Abstract
MiRNAs regulate cardiac development, hypertrophy, and angiogenesis, but their role in cardiac hypertrophy (CH) induced by aerobic training has not previously been studied. Aerobic training promotes physiological CH preserving cardiac function. This study assessed involvement of miRNAs-29 in CH of trained rats. Female Wistar rats (n = 7/group) were randomized into three groups: sedentary (S), training 1 (T1), training 2 (T2). T1: swimming sessions of 60 min/5 days/wk/10 wk. T2: similar to T1 until 8th wk. On the 9th wk rats swam 2×/day, and on the 10th wk 3×/day. MiRNAs analysis was performed by miRNA microarray and confirmed by real-time PCR. We assessed: markers of training, CH by ratio of left ventricle (LV) weight/body wt and cardiomyocytes diameter, pathological markers of CH (ANF, skeletal α-actin, α/β-MHC), collagen I and III (COLIAI and COLIIIAI) by real-time PCR, protein collagen by hydroxyproline (OH-proline) concentration, CF and CH by echocardiography. Training improved aerobic capacity and induced CH. MiRNAs-1, 133a, and 133b were downregulated as observed in pathological CH, however, without pathological markers. MiRNA-29c expression increased in T1 (52%) and T2 (123%), correlated with a decrease in COLIAI and COLIIIAI expression in T1 (27%, 38%) and T2 (33%, 48%), respectively. MiRNA-29c was inversely correlated to OH-proline concentration (r = 0.61, P < 0.05). The E/A ratio increased in T2, indicating improved LV compliance. Thus, these results show that aerobic training increase miR-29 expression and decreased collagen gene expression and concentration in the heart, which is relevant to the improved LV compliance and beneficial cardiac effects, associated with aerobic high performance training.
Keywords: cardiac hypertrophy, collagen, molecular markers, swimming training, physiological cardiac hypertrophy, diastolic function
microRNAs (miRNAs) are recognized as a new class of gene expression regulators, consisting of short RNAs, single-stranded, that do not synthesize proteins.
MiRNAs are currently potential therapeutic targets and biomarkers in cardiovascular research of various physiological and pathological processes (2, 3, 33–37, 40). The action of miRNAs occurs at the posttranscriptional level. MiRNAs negatively regulate the expression of their target genes by coupling to the 3′-untranslated regions (3′-UTR) of mRNA expressed by the target gene (direct modulation) or in the 3′-UTR of mRNA related with the expression of target gene (indirect modulation), which represses its translation into protein (18, 19).
There are several studies involving miRNAs in pathological cardiac hypertrophy (CH) (2, 3, 29, 35, 36). MiRNAs-1, 133a, and 133b are the best known and highly expressed in heart (36) and already have several targets genes validated, as transcription factors, proteins involved in cellular growth and division, rearrangement of myofibrils and cardiac contractility (34). Carè et al. (2) reported that the decreased expression of miRNA-1 and 133 in vivo and in vitro has a critical role in hypertrophic response. Inversely, in vitro, overexpression of both miRNAs inhibited CH (2). Studies of miRNA target genes for proteins, enzymes, and nuclear factors involved in cardiac growth, differentiation, and cardiogenesis suggest that the miRNAs are key regulators of a general program for cardiac growth (2, 31, 40).
The family of miRNA-29 (29a, 29b, and 29c) has validated target genes involved in cardiac fibrosis, a process that occurs concurrently with pathological CH, broadly related to cardiovascular disease. The downregulation of miRNA with anti-miRNAs in vivo and in vitro increases expression of collagens type I and III (COLIAI, COLIIIAI), whereas increased expression of miRNA-29 in fibroblasts reduces collagen expression (37).
The present study was aimed at understanding the role of miRNA regulation in aerobic exercise training-induced CH and the underlying molecular mechanisms. We hypothesized that aerobic exercise training modulates specific miRNAs.
The physiological adaptations to aerobic training consist of a set of morphological and functionally beneficial effects on skeletal muscle metabolism, blood circulation, and heart performance. These adaptations include hemodynamic, metabolic, and cardiovascular adaptations, such as CH. CH is a physiological increase in left ventricle (LV) mass that contributes to increased ventricular stroke volume and cardiac output (32, 38).
This CH phenotype is associated with sarcomeres added in series to lengthen the cardiac cell, as well as in parallel. In contrast to pathological CH, physiological CH induced by aerobic training occurs in response to intermittent volume workload imposed to the heart, resulting in an increased biosynthesis of contractile components and in higher expression of isoforms of the fastest myosin heavy chain (α-MHC) and lower expression of slower isoforms (β-myosin heavy chain or β-MHC). Such changes are not concomitant with expression of genes for fetal reprogramming, such as atrial natriuretic factor (ANF) and pathological CH markers including skeletal α-actin (14, 23, 24). The gene expression of these markers is an important criterion to distinguish if the stimuli are simply trophic (growth fast or normal) or stimuli that are primarily hypertrophic, indicating the beginning of a pathological response (4, 5). Akt1 is other critical determinant of intracellular signaling pathway in physiological CH induced by aerobic exercise training, thus the Akt1 pathway also differentiates between physiological and pathological CH (8, 17, 23–25).
A differential characteristic to distinguish the physiological from pathological CH is a disproportional expression of collagens and matrix extracellular proteins (19, 37).
Thus, in the present study, we investigated the role of miRNA-29 family, which has collagens as target genes (37), on the improvement of ventricular function promoted by two different levels of swimming training.
MATERIALS AND METHODS
Animal Care
All protocols and surgical procedures were used in accordance with the guidelines of the Brazilian College for Animal Experimentation (COBEA) and were approved by the Ethics Committee of the School of Physical Education and Sport of the University of São Paulo. Female adults normotensive Wistar rats (190–220 g, n = 21) were used and handled according to approved institutional guidelines. In our experience, female rats respond to exercise training with greater CH development than male rats. The animals were weighed weekly and housed 3–5 per cage in a controlled room temperature (22°C) with a 12-h dark-light cycle and fed standard rat chow ad libitum. The rats were randomly assigned into three experimental groups: sedentary control (S, n = 7), swimming trained following protocol 1 (T1, n = 7), and swimming trained following protocol 2 (T2, n = 7), as described below.
Exercise Training Protocols
Protocol 1.
Low-intensity, long-duration exercise training consisted of swimming sessions of 60-min duration, 5 days/wk, for 10 wk, that were carried out between 11:30 AM and 1:30 PM. This low-intensity long-period training protocol [protocol 1 (T1)] has already been used previously in our laboratory and is effective for promotion of cardiovascular adaptations and for increase of muscle oxidative capacity (26).
