was assessed retrospectively using the Canadian neurologic scale.
Results
Based on the findings of VSE/FEES, 33 patients (34%) were at high risk of aspiration and (66%) were nonaspirators. Clinical signs (eg, absent swallow, difficulty handling secretions, or reflexive cough after water bolus) had an overall accuracy of 74% with a sensitivity of 58% and a specificity of 83% for the detection of aspiration. Three objective measures of voluntary cough (expulsive phase rise time, volume acceleration, and expulsive phase peak flow) were each associated with an aspiration risk category (areas under the curves were 0.93, 0.92, and 0.86, respectively). Expulsive phase rise time > 55 m/s, volume acceleration < 50 L/s/s, and expulsive phase peak flow < 2.9 L/s had sensitivities of 91%, 91%, and 82%, respectively; and specificities of 81%, 92%, and 83%, respectively for the identification of aspirators.
Conclusion
Objective measures of voluntary cough can identify stroke patients who are at risk for aspiration and may be useful as an adjunct to the standard bedside clinical assessment.
Keywords: aspiration, cough, deglutition, diagnosis, dysphagia, pneumonia, stroke, voluntary cough
Poststroke dysphagia is associated with several complications, including acute malnutrition, dehydration, pneumonia, and airway obstruction, which can lead to longer hospitalizations and poorer quality of life.1-3 Up to 50% of stroke patients studied with videofluoroscopic evaluation of swallow (VSE) have dysfunctional swallowing, and up to a third of these dysphagic patients aspirate.4-6 Pneumonia develops in more than one third of aspirators, and 3.8% of patients die as result of this complication. Importantly, early detection of swallowing deficits is associated with lower pneumonia rates.1
Screening stroke patients for dysphagia within 24 h of hospital admission is mandated within the Veterans Health Administration System and is one of the quality indicators for Joint Commission primary stroke center certification.7,8 Subjectively assessed clinical signs and patient self-report of swallowing difficulties are commonly used to screen for aspiration risk, but have limited sensitivity and specificity.6,9-11 Clinical evaluation of swallowing may be further limited by cognitive impairments and language deficits, but little is known about their relationship to poststroke dysphagia or aspiration risk.12
Cough has traditionally been a key component of the bedside clinical evaluation of swallowing. Cough and swallow behaviors share anatomic structures, and functionally both keep the airway clear of food and secretions. Upper airway laryngeal motor functions are important components of both cough and swallowing. Involuntary cough or reflexive cough in response to the presence of liquids or solid food in the laryngeal area protects against tracheobronchial aspiration. The term voluntary cough refers to a cough that is produced on command and is not related to eating or drinking.13
Objective diagnostic studies including VSE14 or fiberoptic endoscopic evaluation of swallow (FEES)15 are equally sensitive to the detection of aspiration and are considered the current “gold standards” for swallowing evaluation. The tests also provide information that is helpful in designing treatment programs. These tests, however, are often not readily available. In combination with other clinical indicators, subjective assessment of voluntary cough can be used to determine which stroke patients need to undergo VSE or FEES.11 The subjective nature of the assessment leads to observer variability that can be reduced through the use of objective measures of voluntary cough that can be easily performed in a variety of settings and readily included in the clinical swallowing evaluation.16 Objective assessment of voluntary cough, however, is not currently part of routine aspiration screening, and its clinical utility is unknown.
We hypothesized that objective measures of voluntary cough would have greater sensitivity and specificity in predicting aspiration compared to the findings on clinical examination that are often used to identify patients with elevated aspiration risk, and that the addition of these cough measures to the clinical evaluation would improve its accuracy. A secondary study objective was to extend the current understanding of the clinical profile of dysphagic stroke patients by determining the proportion of patients having concomitant speech and cognitive deficits that could affect the swallowing evaluation.
Materials and Methods
Subjects were consecutive consenting patients who had recently experienced an ischemic stroke who were admitted to the Durham (NC) Veterans Affairs Medical Center (DVAMC) between November 2000 and November 2002 in whom the necessary tests could be scheduled (n = 96). Patients with a history of radiation therapy to the head and neck, brain tumor, or brain surgery were excluded. The Institutional Review Board of the DVAMC approved the study protocol.
Subject characteristics including comorbidities were abstracted from the medical record. The location of the stroke was classified as cortical, subcortical, brainstem, multiple (if more than one area was affected), and hemisphere based on clinical features as indicated in the medical record and verified by a review of brain CT scans and/or MRI scans by a neuroradiologist who was unaware of the results of the swallowing evaluations. Initial stroke severity and stroke severity on the day of the swallowing evaluation were retrospectively measured with the Canadian neurologic stroke scale (CNSS), the scores of which range from 11.5 (least severe) to 0 (most severe).17 The presence or absence of speech deficits and orientation at hospital admission were abstracted from the medical record.
Speech language pathologists performed a clinical swallowing evaluation, cognitive screening, and aerodynamic and sound pressure level measurements of voluntary cough immediately before or after the VSE (n = 91) or FEES (n = 5), the order of which was determined solely by the clinical schedules of the Speech Pathology and Radiology Services at the DVAMC. The speech language pathologist was unaware of the results of the VSE or FEES when conducting the objective assessment of cough in those subjects who had already undergone the test. The clinical swallowing evaluation, which was performed at the bedside, included assessments of reflexive cough or choking after water and/or ice chip boluses and noted an absent swallow reflex on any trial for which the command to swallow was given. The speech language pathologist administered an ice chip bolus then observed the subject for reflexive cough and choking. If there was no reaction, the subject was instructed to take a small sip of water from a cup and the clinician repeated observation for coughing or choking. The speech language pathologist placed a hand on the laryngeal area to ensure that a swallow occurred. Subjects were classified as aspirators based on the clinical assessment if any of the three assessments were judged as abnormal.
The neurobehavioral cognitive status examination (NCSE) was used for cognitive screening and was performed on the day of the clinical swallowing evaluation.18 NCSE scores reflect normal function, or mild, moderate, or severe cognitive impairment through the assessment of the following: orientation (mild problem, 8/12 items correct; moderate problem, 6/12 items correct; and severe problem, ≤ 4/12 items correct); attention (mild problem, 5/8 items correct; moderate problem, 3/8 items correct; and severe problem, ≤ 1/8 items correct); auditory comprehension (mild problem, 4/6 items correct; moderate problem, 3/6 items correct; or severe problem, ≤ 2/6 items correct); repetition (mild problem, 9/12 items correct; moderate problem, 7/12 items correct; severe problem, ≤ 5/12 items correct); naming (mild problem, 5/8 items correct; moderate problem, 3/8 items correct; or severe problem, ≤ 2/8 items correct); memory (mild problem, 8/12 items correct; moderate problem, 6/12 items correct; or severe problem, ≤ 4/12 items correct); reasoning, which included similarities (mild problem, 4/8 items correct; moderate problem, 3/8 items correct; severe problem, ≤ 2/8 items correct); and judgment (mild problem, 3/6 items correct; moderate problem, 2/6 items correct; severe problem, ≤ 1/6 items correct). Patients were categorized as “cognitively impaired” if there was a moderate or severe impairment for any of the cognitive domains assessed by the NCSE. The potential impact of cognitive impairments was also analyzed based on a notation of “disorientation” in the patient’s medical record.
Methods for the objective measurement of sound pressure level and aerodynamic characteristics of cough have been described previously.16 Briefly, cough characteristics include duration, volume and peak flow of the inspiration phase, compression phase duration, peak flow and rise time for the expulsive phase, and volume acceleration (expulsive phase peak flow/expulsive phase rise time).16 Measurements of aerodynamic and sound pressure levels of voluntary cough were obtained by one speech language pathologist using a computerized software/hardware analysis system (Perci SAR system; Microtronics, Corp; Chapel Hill, NC). An airtight mask was fitted over the oral-nasal area (Glottal Enterprises; Syracuse, NY). Airflow was recorded with a differential pressure transducer connected to the pneumotacho-graph built into the facemask. Cough airflows were low-pass filtered to 50 Hz with a 3-dB rolloff at 90 dB per octave. A calibrated microphone was attached to the facemask to record the sound pressure level during cough. Subjects were evaluated for their ability to follow commands by verbal cue or imitation, and all were awake and alert at the time of all evaluations. Initially, subjects were instructed to breathe quietly for 30 s with the mask in place to obtain the respiratory rate. Subjects were subsequently requested to voluntarily produce a “strong” cough for three trials. The airflow and microphone signals were amplified, displayed in real time and on a video monitor, digitized, and stored for later analysis.
VSE or FEES was performed with the subjects seated in the upright position, and the study was videotaped for later analysis. The liquids were prepared to match the following liquids available to the inpatient at volumes of 5 and 15 mL, and unregulated drinks from a cup: thin liquids (3 to 8 centipoise [cP]); nutritional liquids (Ensure Plus; Abbott Laboratories; Columbus, OH) [30 to 35 cP]; and thickened liquids (250 to 300 cP). Further administration of a bolus of liquid of a particular consistency was terminated if aspiration occurred, despite the application of therapeutic interventions (eg, use of chin-tuck or head-turn maneuvers). The VSE was performed in the radiology suite, and the subjects were seated in a chair designed for this purpose. The VSE appears as a moving picture radiograph of the bone, cartilage, and soft-tissue swallowing structures, as the food and liquid mixed with barium passed through the oral, pharyngeal, and esophageal stages of the swallow. The FEES involved passing a flexible nasopharyngo-scope transnasally to allow a superior view of the pharynx and larynx, from which swallowing may be assessed. Because pharyngeal contraction obstructs the lumen of the scope, the motion of the pharynx “during the swallow” is not visualized. However, this procedure can identify laryngeal penetration and aspiration with direct observation of bolus material in the larynx or trachea.