Protocol 2.
Low-intensity, high long duration exercise: Animals assigned to [protocol 2 (T2)] performed the same swimming training protocol as T1 until the end of the 8th wk. On the 9th wk rats trained twice a day, swimming sessions of 60-min duration with 4 h interval between sessions. In that week swimming sessions were carried out from 07:30 to 08:30 AM and from 12:30 to 1:30 PM. On the 10th wk rats trained three times a day, with sessions of 60-min duration with 4 h interval between sessions. In that week swimming sessions were carried on from 07:30 to 08:30 AM, from 12:30 to 1:30 PM, and from 5:30 to 6:30 PM. The aim of increasing training volume (T2) was to induce the magnitude of CH and aerobic high performance.
Graded Treadmill Exercise Test
Exercise tolerance (ET), estimated by total effort time, was evaluated with a graded treadmill exercise protocol as previously described (12). In brief, after being adapted to treadmill exercises over 1 wk (10 min each session), rats were allowed to acclimatize to exercise for at least 30 min. Exercise intensity was increased by 3 m/min (6–33 m/min) every 3 min at 0% grade until failure to maintain exercise. This graded treadmill exercise test as performed after the training protocols.
Oxygen Uptake Measurements
Oxygen uptake (VO2) was measured by means of expired gas analysis during the graded treadmill exercise described above, after training protocols. Gas analysis was performed using an oxygen and carbon oxide analyzer (Sable Systems SS3, FC-10a O2/CO2 analyzer). VO2 was calculated using the measured flow through the metabolic chamber, the expired fraction of effluent oxygen, and the fraction of oxygen in room air.
Hemodynamic Measurements
Arterial blood pressure and heart rate.
Twenty-four hours after the last training session (24 h), the rats were anesthetized (ketamine 90 mg/kg and xylazine 10 mg/kg, intraperitoneally), and a catheter (PE-50) was inserted into the carotid artery, for direct measurement of arterial blood pressure (BP) and heart rate (HR). The catheter was heparinized and filled with saline, and external extremity was occluded. The catheter was placed subcutaneously and exited caudally. For the arterial blood pressure register, animals were individually maintained in cages for at least 24 h before the experimental procedures. Forty-eight hours after the last training session, the arterial blood pressure was recorded for 30 min in quiet, conscious, unrestrained rats. The catheter was connected to a polyethylene tube (PE-100), and this to a pressure transducer (P23Db; Gould-Statham, Oxnard, CA) connected to an amplifier (General Purpose Amplifier-Stemtech). The arterial pressure was recorded by an analog-digital system (Stemtech, Klamath Lake, OR), registered in real time in a microcomputer with a CODAS System and analyzed through a Windows-compatible system, with a sampling frequency of 1,000 Hz/channel. Through this program, the values of systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean blood pressure (MBP) were obtained beat to beat.
Echocardiography
Echocardiography followed the guideline recommendations of the American Society of Echocardiography (28). Transthoracic echocardiography was performed after the training period based on the average of three consecutive cardiac cycles. The equipment was a Sequoia 512 equipment (ACUSON, Mountain View, CA) and a 10- to 14-MHz multifrequency linear transducer. Images were obtained with the transducer placed on the animal's shaved chest (lateral recumbence). To optimize the image, a transmission gel was used between the transducer and the animal's chest (general imaging gel; ATL, Reedsville, PA). Animals were scanned from below at a depth of 2 cm with the focus optimized at 1 cm. Rats were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg). Wall thickness and LV dimensions were obtained from a short-axis view at the level of the papillary muscles. LV mass was calculated by the use of the following formula, assuming a spherical LV geometry and validated in rats: LV mass = 1,047×[(LVd + PWd + IWd)3 − LVd3], where 1,047 is the specific gravity of muscle, LVd is LV end-diastolic diameter, and PWd and IWd are end-diastolic posterior and interseptum wall thickness, respectively. LV shortening was calculated as (LVd − LVs)/LVd × 100, where LVs is LV end-systolic diameter. LV ejection fraction was calculated according to the Teichholz formula. Two-dimensionally guided pulsed Doppler recordings of LV transmitral flow were obtained from the apical 4-chamber view. Isovolumic relaxation time was taken as the time from aortic valve closure to the onset of mitral flow. Velocity of circumferential shortening was measured using the formula (LVd − LVs)/(Lvd × ET), where ET is the ejection time.
Measurements of CH
To measure cardiac mass, the LV was dissected corresponding to the remaining tissue upon removal of both atria and the free wall of the right ventricle. The interventricular septum remained as part of the LV. The CH was assessed by the measurement of the ratio of LV in milligrams to animal body weight (BW) in grams (LV/BW and RV/BW in mg/g). The hearts were stopped at diastole by perfusion of 14 mM KCl. After the heart was weighed, the LV was fixed in 6% formaldehyde, embedded in paraffin, cut in 5 μm sections, the level of papillary muscle, and subsequently stained with hematoxylin and eosin for the visualization of cellular structures. Two randomly selected sections from each animal were visualized by light microscopy using an oil immersion objective with a calibrated magnification (×400). Myocytes with visible nucleus and intact cellular membranes were chosen for diameter determination. The diameter of individually isolated cardiomyocyte displayed on a viewing screen was manually traced, across the middle of the nuclei, with a digitizing pad and determined by a computer-assisted image analysis system (Quantimet 520, Cambridge Instruments). For each animal, 20 visual fields were analyzed.
Biochemical Analysis
Citrate synthase activity measurement.
Citrate synthase (CS) activity was determined spectrophotometrically in mixed right soleus according to the method previously described (30) and used as a marker of muscle oxidative activity. The enzyme activity was measured in whole muscle homogenates, and the amount of the complex resulting from acetyl-CoA and oxaloacetate was determined at 412 nm and 25°C, at an interval of 10 min. CS activity was expressed as μmol·ml−1·mg−1 of protein, and it was measured in these groups to show the effectiveness of the swimming training protocols by a biochemical parameter.
Hydroxyproline determination.
LV collagen was quantified from the hydroxyproline (OH-proline) concentration by modified method, as described previously (1, 13). All tissue samples (∼100 mg wet weight) were taken from the same area of the LV wall. The tissues were hydrolyzed in 1 ml of 8 N HCl at 115°C for 18 h under vacuum. Hydrolysis samples were filtered and vacuum dried. Oxidant (chloramine T; 0.5 ml) was added, vortexed, and allowed to stand for 4 min. To this was added 1.0 ml Ehrlich reagent (3 ml of Ehrlich reagent plus 16 ml of isopropanol). The tubes were vortexed for 4 min and afterwards kept at 60°C for 21 min. The intensity of the coloration was measured at 558 nm after 1 h at room temperature. OH-proline content was determined from duplicate samples of 150 μl using a calibration curve of 0.5–5 μg of 1-OH-proline. The data are expressed as milligrams per gram of OH-proline.
Molecular Analysis
mRNA quantitation using real-time PCR.
The relative gene expression of COLIAI, COLIIIAI, ANF, skeletal α-actin, α-MHC, β-MHC, and miRNA-1, miRNA-133a, miRNA-133b, and miRNA-29c was analyzed by real-time polymerase chain reaction (real-time PCR) as described follow.
RNA extraction and cDNA synthesis.
Frozen LV samples (100 mg) were homogenized in TRIzol (1 ml), and ribonucleic acid (RNA) was isolated according to the manufacturer's instructions (Invitrogen Life Technologies, Strathclyde, UK). Samples were quantified by spectrophotometer at 260 nm and checked for integrity by EtBr-agarose gel electrophoresis.
RNA were primed with 0.5 μg/μl oligo(dT) (12–18 bp) (Invitrogen Life Technologies) to generate the first strand of DNA. Reverse transcription (RT) was performed using SuperScript II Reverse Transcriptase (Invitrogen Life Technologies).
Real-time PCR.
Primers were designed using Primer 3 software (http://frodo.wi.mit.edu/primer3/). DNA sequence was obtained from GenBank, and primers were made in separate exons to distinguish by size PCR products derived from cDNA from those derived from genomic DNA contaminants. The mRNA expression of pathological markers of CH and type I/III collagen were assessed by oligonucleotides primers as follows: for COLIA1, 5′-AgA gAg CAT gAC CgA TggA-3′ and 5′-gAggTTgCC AgT CTg TTg g-3′; for COLIIIA1, 5′-AAg gTC CAC gAggTg ACA A-3′ and 5′-Agg gCC TggACT ACC AAC T-3′; for ANF, 5′-CTT CGG GGG TAG GAT TGA C-3′ and 5′-CTT GGG ATC TTT TGC GAT CT-3′; for skeletal α-actin, 5′-ACC ACA GGC ATT GTT CTG GA-3′ and 5′-TAA GGT AGT CAG TGA GGT CC-3′; for α-MHC, 5′-CGA GTC CCA GGT CAA CAA G-3′ and 5′-AGG CTC TTT CTG CTG GAC C-3′; and for β-MHC 5′-CAT CCC CAA TGA GAC GAA G-3′ and 5′-AGG CTC TTT CTG CTG GAC A-3′.
Real-time quantification of the target genes was performed with a SYBRgreen PCR Master Mix, (Applied Biosystem, PE, Foster City, CA) using ABI PRISM 7700 Sequence Detection System (Applied Biosystem). The expression of cyclophilin A (5′-AAT gCT ggA CCA AAC ACA AA-3′ and 5′-CCT TCT TTC ACC TTC CCA AA-3′) was measured as an internal control for sample variation in RT reaction. An aliquot of the RT reaction was used for 50-cycle PCR amplification in the presence of SYBRgreen fluorescent dye, according to a protocol provided by the manufacturer (Applied Biosystems).
To accurately detect mature miRNAs and confirm array results, a real-time quantification method was performed using Ambion primers (PN 4427975) for miRNAs-1(ID2222), 133a (ID2246), 133b (ID2247), and 29c (ID415) (Ambion, Austin, TX). We used the TaqMan MicroRNA Assays from Applied Biosystems (#4373142). Samples were normalized by evaluating U6 expression (#4373381). For miRNA reactions we used Kit MirvanaT-QRT-PCR miRNA detection Kit and SuperTaq Plus Polymerase (Applied Biosystem).
PCR product generation was monitored by measuring the increase in fluorescence caused by the SYBRgreen binding to double-stranded DNA at each annealing phase. A dissociation curve was generated at the end of the reaction to verify that a single product was amplified. Each heart sample was analyzed in triplicate. Relative quantities of target gene expressions of S rats vs. T1 and T2 groups were compared after normalization to the values of cyclophilin [change in threshold cycle (ΔCT)]. Fold change in mRNA expression was calculated using the differences in ΔCT values between the two samples (ΔΔCT) and the equation 2−ΔΔCT. Data are expressed in percentage related to S group (control).
MiRNA Microarray
MiRNA was isolated from LV tissue by using the mirVana qRT-PCR miRNA. Isolation Kit (Ambion, Austin, TX). Following extraction, samples were diluted 1:100 with TE buffer and quantified using the Beckman Coulter DU 640 Spectrophotometer. RNA from two animals in each group was pooled and used for miRNA expression analysis (LC Science, Houston, TX) with the Agilent platform. The arrays consist of 15,000 features including rat probes for 349 miRNAs based on Sanger miRBASE 13.0. The Agilent miRNA platform requires 100 ng of total RNA per labeling reaction. The quality of RNA samples were checked using the miRMAX microarray. Results are expressed percentage values, calculated by results as arbitrary units (au) and were calculated from these values using ratio between T1 and T2 groups and S group.
Western Blotting Analysis
The protein expression of Akt1 and phospho-Akt (Ser 473) in the LV was analyzed by Western blotting. The frozen ventricles (100 mg) were homogenized in cell lysis buffer containing 100 mM Tris, 50 mM NaCl, 1% Triton X-100, and protease and phosphatase inhibitor cocktail (1:100; Sigma-Aldrich, St. Louis, MO). Insoluble heart tissues were removed by centrifugation at 3,000 g, 4°C, 10 min. Samples were loaded and subjected to SDS-PAGE in 8% polyacrylamide gels. After electrophoresis, proteins were electro-transferred to nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ). Equal loading of samples (50 μg) and even transfer efficiency were monitored with the use of 0.5% Ponceau S staining of the blot membrane. The blot membrane was then incubated in a blocking buffer (5% nonfat dry milk, 10 mM Tris·HCl, pH 7.6, 150 mM NaCl, and 0.1% Tween 20) for 2 h at room temperature and then incubated overnight at 4°C with rabbit anti-Akt1 polyclonal antibody (1:1,000, Upstate Cell Signaling Solutions) and rabbit anti-phosphoSer473-Akt polyclonal antibody (1:1,000; Cell Signaling Technology, Hitchin, UK) at room temperature. Binding of the primary antibody was detected with the use of peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagents (Amersham Biosciences) were used to visualize the autoradiogram, which was later exposed to photographic film. The film was developed, and the bands were analyzed using Scion Image software (Scion based on NIH Image). α-Tubulin expression levels were used to normalize the results.