A speech language pathologist who was unaware of the results of the cough evaluation analyzed the VSE and FEES results using the penetration-aspiration scale score (PASS), which is an 8-point scale designed to quantify selected aspects of swallowing (Table 1). This scale measures the depth of upper airway (laryngeal and proximal tracheal area) invasion and whether material entering the airway (trachea) is expelled.19 Patients were classified based on the highest level of the penetration aspiration scale that occurred for any swallow. Subjects were classified as being at high aspiration risk if the score was ≥ 5 (Table 1).
Table 1.
Patients in Each PASS Category*
| PASSs | Description (Based on VSE or FEES) | Patients, No. (%) |
|---|---|---|
| Nonaspirated patients | ||
| 1 | No material entered larynx | 33 (34.38) |
| 2 | Material entered laryngeal area above the TVCs with no residue |
12 (12.50) |
| 3 | Material enters laryngeal area above the TVCs and residue remained in the laryngeal area after the swallow |
3 (3.13) |
| 4 | Material contacts TVCs with no residue |
15 (15.63) |
| Total | 63 (65.63) | |
| Aspirated patients |
||
| 5 | Material contacts TVCs with visible residue |
5 (5.21) |
| 6 | Material passes the glottis with no subglottic residue |
0 (0.00) |
| 7 | Material passes glottis with visible subglottic residue despite patient’s response (spontaneous reflexive cough, throat clear) |
10 (10.41) |
| 8 | Material passes glottis, visible subglottic residue in proximal trachea and absent patient spontaneous reflexive cough (silent aspiration) |
18 (18.75) |
| Total | 33 (34.37) | |
TVC = true vocal cord. Description of the PASS and scores for all subjects.
Statistical Analysis
Continuous variables were summarized as means. Binary and categorical variables were summarized as frequencies (ie, percentages). Means were compared between patients who were at risk for aspiration (penetration aspiration scale score ≥ 5) and those who were not at risk (penetration aspiration scale score < 5) by analysis of covariance to adjust for demographic and clinical characteristics. Stroke severity was analyzed based on the CNSS score as both an ordinal scale and a trichotomized scale (severe, 0 to 5.5; moderate, 6 to 8.5; mild, 9 to 11.5). Associations between aspiration and other categorical variables were assessed using a Pearson χ2 test. Logistic regression models were used to predict the odds of aspiration using objective cough measures after adjusting for demographic and clinical characteristics. The diagnostic accuracy of each objective cough measure was assessed using a receiver operator characteristic (ROC) curve. An overall sensitivity (and specificity) given all possible specificities (sensitivities) was evaluated using the area under the curve (AUC). The overall sensitivity and specificity were considered “excellent,” “very good,” “good,” “moderate,” and “poor” if the AUC was 90 to 100%, 80 to 89%, 70 to 79%, 60 to 69%, and < 60%, respectively.20 The sensitivities and specificities of specific values (thresholds) of the objective cough measures were compared with that of the clinical evaluation using the McNemar test. To assess the interrater reliability of the swallowing evaluations, a speech language pathologist who was unaware of the original evaluation results reviewed a random 10% sample of these evaluations. Agreement between raters was assessed using κ statistics. All statistical tests were performed using a statistical software package (SAS, version 9.1; SAS Institute; Cary, NC). ROC curves were plotted and analyzed using other software (Splus, version 6.2; Insightful Corp; Durham, NC). A p value <0.05 was considered to be statistically significant.
Results
The results of the VSE or FEES are listed in Table 1. Thirty-three subjects (34%) were classified as being at high risk for aspiration (penetration aspiration scale score, ≥ 5), and 63 subjects were nonaspirators (penetration aspiration scale score, ≤ 4). The interrater reliability for the instrumental swallow evaluations was 0.88 (95% confidence interval [CI], 0.82 to 0.92), reflecting excellent agreement.
Table 2 summarizes the subjects’ demographic and clinical characteristics categorized by the presence or absence of risk for aspiration. The mean ± SE interval between stroke onset and the swallowing evaluation was 6.0 ± 0.7 days (median interval, 4.0 days; range, 1 to 33 days). A total of 46% of evaluations were conducted within 3 days of stroke onset (within 7 days, 74%; within 10 days, 86%; within 30 days, 95%). Over one third of patients had a history of coronary artery disease, diabetes, or cerebrovascular disease. Clinical signs of aspiration, speech problems, disorientation, cognitive deficits, and mortality were substantially more likely to be present among those patients at high risk of aspiration compared to the nonaspirating subjects (Table 2). When those patients who were at high risk of aspiration were compared with those who were not at risk for aspiration, there was no difference for the clinical characteristics of shortness of breath (18.2% vs 7.9%, respectively; p = 0.13) or lung disease (18.2% vs 19.1%, respectively; p = 0.92). A clinical history of the following conditions was not related to aspiration, including: alcoholism (24.2% vs 14.3%, respectively; p = 0.22); arthritis (9.1% vs 15.9%, respectively; p = 0.36); coronary artery bypass grafting (18.2% vs 15.9%, respectively; p = 0.77); cancer (33.3% vs 15.9%, respectively; p = 0.07); cardiac problems (39.4% vs 28.6%, respectively; p = 0.28); carotid end-arterectomy (3.0% vs 3.2%, respectively; p = 0.97); cerebellar signs (6.4% vs 6.1%, respectively; p = 0.96); COPD (21.2% vs 15.9%, respectively; p = 0.51); cerebrovascular accident (48.5% vs 36.5%, respectively; p = 0.26); dementia (21.9% vs 11.11%, respectively; p = 0.21); depression (27.3% vs 15.9%, respectively; p = 0.18); diabetes (39.4% vs 39.7%, respectively; p = 0.98); esophageal problems (15.1% vs 25.4%, respectively; p = 0.25); gastroesophageal reflux disease (15.2% vs 24.2%, respectively; p = 0.30); hepatitis C (3.0% vs 0%, respectively; p = 0.16); hypertension (84.9% vs 79.4%, respectively; p = 0.51); hypoglycemia (3.0% vs 0%, respectively; p = 0.16); pulmonary edema (12.1% vs 4.8%, respectively; p = 0.19); renal problems (15.2% vs 19.1%, respectively; p = 0.63); and a history of transient ischemic attack (15.2% vs 15.98%, respectively; p = 0.934).
Table 2.
Summary of Patient Demographic and Clinical Characteristics*
| Aspiration |
||||
|---|---|---|---|---|
| Characteristics | Total (n = 96) | No (n = 63) |
Yes (n = 33) |
p Value† |
| Age, yr | 67.77 (1.19) | 67.00 (1.51) | 69.24 (1.94) | 0.3747 |
| Black race | 40.43 | 40.32 | 40.63 | 0.9774 |
| SOB | 11.46 | 7.94 | 18.18 | 0.1344 |
| Lung disease | 18.75 | 19.05 | 18.18 | 0.9178 |
| Level of lesion | ||||
| Cortical | 29.03 | 24.19 | 38.71 | 0.2897 |
| Subcortical | 12.90 | 14.52 | 9.09 | |
| Brain stem | 3.23 | 4.84 | 0.00 | |
| Cerebellum | 4.30 | 6.45 | 0.00 | |
| Multiple levels | 44.09 | 45.16 | 39.39 | |
| Do not know | 6.45 | 4.84 | 9.09 | |
| Laterality of lesion | ||||
| Left | 30.11 | 29.03 | 32.26 | 0.8208 |
| Right | 18.28 | 20.97 | 12.12 | |
| Bilateral | 43.01 | 41.94 | 42.42 | |
| Do not know | 8.60 | 8.06 | 9.09 | |
| Cognitive deficit | 53.13 | 42.86 | 72.73 | 0.0053 |
| Patient’s clinical history | ||||
| Aspiration pneumonia | 9.47 | 1.61 | 24.24 | 0.0003 |
| Pneumonia | 7.29 | 1.59 | 18.18 | 0.0030 |
| Mortality | ||||
| Death in 3 mo | 14.58 | 4.76 | 33.33 | 0.0002 |
| Death in 18 mo | 27.08 | 17.46 | 45.45 | 0.0034 |
Values are given as the mean (SE) or %, unless otherwise indicated. Yes = PASS ≥ 5; No = PASS < 5; SOB = shortness of breath; Death in 3 mo = death within 3 mo of swallowing evaluation; Death in 18 mo = death within 18 mo of swallowing evaluation.
From t tests for continuous variables and Pearson χ2 tests for categorical and binary variables.
Only 1 of the 63 nonaspirators (1.6%) had a history of pneumonia compared to 24.2% of those patients who were at high aspiration risk (8 of 33 patients). The mortality rates for the 3 months following the swallowing evaluation were 4.8% in nonaspirators (3 of 63 patients) and 33.3% in aspirators (11 of 33 patients; p = 0.0002). The mortality rates after 18 months were 17.5% in nonaspirators (11 of 63 patients) and 45.5% in aspirators (15 of 33 patients; p = 0.003).
All objective cough measures except inspiration phase duration and glottic closure phase duration were associated with aspiration risk (Table 3). In particular, patients with a high risk of aspiration had lower inspiration phase volume, inspiration phase peak flow, sound pressure level, expiration phase peak flow, and volume acceleration, and higher expulsive phase rise times than nonaspirating patients. CNSS scores were lower (ie, more severe) in aspirating patients at both the time of stroke and the time of the swallowing evaluations. Logistical regression showed that the odds of aspiration were independently associated with inspiratory phase peak flow, sound pressure level, expiratory phase peak flow, expulsive phase rise time, volume acceleration, and CNSS scores at the time of swallowing evaluation after adjusting for age, race, and various clinical factors (Table 4).
Table 3.