Statistical Analysis
Results are represented as means ± SD. Statistical analysis was performed using randomized one-way ANOVA. Tukey's post hoc test was used for individual comparisons between means when a significant change was observed with ANOVA. To indicate how closely two variables change in relationship to each other Pearson's correlation coefficient was used. P < 0.05 were accepted as statistically significant.
RESULTS
Aerobic Training Markers
To evaluate the effectiveness of swimming training, hemodynamic and biochemical assessments were performed as well as the graded test for the experimental groups. Table 1 summarizes values of MBP, HR, ET, VO2, and CS activity results of the groups S, T1, and T2. There was no difference of MBP among groups. However, HR decreased significantly after the swimming training protocol in T1 [301.2 ± 15.3 beats/min (bpm), P < 0.05] and T2 (309 ± 14 bpm, P < 0.05) related to S group (345.0 ± 12.1 bpm). The ET is given by the total duration (minutes) of the graded treadmill exercise test at the end of training protocols. Trained groups had an increase of 20.7 and 29.4% for T1 and T2, respectively (P < 0.05) vs. S. VO2max increased 12% in T1 and 18% in T2 compared with S group (T1: P < 0.05 vs. S and T2: P < 0.01 vs. S). CS in soleus muscle of rats was significantly higher in both T1 (262.0 ± 59.6 μmol·ml−1·mg−1, P < 0.05) and T2 (366.2 ± 46.4 μmol·ml−1·mg−1, P < 0.05) than S group (178.6 ± 63.0 μmol·ml−1·mg−1). Resting bradycardia, increase in VO2max, ET, and CS confirm effectiveness of training protocols adopted, being markers of aerobic work capacity.
Table 1.
Hemodynamic measurements and aerobic training markers
| S (n = 7) | T1 (n = 7) | T2 (n = 7) | |
|---|---|---|---|
| Mean blood pressure, mmHg | 113 ± 7 | 110 ± 6 | 108 ± 9 |
| Heart rate, bpm | 345 ± 12 | 301 ± 15* | 309 ± 14* |
| VO2max, ml·kg−1·min−1 | 67.6 ± 5.7 | 75.63 ± 5.1* | 80.05 ± 6.2* |
| Exercise tolerance, min | 26.12 ± 3.7 | 35.99 ± 5.1** | 35.7 ± 4.1** |
| Citrate synthase activity, μmol·ml−1·mg−1 | 178.6 ± 63.0 | 262.0 ± 59.6* | 366.2 ± 46.4* |
Effects of swimming training protocols [protocol 1 (T1), protocol 2 (T2)] on mean blood pressure, heart rate [beats per minute (bpm)]; oxygen uptake, exercise capacity, and cytrate sinthase activity. Significant difference vs.
sedentary group (S), P < 0.05;
S, P < 0.01.
CH
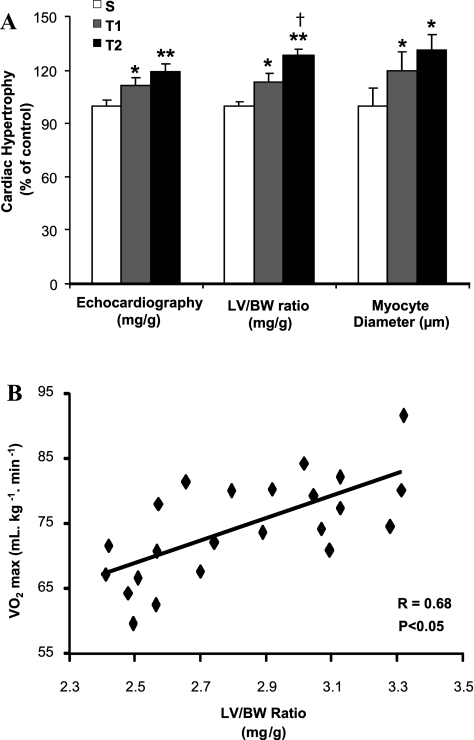
BW before and after swimming training was similar among groups (data not showed). LV hypertrophy (LV/BW ratio), cardiomyocytes diameter, and echocardiography analysis were used as indexes of CH. Figure 1A shows significantly differences in all three measurements of CH. LV/BW ratio obtained by T1 and T2 was 13% (2.8 ± 0.14 mg/g) and 27% (3.2 ± 0.12 mg/g) (P < 0.01), respectively, compared with S group (2.5 ± 0.06 mg/g). This increase in LV/BW ratio observed with swimming training was further confirmed by the increase of LV cardiomyocytes diameter in T1 (13.2 ± 1.3 μm, 20%) and T2 group (14.4 ± 1.3 μm, 30%) (P < 0.05) compared with S group (11 ± 1.1 μm) and also by results obtained from echocardiography (S: 4.150 ± 0.18, T1: 4.593 ± 0.21 mg/g, P < 0.05 vs. S; T2: 4.945 ± 0.42 mg/g, P < 0.01 vs. S) that presented 11 and 19% of CH to T1 and T2, respectively. Additionally, Fig. 1B shows a high positive correlation of LV/BW ratio with VO2max results (r = 0.68, P < 0.05).
Fig. 1.
Effect of different aerobic exercise training volume on cardiac hypertrophy (CH) of Wistar rats. Data are presented as means ± SD. A: CH was displayed by echocardiography (mg/g), left ventricle (LV) weight-to-body weight ratio (LV/BW, mg/g) and cardiomyocyte diameter analysis in sedentary (S, n = 7) and trained groups (T1, n = 6; T2, n = 6). Significant difference vs. *S, †T1 P < 0.05; **T1, P < 0.01. B: the increase of CH was positively correlated with the increased of VO2max (S, n = 7; T1, n = 7; T2, n = 8) (r = 0.68, P < 0.05).
Molecular Markers of Pathological CH
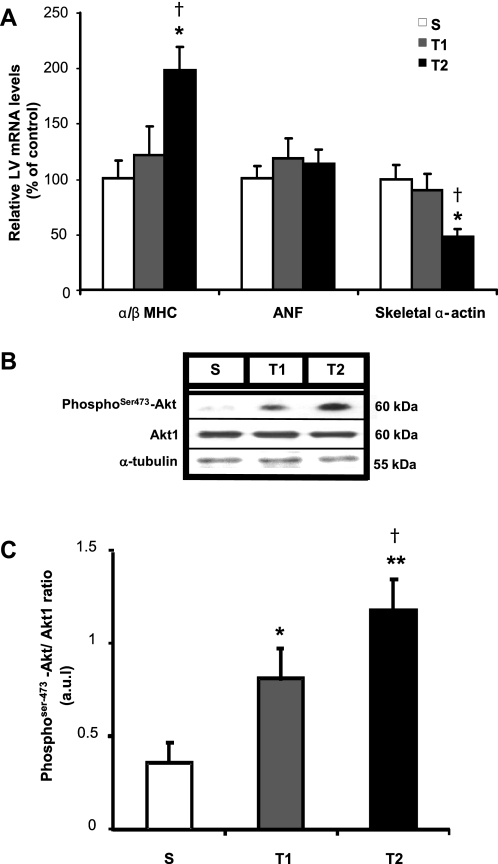
Pathological CH is characterized by the induction of genes normally expressed during fetal development, such as ANF, and pathological CH markers as skeletal α-actin and decreased of ratio α/β-MHC. Additionally, Akt1 is a critical determinant of intracellular signaling pathway in physiological CH induced by aerobic exercise training. To assess whether these markers were expressed and thus confirm the order of physiological CH, the mRNA levels of these four genes were assessed in the LV in S, T1, and T2 groups by real-time PCR, and the protein expression of phosphoSer473-Akt and Akt1 by Western blot. Figure 2 shows that swimming training did not modify the gene expression of ANF. Similarly, T1 did not change the gene levels of skeletal α-actin and α/β-MHC compared with S; however, T2 significantly lowered 53% the LV levels of skeletal α-actin (P < 0.05 vs. S and P < 0.01 vs. T1), and it increased 98% the LV levels of ratio α/β-MHC (P < 0.05 vs. S and P < 0.01 vs. T1). The activation of Akt pathway, given by the ratio phosphoSer473-Akt/Akt, increased 125% in T1 (P < 0.05) and 228% in T2 groups (P < 0.01) related to S.
Fig. 2.
Effect of swimming exercise training on classical molecular markers of cardiac hypertrophy. A: α/β-myosin heavy chain (MHC) ratio, atrial natriuretic factor (ANF), and skeletal α-actin evaluated by real-time PCR. Targeted genes were normalized by cyclophilin mRNA. B: representative blots of cardiac phosphoSer473-Akt, Akt1, and α-tubulin from S, T1, and T2 groups. C: cardiac Akt activation (represented by phosphoSer473-Akt/Akt1 ratio). Targeted bands were normalized to cardiac α-tubulin. Groups: S (n = 7), T1 (n = 6), and T2 (n = 6). Data are reported as means ± SD. Significant difference vs. *S, †T1, P < 0.05; **S, P < 0.01.
MiRNA Analysis
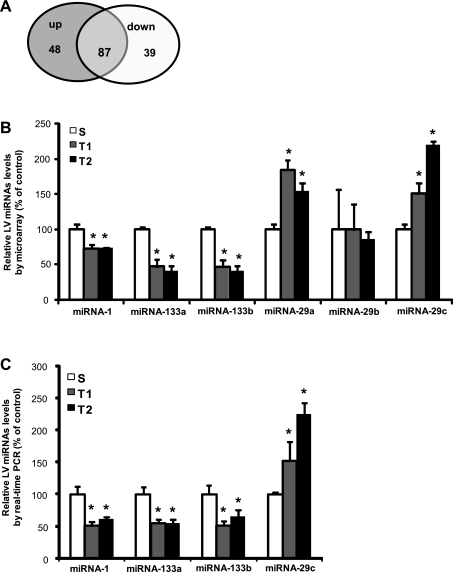
To investigate the potential involvement of miRNAs in physiological CH induced by training we performed microarray analysis in two different protocols of swimming training, using a microarray representing 349 different miRNAs. The analysis was restricted to those miRNAs that had a significant change from baseline (S). From these 349 miRNAs, 87 presented differential expression compared with S group, the expression for 48 were upregulated and for 39 were downregulated compared with S group (P < 0.01, Fig. 3A). The miRNAs that were differentially expressed and selected in this study were miRNAs-1, 133a, 133b, 29a, 29b, and 29c, previously validated as involved in pathological and stress responsive CH response (2, 31, 37). Figure 3B shows that miRNAs-1, 133a, and 133b presented decreased expression in T1 and T2 compared with S group. MiRNA-1 expression was 28% lower in T1 and 27% in T2 (S: 57,816 ± 3,647, T1: 41,555 ± 2,440, T2: 42,009 ± 650 au, P < 0.01). MiRNAs-133a (S: 24,858 ± 752, T1:11,661 ± 1,298, T2: 9,987 ± 776) and 133b (S: 22,779 ± 773, T1: 10,536 ± 1,054, T2: 9,178 ± 672 au, P < 0.01) were 53% in T1 and 59% lower in T2 related to S group, presenting a similar change of expression pattern observed in pathological hypertrophy (2). There were no differences among groups in miRNA-29b expression. However, there was upregulation of miRNA-29a, 84% in T1 and 53% in T2 groups compared with S group (S: 5,572 ± 408, T1: 10,255 ± 1,495, T2: 8,563 ± 445 au P < 0.01) and miRNA-29c (51% in T1 and 119% in T2 - S: 744 ± 384, T1: 1,125 ± 172, T2: 1,624 ± 206 au, P < 0.01) compared with S group, respectively. Interestingly, the pattern of expression changes contrasts with previously observed patterns in pathological hypertrophy regulation (33, 37).
Fig. 3.
Differential expression of microRNAs (miRNAs) in LV induced by swimming exercise training. A: total of miRNAs differentially expressed by microarray: 87 miRNAs presented significant difference, 48 upregulated, 39 downregulated (P < 0.01 vs. *S). B: relative expression of miRNAs-1, 133a, 133b, 29a, 29b, and 29c related to S group by microarray (S, n = 2; T1, n = 2; T2, n = 2) (P < 0.01 vs. S). C: confirmation of differential expression of miRNAs-1, 133a, 133b, and 29c by real-time PCR reaction, percentage related to S group (%) S (n = 5), T1 (n = 5). and T2 (n = 5).