Summary of Objective Cough Measures and CNS*
| Aspiration |
||||
|---|---|---|---|---|
| Variables | All (n = 96) | No (n = 63) | Yes (n = 33) | p Value† |
| Inspiration phase duration, s | 0.94 (0.03) | 0.91 (0.04) | 1.00 (0.07) | 0.1872 |
| Inspiration phase volume, L | 0.61 (0.05) | 0.69 (0.06) | 0.45 (0.08) | 0.0284 |
| Inspiration phase peak flow, L/s | 1.23 (0.08) | 1.44 (0.09) | 0.82 (0.11) | 0.0001 |
| Glottic phase duration, s | 0.25 (0.02) | 0.25 (0.02) | 0.25 (0.03) | 0.8914 |
| Sound pressure level, dB | 91.96 (1.41) | 96.41 (1.01) | 83.74 (3.10) | < 0.0001 |
| Expulsive phase peak flow, L/s | 4.36 (0.32) | 5.62 (0.38) | 1.98 (0.27) | < 0.0001 |
| Expulsive phase rise time, ms | 95.06 (10.62) | 14.05 (28.26) | 161.50 (27.73) | < 0.0001 |
| Volume acceleration, L/s/s | 97.42 (9.39) | 136.15 (11.20) | 23.49 (6.10) | < 0.0001 |
| CNSS score onsite | 8.85 (0.20) | 9.40 (0.19) | 7.80 (0.40) | < 0.0001 |
| CNSS at swallowing | 9.01 (0.20) | 9.56 (0.19) | 7.95 (0.38) | < 0.0001 |
Values are given as the mean (SE), unless otherwise indicated. CNSS score onsite = CNSS score at the time of administration (ie, stoke onset); CNSS at swallowing = CNSS score at the time of swallowing evaluation.
From analyses of covariance adjusting for age and race.
Table 4.
Summary of Predictors in Logistic Regression Models*
| Unadjusted Model |
Adjusted Model |
|||
|---|---|---|---|---|
| Predictors | OR (SE) | p Value† | OR (SE) | p Value† |
| Inspiration phase duration, s | 2.35 (1.54) | 0.1933 | 3.26 (2.91) | 0.1851 |
| Inspiration phase volume, L | 0.23 (0.15) | 0.0225 | 0.33 (0.25) | 0.1509 |
| Inspiration phase peak flow, L/s | 0.21 (0.09) | 0.0003 | 0.28 (0.17) | 0.0334 |
| Glottic phase duration, s | 1.01 (1.16) | 0.9948 | 0.84 (1.50) | 0.9211 |
| Sound pressure level, dB | 0.87 (0.03) | < 0.0001 | 0.90 (0.04) | 0.0219 |
| Expulsive phase peak flow, L/s | 0.45 (0.08) | < 0.0001 | 0.52 (0.12) | 0.0034 |
| Expulsive phase rise time, ms | 1.08 (0.02) | 0.0002 | 1.11 (0.04) | 0.0037 |
| Volume acceleration, L/s/s | 0.96 (0.01) | < 0.0001 | 0.96 (0.01) | 0.0006 |
| CNSS score onsite | 0.64 (0.09) | 0.0009 | 0.79 (0.12) | 0.1053 |
| CNSS score at swallowing | 0.62 (0.08) | 0.0004 | 0.75 (0.11) | 0.0582 |
The prediction using objective cough measures and CNSS score were unadjusted (adjusted) by demographic and clinical characteristics (eg, age, race, clinical signs of aspiration, speech problems, disorientation, and cognitive deficit) in the unadjusted (adjusted) logistic regression model. See Table 3 for abbreviations not used in the text.
From logistic regression models.
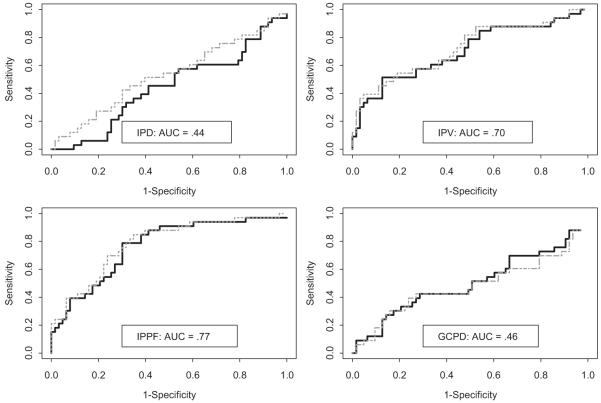
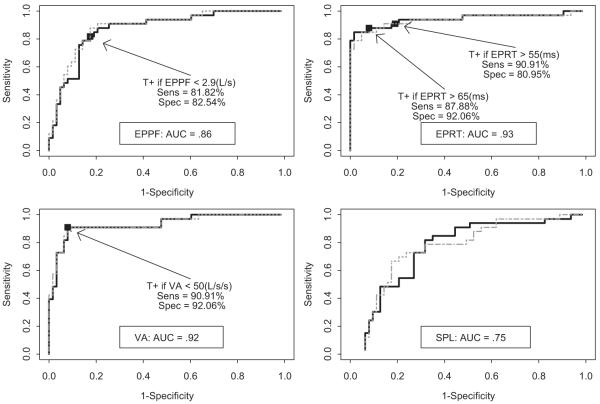
ROC curves using objective cough measures with and without adjustment for CNSS scores are presented in Figures 1 and 2. Only inspiratory phase duration and glottic closure phase duration were found to have poor diagnostic accuracy for identifying those patients at high aspiration risk (AUC < 50%) [Fig 1]. Expulsive phase peak flow, expulsive phase rise time, and volume acceleration had AUCs of 0.86, 0.93, and 0.92, respectively, indicating good diagnostic accuracy (Fig 2).