MiRNAs Expression by Real-time PCR
To confirm the involvement of miRNAs in physiological CH we performed quantification of miRNAs-1, 133a, 133b, and 29c by real-time PCR. MiRNAs-1 (S:1.02 ± 0.23, T1: 0.52 ± 0.14, T2: 0.61 ± 0.15 au), 133a (S: 1.02 ± 0.24, T1: 0.57 ± 0.07, T2: 0.55 ± 0.16 au), and 133b (S:1.03 ± 0.31, T1: 0.54 ± 0.12, T2: 0.74 ± 0.24 au) were downregulated, while miRNA-29c (S:1.00 ± 0.07, T1: 1.54 ± 0.59, T2: 2.23 ± 0.45 au) was upregulated in T1 and T2 related to S. The expression of miRNA-29c increased 52% in T1 and 123% in T2 related to control group, which confirmed the microarray results.
Collagen Expression and OH-proline Concentration
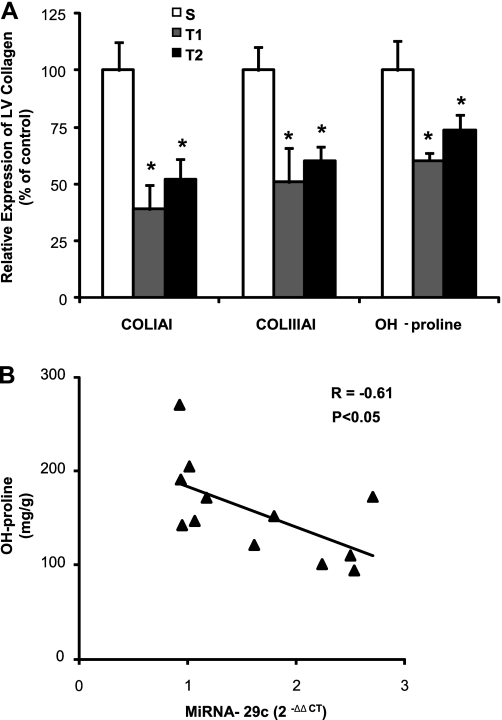
Additionally, to evaluate the participation of collagen in CH induced by exercise training, we quantified the gene expression of COLIAI and COLIIIAI by real-time PCR and the cardiac OH-proline concentration. COLIAI was decreased 61% in T1 (P < 0.05) and 48% (P < 0.05) in T2 compared with S group. Similarly, COLIIIAI expression was 49% (P < 0.05) and 40% (P < 0.05) lower in T1 and T2 compared with S group. The OH-proline concentration confirmed that total LV collagen content was 40% lower in T1 (142 ± 29 mg/g, P < 0.05) and 27% decreased in T2 (125 ± 34 mg/g, P < 0.05) compared with S group (235 ± 45 mg/g). Additionally Fig. 4B shows a high negative correlation of miRNA-29c expression with OH-proline concentration (r = 0.61, P < 0.05).
Fig. 4.
Gene and protein expression of collagen. A: collagen type I gene relative expression by real-time PCR, COLIAI (S, n = 5; T1, n = 5; T2, n = 5), collagen type III gene relative expression by real-time PCR, COLIIIAI (S, n = 5; T1, n = 5; T2, n = 5), LV collagen quantified from the hydroxyproline (OH-proline) concentration (mg/g) (S, n = 4; T1, n = 4; T2, n = 4). Values are expressed in percentage (%) from control group (Significant difference vs. *S, P < 0.05). B: the increase of miRNA-29c expression was negatively correlated with the decreased OH-proline concentration in LV (r = −0.61, P < 0.05). Significant difference vs.*S, P < 0.05.
These results (Fig. 4) show evidence that COLIAI and COLIIIAI, gene targets of miRNAs-29a and 29c, are decreased in both trained groups compared with S group.
Cardiac Function
Finally, Table 2 summarizes systolic and diastolic function data, obtained by echocardiography, performed to assess the cardiac function after the aerobic training. There was no difference among groups in cardiac systolic function. However, the ratio E/A was increased in T2 (1.644 ± 0.11 P < 0.05) and presented no difference in T1 (1.504 ± 0.19) related to the S group (396 ± 0.16). Results of E/A suggests an improved ventricular compliance in trained animals.
Table 2.
Echocardiography measurements
| S (n = 7) | T1 (n = 6) | T2 (n = 6) | |
|---|---|---|---|
| Systolic Function | |||
| LVEF, % | 77 ± 5 | 75 ± 2 | 73 ± 2 |
| LVFS, % | 39 ± 4 | 38 ± 3 | 36 ± 2 |
| VCF, m/s | 0.204 ± 0.02 | 0.211 ± 0.2 | 0.222 ± 0.04 |
| Diastolic Function | |||
| Peak E, m/s | 0.451 ± 0.05 | 0.499 ± 0.05 | 0.458 ± 0.03 |
| Peak A, m/s | 0.328 ± 0.03 | 0.333 ± 0.03 | 0.279 ± 0.01 |
| Ratio E/A | 1.396 ± 0.16 | 1.504 ± 0.19 | 1.644 ± 0.11* |
| IVRT, ms | 31.0 ± 1.7 | 30.7 ± 1.8 | 28.2 ± 1.6 |
Evaluation of cardiac function by echocardiography. LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; VCF, velocity of circumferential fiber shortening; peak E, peak E wave; peak A, peak A wave; ratio E/A, ratio of peak E wave per peak A wave; IVRT, isovolumetric relaxation time. Significant difference vs.
S, P < 0.05.
DISCUSSION
The main findings of the present study show that swimming training induced upregulation of the expression of miRNAs-29a and 29c, which was related with a significant decrease in LV collagen gene (COLIAI and COLIIIAI) and protein concentration. Also, miRNA-29c was inversely correlated to OH-proline concentration in physiological CH. These effects are associated with improvement of ventricular compliance shown by the T2 group. Additionally, there was downregulation of miRNAs-1, 133a, and 133b that can also be inversely associated with CH phenotype displayed by the T1 and T2 groups.
Several studies have showed that the miRNA-29 family is involved in fibrosis regulation in diseases such as diabetic nephropathy, myocardial infarction, systemic sclerosis, and chondropathy (4, 10, 22). These miRNAs already have validated targets in vitro and in vivo to extracellular matrix protein mRNAs of collagen IAI, IAII, IAIII, IIIAI, fibrilin, and elastin in heart and several tissues. Transforming growth factor-β and platelet-derived growth factor B pathways are targets of miRNA-29. The miRNA-29 family are regulators of the production and deposition of collagens in the heart and tendon (4, 22, 37). Moreover, in skin fibrosis, cotransfection with pre-miRNA-29 significantly decreased the relative luciferase activity of the reporter gene, suggesting a direct regulation of collagen by miRNA-29 (10).
Similarly, miRNAs-1, 133a, and 133b also play significant roles in cardiac remodeling and hypertrophy and function as part of a general program of physiological and pathological CH (2, 3, 19, 33, 34, 36). There was decreased expression of these miRNAs in patients with hypertrophic cardiomyopathy and atrial dilation and in three different murine models of CH (interval-trained rats, transverse aortic constriction mice, and Akt E40K transgenic mice, which overexpress cardiac Akt kinase). When the authors overexpressed miRNA-133, protein synthesis and hypertrophic response were suppressed and inversely, buffering of the same miRNA with a targeted 3′-UTR decoy resulted in marked hypertrophy, protein synthesis, increased fetal gene expression, and pathological CH markers (2). Another study using mice with combined deletion of miRNAs-133a and 133b resulted in severe malformations such as ventricular septal defects, enlargement of right ventricle and atria due cardiomyopathy and excessive fibrosis (20). The main validated targets are Cdc-42 and Rho-A, two GTP-GDP binding molecules involved in cell growth, myofibrillar organization, and contractility regulation, as well as WHSC2/NELF-A, a nuclear factor that participates in the regulation of RNA polymerase II transcription elongation (3).
The expression pattern observed in these previous studies on miRNA-29, 1, 133a, and 133b are consistent with an inhibition or downregulation of the miRNA-29 family causing increased fibrosis and, miRNAs-1, 133a, and 133b causing remodeling and hypertrophy (2, 3, 20, 33). In the present study, we noted the effect on miRNAs-29 was the reverse for pathological CH regulation compared with physiological CH regulation. The effect of swimming aerobic training caused a change of expression pattern similar that observed in pathological CH to miRNAs-1, 133a, and 133b (34–36). Although chronic aerobic exercise is a known and potent inducer of CH coupled with preserved function and physiological phenotypic features, few studies so far have studied the relationship to miRNAs. A study in humans related aging and miRNAs in response to exercise training (9); however, the study focused on skeletal muscle miRNAs. There is a lack of research about the effect of aerobic training on the expression of miRNAs in the heart. Our results suggest that the miRNAs-29 have a key role in the differentiation of physiological and pathological phenotypes. In this respect, studies involving exercise training as an inducer of physiological CH open the door for studies of other miRNAs for discriminating between these phenotypes and determining therapeutic components useful in cardiovascular therapy.
As expected, swimming training protocols increased aerobic capacity, and this increase was proportional to the volume of training, modulated by a greater number of exercise sessions in T2 compared with T1. Resting bradycardia and increased ET occurred in both protocols compared with control group as expected. Previous studies showed that both protocols used in the present study promoted similar adaptations such as a linear increase in peak oxygen consumption and in the skeletal muscle oxidative enzyme activity represented by CS in soleus muscle, CH, and resting bradycardia. These results show that aerobic conditioning improved physiological and biochemical variables relevant to the assessment of cardiovascular health (27).
The two training protocols reveal there is a close positive correlation (r = 0.68, P < 0.05) of LV/BW ratio compared with VO2max. This finding suggests that the physiological CH phenotype depends of increased aerobic performance. Previous studies showed that endurance training caused eccentric CH, in which the adaptive responses are distributed across the LV wall and considered as physiological occurrence in response to intermittent volume workload applied on the cardiovascular system (11, 26, 27).
The results show that CH induced by training is not concomitant with gene expression of pathological markers of CH such as ANF, skeletal muscle α-actin, and β-MHC. Additionally, CH is concomitant with an increase in gene expression of α-MHC, activation of the phosphorylated Akt signaling pathway, and also preserved systolic and improved ventricular compliance, which have a physical influence on diastolic function (16, 25). These molecular characteristics in conjunction with preserved contractility and ventricular function can distinguish the physiological from pathological CH (14, 23, 24).
On the other hand, the training protocols used in present study are inducers of physiological CH and exclude fetal reprogramming genes, regarded as molecular markers of cardiac pathology, and also includes linear activation of Akt signaling pathway, a classical pathway of physiological CH related to exercise training (14, 21, 23, 25, 39). Both training protocols are useful to investigate biochemical and physiological adaptations related to aerobic exercise training.
The main finding of this study is that the decreases of gene expression of COLIAI and COLIIIAI and total collagen protein expression (OH-proline) in the LV differentiates pathological CH from the physiological phenotype. It links the effects of aerobic training, cardiac function, hypertrophic response, and increase of expression of miRNAs-29. Additionally, there was close negative correlation (r = −0.61, P < 0.05) with miRNA-29c and protein expression of collagen in LV, which shows that the increase of miRNA-29c is related with decreased collagen expression. The regulation of collagen by the family of miRNAs-29 has been associated, in rodents, with disorders such as infarction and heart failure (36, 37). Our model of exercise-induced physiological CH shows that in healthy individuals this regulation also occurs; however, it is contrary to what has been shown in disease status, i.e., the increase in miRNA-29, which is correlated with a decrease in the expression of collagen and ventricular compliance that appears to contribute to the healthy phenotype and cardiovascular function.
Cardiac collagen expression increases in response to injury or constant pressure overloads caused by all cardiac diseases (33, 37). Cardiac fibroblasts, in response to pathological stress, start to express extracellular matrix proteins disproportionately producing fibrosis, which causes mechanical stiffness, contributes to dysfunction in cardiac contractility, and participates in pathological remodeling, which is partly responsible for increase of heart size (33, 37). In contrast, under physiological conditions, cardiac myocytes are surrounded by a fine network of collagen fibers that functions as part of the scaffold of connective tissue to sustain the cardiac muscle fibers. Aerobic training consists of a volume workload intermittently applied to the heart, and the hypertrophic response in this case was accompanied by a decrease in the expression of collagen. We suggest that this key difference protected the heart from fibrosis and pathological remodeling and miRNAs-29 were involved in this process.
The decrease in gene and protein collagen expression was concomitant with less collagen deposition in heart tissue and greater ventricular compliance as shown by the increase in E/A ratio obtained by echocardiography measurement. This suggests that ventricular compliance is indirectly regulated by miRNAs-29, which was upregulated in T1 and T2 groups.