Figure 1.
ROC curves for objective cough measures. IPD = inspiration phase duration; IPV = inspiration phase volume; IPPF = inspiration phase peak flow; GCPD = glottic closure phase duration. Solid (dotted) curves are ROC curves unadjusted (adjusted) for CNSS score. Aspiration is defined as a PASS ≥ 5.
Figure 2.
ROC curves for objective cough measures. EPPF = expulsive phase peak flow; EPRT = expulsive phase rise time; VA = volume acceleration (or EPPF/EPRT); SPL = sound pressure level; T+ = test positive; Sens = sensitivity; Spec = specificity. Solid (dotted) curves are ROC curves unadjusted (adjusted) for CNSS score. Aspiration is defined as a PASS ≥ 5.
The sensitivity and specificity to predict the risk for aspiration on VSE or FEES associated with the set of commonly used clinical signs of aspiration were 57.6% (95% CI, 39.3 to 75.9%) and 82.5% (95% CI, 73.7 to 91.4%), respectively. Reflexive cough after water or ice chip bolus had a sensitivity of 39% (95% CI, 22.4 to 55.6%) and a specificity of 82% (95% CI, 72.7 to 91.3%). Cutoffs of expulsive phase peak flow < 2.9 L/s, expulsive phase rise time > 65 ms, and volume acceleration < 50 L/s had specificities of 82.5% (95% CI, 73.3 to 91.8%), 92.1% (95% CI, 85.5 to 98.6%), and 92.1% (95% CI, 85.5 to 98.6%), respectively, and sensitivities of 81.8% (95% CI, 68.7 to 95%), 87.9% (95% CI, 76.7 to 99%), and 90.9% (95% CI, 81.1 to 100%), respectively. These sensitivities were each higher than the clinical assessment (p < 0.05, 0.02 and 0.01, respectively) [Table 5]. Similar results were obtained with a more rigorous definition of aspiration (PASS, ≥ 6vs ≥ 5, respectively; results not shown).
Table 5.
Diagnostic Improvement Using Voluntary Cough Examination
| Variables | Volume Acceleration* |
Expulsive Phase Rise Time† |
Expulsive Phase Peak Flow‡ |
|---|---|---|---|
| Probability of correcting mistakes caused by using clinical signs | |||
| Types of mistakes made by using clinical signs§ | |||
| False negative (n = 14) | 100% (14/14 patients) | 93% (13/14 patients) | 93% (13/14 patients) |
| False positive (n = 11) | 100% (11/11 patients) | 100% (11/11 patients) | 91% (10/11 patients) |
| Probability of making mistakes in patients receiving correct diagnoses by use of clinical signs | |||
| Types of correctness made by using clinical signs∥ | |||
| True negative (n = 52) | 10% (5/52 patients) | 10% (5/52 patients) | 19% (10/52 patients) |
| True positive (n = 19) | 19% (3/19 patients) | 19% (3/19 patients) | 31% (5/19 patients) |
Positive test result is defined as volume acceleration < 50 L/s/s.
Positive test result is defined as expulsive phase rise time > 55 ms.
Positive test result is defined as expulsive phase peak flow < 2.9 L/s.
False negative or false positive indicates patients who received a false-negative or false-positive diagnosis by using the clinical signs.
True negative or true positive indicates patients who received a correct negative or positive diagnosis by using the clinical signs.
Discussion
The primary finding of this analysis is that two of the objective measures of voluntary cough, expulsive phase rise time and volume acceleration, are independently associated with aspiration risk as measured by the VSE or FEES, which are tests generally considered to be “gold standards.” The prevalence of aspiration in this cohort was consistent with that reported in other studies4-6 of similarly aged acute stroke patients that also used the VSE, suggesting that our results are likely generalizable. Of note, these objective measures of cough appear to be better in identifying stroke patients who are at risk of aspiration than routinely used clinical assessments.
The relationship of abnormal voluntary cough to aspiration risk in patients with acute stroke is likely due to deficits of the systems that underlie both swallowing and cough. The results suggest that that the efficacy of cough may be as important if not more important than deficits in swallowing in determining aspiration risk.