We conclude that miRNAs-29 regulates physiological cardiac responses to cardiac hypertrophy resulting from aerobic exercise. The effect of increased miRNAs-29 by exercise is to decrease collagen and protect the extracellular matrix of the heart. This posttranscriptional regulatory mechanism may provide a logical basis and expand the understanding about therapeutic effects of both exercise and miRNAs.
GRANTS
The present investigation was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) Grant 2009/18370-3. U. P. R. Soci is the recipient of a social demand Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Fellowship by the Medicine Faculty of University of São Paulo. T. Fernandes was the recipient of FAPESP Fellowship 07/56771-4. N. Y. Hashimoto was recipient of a CAPES Fellowship. E. M. Oliveira holds scholarships from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil. M. I. Phillips was supported by National Heart, Lung, and Blood Institute Grant 1 R01 HL-077602.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bergman I, Loxley R. New spectrophotometric method for the determination of proline in tissue hydrolyzates. Anal Chem 42: 702–706, 1970 [DOI] [PubMed] [Google Scholar]
- 2. Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW, 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med 13: 613–618, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Catalucci D, Gallo P, Condorelli G. MicroRNAs in cardiovascular biology and heart disease. Circ Cardiovasc Genet 2: 402–408, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Chen CH, Zhou YL, Wu YF, Cao Y, Gao JS, Tang JB. Effectiveness of microRNA in Down-regulation of TGF-beta gene expression in digital flexor tendons of chickens: in vitro and in vivo study. J Hand Surg Am 34: 1777–1784. e.1, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Chien KR, Knowlton KU, Zhu H, Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiological response. FASEB J 5: 3037–3046, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Chien KR, Zhu H, Knowlton KU, Miller-Hance W, van-Bilsen M, O'Brien TX, Evans SM. Transcriptional regulation during cardiac growth and development. Annu Rev Physiol 55: 77–95, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Crimi E, Ignarro LJ, Cacciatore F, Napoli C. Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol 6: 292–300, 2009 [DOI] [PubMed] [Google Scholar]
- 8. DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation 113: 2097–2104, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Drummond MJ, Mccarthy JJ, Fry CS, Esser KA, Rasmussen BB. Aging differentially affects human skeletal muscle microRNA expression at rest and following resistance exercise and essential amino acid ingestion. Am J Physiol Endocrinol Metab 295: E1333–E1340, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Du B, Ma LM, Huang MB, Zhou H, Huang HL, Shao P, Chen YQ, Qu LH. High glucose down-regulates miR-29a to increase collagen IV production in HK-2 cells. FEBS Lett 584: 811–816, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Evangelista FS, Brum PC, Krieger JE. Duration-controlled swimming exercise training induces cardiac hypertrophy in mice. Braz J Med Biol Res 36: 1751–1759, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Ferreira JC, Rolim NP, Bartholomeu JB, Gobatto CA, Kokubun E, Brum PC. Maximal lactate steady state in running mice: effect of exercise training. Clin Exp Pharmacol Physiol 34: 760–765, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Gunja-Smith Z, Lin J, Woessner JF., Jr Changes in desmosine and pyridinoline crosslinks during rapid synthesis and degradation of elastin and collagen in the rat uterus. Matrix 9: 21–27, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signaling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Iemitsu M, Miyauchi T, Maeda S, Sakai S, Kobayashi T, Fujii N, Miyazaki H, Matsuda M, Yamaguchi I. Physiological and pathological cardiac hypertrophy induce different molecular phenotypes in the rat. Am J Physiol Regul Integr Comp Physiol 281: R2029–R2036, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci USA 85: 339–343, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL, Condorelli G, Ellingsen O. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol 214: 316–321, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 6: 376–385, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol 6: 419–429, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN. MicroRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev 22: 3242–3254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumo S, Cantley LC. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol 25: 9491–9502, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maurer B, Stanczyk J, Jüngel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum 62: 1733–1743, 2010 [DOI] [PubMed] [Google Scholar]
- 23. McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, Buerger A, Izumo S, Jay PY, Jennings GL. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci USA 104: 612–617, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol 34: 255–262, 2007 [DOI] [PubMed] [Google Scholar]
- 25. McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase (p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA 100: 12355–12360, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Medeiros A, Oliveira EM, Gianolla R, Casarini DE, Negrão CE, Brum PC. Swimming training increases cardiac vagal activity and induces cardiac hypertrophy in rats. Braz J Med Biol Res 37: 1909–1917, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Oliveira EM, Sasaki MS, Cerêncio M, Baraúna VG, Krieger JE. Local renin-angiotensin system regulates left ventricular hypertrophy induced by swimming training independent of circulating renin: a pharmacological study. J Renin Angiotensin Aldosterone Syst 10: 15–23, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58: 1072–1083, 1978 [DOI] [PubMed] [Google Scholar]
- 29. Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res 100: 416–424, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Srere PA. Studies on purified citrate-enzymes: metabolic interpretations. Biochem Soc Symp 27: 11–21, 1968 [PubMed] [Google Scholar]
- 31. Takaya T, Ono K, Kawamura T, Takanabe R, Kaichi S, Morimoto T, Wada H, Kita T, Shimatsu A, Hasegawa K. MicroRNA-1 and MicroRNA-133 in spontaneous myocardial differentiation of mouse embryonic stem cells. Circ J 73: 1492–1497, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Urhausen A, Kindermann W. Sports-specific adaptations and differentiation of the athlete's heart. Sports Med 4: 237–244, 1999 [DOI] [PubMed] [Google Scholar]
- 33. van Rooij E, Marshall WS, Olson EN. Toward microRNA-based therapeutics for heart disease. Circ Res 103: 919–928, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest 117: 2369–2376, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Rooij E, Sutherland LB, Liu N, Willians AH, Macanally Gerard RD J, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 103: 18255–18260, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316: 575–579, 2007 [DOI] [PubMed] [Google Scholar]
- 37. van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA 105: 13027–13032, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vollaard NB, Constantin-Teodosiu D, Fredriksson K, Rooyackers O, Jansson E, Greenhaff PL, Timmons JA, Sundberg CJ. Systematic analysis of adaptations in aerobic capacity and submaximal energy metabolism provides a unique insight into determinants of human aerobic performance. J Appl Physiol 106: 1479–1486, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 83: 1849–1865, 1991 [DOI] [PubMed] [Google Scholar]
- 40. Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 436: 214–220, 2005 [DOI] [PubMed] [Google Scholar]