We found that three of four aspirators had cognitive deficits and nearly 90% had speech and/or language deficits. These deficits may compromise a patient’s ability to report swallowing problems and to understand questions during the clinical evaluation. Cognitive problems have been implicated as a confounder in a retrospective study12 of patients with acute stroke in which hemispatial neglect and aphasia were found in 73% and 44% of aspirators, respectively.
Similar to the present results, other studies have reported that subjective clinical assessments have limited capacity to identify stroke patients who are at high aspiration risk. For example, one study6 found low sensitivities and specificities associated with those characteristics of reflexive cough after water swallow that are commonly thought to predict aspiration, including its strength (sensitivity, 24%; specificity, 80%) and quality (sensitivity, 38%; specificity, 77%). Subjective assessments of voluntary cough had similar poor performance, as follows: strength (sensitivity, 42%; specificity, 79%); and quality (sensitivity, 26%; specificity, 89%).6 Oral motor signs associated with aspiration included unilateral weak palatal movement during gag (sensitivity, 56%; specificity, 51%) and weak bilateral movement (sensitivity, 56%; specificity, 60%). The voice behaviors tested were strained voice (sensitivity, 30%; specificity, 92%), wet/gurgly voice (sensitivity, 22%; specificity, 96%), and dysphonia (sensitivity, 54%; specificity, 86%).6 Based on such findings, up to 40% of patients who might benefit from interventions to avoid aspiration would not be identified based on clinical bedside assessments alone, and ≥ 15% might be given unnecessary dietary restrictions.
We found that stroke severity on the day of the swallowing evaluation, but not on the day of hospital admission, was independently associated with aspiration risk. Stroke severity, however, is a poor marker of those patients who are at-risk for aspiration. Aspirators (n = 33) were represented at all levels of stroke severity at hospital admission (mild stroke, 39.4% [13 of 33 patients]; moderate stroke, 42.4% [14 of 33 patients]; and severe stroke, 18.2% [6 of 33 patients]), as were the 63 nonaspirators (mild stroke, 71.4% [48 of 63 patients]; moderate stroke, 20.6% [12 of 63 patients]; and severe stroke, 4.8% [3 of 63 patients]). Those patients who were at high risk for aspiration and nonaspirators were also similarly distributed for stroke severity measured on the day of the swallowing evaluation. There were no other clinical characteristics that could be used as markers of aspiration.
An expulsive phase rise time of > 67 ms or a volume acceleration of < 33 mL/s/s correctly identified > 90% of aspirators and > 90% of those patients not requiring interventions to reduce their aspiration risk. It should be noted that the airflow signal was low-pass filtered, and that the characteristics of this filtering can influence the actual value of rise time. As such, the characteristics of this filtering are critical. Patient reaction to an inhaled irritant (ie, reflexive cough after tartaric acid inhalation) has also been proposed as a predictor of aspiration pneumonia in stroke patients.21 Future studies are required to directly evaluate the comparative utility of such alternative approaches.
The difference in expulsive phase rise time between those patients at high risk for aspiration and nonaspirators may be attributable to impaired function of the laryngeal and/or respiratory neuromuscular complexes. In a previous study,16 we found that expulsive phase rise times of healthy subjects were one third that of stroke patients (0.05 vs 0.15 s, respectively; p = 0.0001). Abnormal muscle function secondary to stroke, either because of weakness (hypotonia) or bradykinesia resulting from hypertonia, affects laryngeal and respiratory muscle movement, which would affect voluntary cough. The slower expulsive phase rise time of the voluntary cough in patients with stroke reported in this study may be due to a physiologic change that can be compared to healthy subjects with more rapid opening of normal function of the true vocal cords and respiratory musculature during the transition between the glottic closure and expulsive phase of cough. The longer expulsive phase time consistently observed in patients at risk for aspiration is likely caused by abnormal laryngeal and/or respiratory muscle function. This mechanism is supported by a study of cough in laryngectomized patients that showed expulsive phase rise time was longer in those without laryngeal muscles compared to control subjects.22
Our results suggest that objective measures of voluntary cough, alone or in conjunction with subjectively assessed clinical signs, may assist the clinician in more accurately identifying acute stroke patients who are at risk of aspiration. These findings will, however, require validation in an independent cohort.
Acknowledgments
This study was supported by the Department of Rehabilitation, Research and Development Service, Veterans Affairs National Headquarters.
Abbreviations
- AUC
area under the curve
- CI
confidence interval
- CNSS
Canadian neurologic stroke scale
- cP
centipoise
- DVAMC
Durham Veterans Affairs Medical Center
- FEES
fiberoptic endoscopic evaluation of swallow
- NCSE
neurobehavioral cognitive status examination
- PASS
penetration-aspiration scale score
- ROC
receiver operating characteristic
- VSE
videofluoroscopic evaluation of swallow
Footnotes
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Doggett DL, Tappe KA, Mitchell MD, et al. Prevention of pneumonia in elderly stroke patients by systematic diagnosis and treatment of dysphagia: an evidence-based comprehensive analysis of the literature. Dysphagia. 2001;16:279–295. doi: 10.1007/s00455-001-0087-3. [DOI] [PubMed] [Google Scholar]
- 2.Foley N, Finestone H, Woodbury MG, et al. Energy and protein intakes of acute stroke patients. J Nutr Health Aging. 2006;10:171–175. [PubMed] [Google Scholar]
- 3.Feinberg MJ, Ekberg O. Deglutition after near-fatal choking episode: radiologic evaluation. Radiology. 1990;176:637–640. doi: 10.1148/radiology.176.3.2389020. [DOI] [PubMed] [Google Scholar]
- 4.Daniels SK, Brailey K, Priestly DH, et al. Aspiration in patients with acute stroke. Arch Phys Med Rehabil. 1998;79:14–19. doi: 10.1016/s0003-9993(98)90200-3. [DOI] [PubMed] [Google Scholar]
- 5.Mann G, Dip PG, Hankey GJ, et al. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;30:744–748. doi: 10.1161/01.str.30.4.744. [DOI] [PubMed] [Google Scholar]
- 6.McCullough GH, Rosenbek JC, Wertz RT, et al. Utility of clinical swallowing examination measures for detecting aspiration post-stoke. J Speech Lang Hear Res. 2005;48:1280–1293. doi: 10.1044/1092-4388(2005/089). [DOI] [PubMed] [Google Scholar]
- 7.Management of patients with swallowing (dysphagia) or feeding disorders. Department of Veterans Affairs Veterans Health Administration; Washington, DC: May 17, 2006. VHA directive 2006-032. [Google Scholar]
- 8.Alberts MJ, Hademenos G, Latchaw RE, et al. Recommendations for the establishment of primary stroke centers. JAMA. 2000;283:3102–3109. doi: 10.1001/jama.283.23.3102. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey DJ, Smithard DG, Kalra L. Early assessment of dysphagia and aspiration risk in acute stroke patients. Stroke. 2003;34:1252–1257. doi: 10.1161/01.STR.0000066309.06490.B8. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson H, Ekberg O, Olsson R, et al. Dysphagia in stroke: a prospective study of quantitative aspects of swallowing in dysphagic patients. Dysphagia. 1998;13:32–38. doi: 10.1007/PL00009547. [DOI] [PubMed] [Google Scholar]
- 11.Leder SB, Espinosa JP. Aspiration risk after acute stroke: comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17:214–218. doi: 10.1007/s00455-002-0054-7. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder MF, Daniels SK, McClain M, et al. Clinical and cognitive predictors of swallowing recovery in stroke. J Rehabil Res Dev. 2006;43:301–310. doi: 10.1682/jrrd.2004.12.0154. [DOI] [PubMed] [Google Scholar]
- 13.Smith Hammond CA, Goldstein LB. Cough and aspiration of food and liquids due to oral-pharyngeal dysphagia: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl):154S–168S. doi: 10.1378/chest.129.1_suppl.154S. [DOI] [PubMed] [Google Scholar]
- 14.Logemann JA. Evaluation and treatment of swallowing disorders. College-Hill Press; San Diego, CA: 1986. [Google Scholar]
- 15.Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia. 1988:216–219. doi: 10.1007/BF02414429. [DOI] [PubMed] [Google Scholar]
- 16.Smith Hammond CA, Goldstein LB, Zajac DJ, et al. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56:502–506. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein LB, Chilkuri V. Retrospective assessment of initial stroke severity with the Canadian neurological scale. Stroke. 1997;28:1181–1184. doi: 10.1161/01.str.28.6.1181. [DOI] [PubMed] [Google Scholar]
- 18.Northern California Neurobehavioral Group . The neurobehavioral cognitive status examination. The Northern California Neurobehavioral Group; Fairfax, CA: 1995. [Google Scholar]
- 19.Robbins JA, Levine R, Wood J, et al. Differentiation of normal and abnormal airway protection during swallowing using the penetration aspiration scale. Dysphagia. 1999;14:228–232. doi: 10.1007/PL00009610. [DOI] [PubMed] [Google Scholar]
- 20.Sackett DL, Haynes RB, Guyatt GH, et al. Clinical epidemiology: a basic science for clinical medicine. 2nd ed. Little, Brown and Co; Boston, MA: 1991. [Google Scholar]
- 21.Addington WR, Stephens RE, Gilliland KA. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke: an interhospital comparison. Stroke. 1999;30:1203–1207. doi: 10.1161/01.str.30.6.1203. [DOI] [PubMed] [Google Scholar]
- 22.Fontana GA, Pantaleo T, Lavorini F, et al. Coughing in laryngectomized patients. Am J Respir Crit Care Med. 1999;160:1578–1584. doi: 10.1164/ajrccm.160.5.9901093. [DOI] [PubMed] [Google Scholar]