Abstract
Objective
The goal was to determine the clinical utility of Doppler echocardiography in predicting the presence and severity of pulmonary hypertension in patients with chronic lung disease who subsequently underwent cardiac catheterization.
Methods
A retrospective review of data for all patients <2 years of age with a diagnosis of bronchopulmonary dysplasia, congenital diaphragmatic hernia, or lung hypoplasia who underwent echocardiography and subsequently underwent cardiac catheterization for evaluation of pulmonary hypertension was performed. The accuracy of echocardiography in diagnosing pulmonary hypertension, on the basis of estimated systolic pulmonary artery pressure, was compared with the detection of pulmonary hypertension with the standard method of cardiac catheterization.
Results
Thirty-one linked measurements for 25 children were analyzed. Systolic pulmonary artery pressure could be estimated in 61% of studies, but there was poor correlation between echocardiography and cardiac catheterization measures of systolic pulmonary artery pressure in these infants. Compared with cardiac catheterization measurements, echocardiographic estimates of systolic pulmonary artery pressure diagnosed correctly the presence or absence of pulmonary hypertension in 79% of the studies in which systolic pulmonary artery pressure was estimated but determined the severity of pulmonary hypertension (severe pulmonary hypertension was defined as pulmonary/systemic pressure ratio of ≥0.67) correctly in only 47% of those studies. Seven (58%) of 12 children without estimated systolic pulmonary artery pressure demonstrated pulmonary hypertension during subsequent cardiac catheterization. In the absence of estimated systolic pulmonary artery pressure, qualitative echocardiographic findings, either alone or in combination, had worse predictive value for the diagnosis of pulmonary hypertension.
Conclusion
As used in clinical practice, echocardiography often identifies pulmonary hypertension in young children with chronic lung disease; however, estimates of systolic pulmonary artery pressure were not obtained consistently and were not reliable for determining the severity of pulmonary hypertension.
Keywords: chronic lung disease, bronchopulmonary dysplasia, pulmonary hypertension, echocardiography, cardiac catheterization
Abnormalities of the pulmonary circulation, including the development of pulmonary hypertension (PH), complicate the course of chronic lung disease (CLD) in newborns and contribute to late morbidity and death during infancy, especially in the settings of bronchopulmonary dysplasia (BPD), congenital diaphragmatic hernia (CDH), and pulmonary hypoplasia.1–4 Diagnosis of pulmonary vascular disease in this population is difficult and requires a high degree of suspicion because the symptoms of PH may be subtle, even in patients with significantly elevated pulmonary artery (PA) pressures. In addition, clinical signs of PH may be masked by or attributed to signs of CLD itself, further delaying recognition of PH. Detection of pulmonary vascular abnormalities is important because it can provide prognostic information and may dictate the need for such therapies as vasodilator treatment, more-aggressive respiratory support, and surgical or interventional cardiac catheterization procedures. Assessment of the magnitude of PH influences decisions regarding medical management and pursuit of additional diagnostic and therapeutic procedures.
Doppler echocardiography is a noninvasive test that is commonly used to screen for PH and to help guide management. Cardiac catheterization, the standard method for assessing pulmonary vascular disease, is a much more invasive test and has been reserved for patients for whom noninvasive assessments are considered inadequate and for assessment of acute vasoreactivity, to guide long-term therapy. Previous studies in adults and children evaluated the ability of echocardiography to diagnose and to determine the severity of PH,5–12 but few studies in infants compared directly the echocardiographic estimates of systolic PA pressure (sPAP) derived from the tricuspid regurgitant jet velocity (TRJV) with direct measurements obtained through cardiac catheterization,7,12 and no study concentrated on infants and young children with CLD.
In addition, most of the previous studies assessed the validity of echocardiographic measurements performed simultaneously with cardiac catheterization measurements, showing good correlation between sPAP estimated with echocardiography and the value measured with cardiac catheterization.5–7,9–12 Although performing simultaneous echocardiographic measurements during cardiac catheterization is the best study design to evaluate the validity and capability of echocardiographic measurements, this approach does not mirror the use of echocardiography in clinical practice, where echocardiographic interpretation serves as the basis for the decision to refer patients for cardiac catheterization (often performed days to weeks later) and guides therapy. Questions remain regarding whether echocardiography identifies correctly patients who should undergo cardiac catheterization and whether it provides sufficient information for effective monitoring of patients with established PH without cardiac catheterization, especially children with CLD.
In our institution, cardiac catheterization is reserved for patients with CLD who have persistent signs of severe cardiorespiratory disease and are suspected of having significant PH after optimal management of their CLD and associated morbidities, to assess the severity of PH; to exclude or to document the severity of associated anatomic cardiac lesions; to define the presence of systemic/pulmonary collateral vessels, pulmonary venous obstruction, or left heart dysfunction; and to assess pulmonary vascular reactivity in patients who fail to respond to oxygen therapy alone. In addition, patients being treated for PH with vasodilator therapy are referred for cardiac catheterization when clinical improvement is in doubt or echocardiographic measurements fail to provide adequate hemodynamic assessment.
In the present study, we sought to determine the effectiveness of echocardiography, as used in clinical practice, to predict the presence and severity of PH in a population of children <2 years of age with CLD attributable to BPD, CDH, or pulmonary hypoplasia who sub-sequently underwent cardiac catheterization. Our objectives were to determine how often the sPAP can be estimated with echocardiography by using the TRJV, whether the sPAP estimated with echocardiography predicts accurately PH severity, as measured during subsequent cardiac catheterization, whether other qualitative echocardiographic signs of PH predict the presence of PH in the absence of a measurable TRJV, and ultimately whether echocardiographic results are of sufficient reliability to diagnose and to manage PH without cardiac catheterization.
Methods
Study Population
We reviewed the medical charts of all patients with a diagnosis of CLD that included BPD, CDH, or pulmonary hypoplasia who underwent cardiac catheterization at <2 years of age for evaluation of PH, between January 1998 and July 2006. Approval for review of the medical charts was granted by the local institutional review board. Patients with atrial septal defects, persistent foramen ovale, patent ductus arteriosus, or ventricular septal defects were included in the study; however, patients with complex congenital heart disease were excluded from analysis, to explore more directly the pulmonary circulation in patients with CLD without these additional hemodynamic factors. Medications were reviewed, to ensure that patients were receiving the same medications at the times of echocardiography and cardiac catheterization.
Patient characteristics recorded included gestational age, birth weight, gender, and primary medical diagnoses. Patient data relevant to each echocardiogram and catheterization, including age, cardiopulmonary support, and medication requirements at the time of each study, were also collected.
Echocardiography
Echocardiographic measurements included TRJV, measurable dimensions of heart chambers, any detectable shunt lesions, and the direction of flow for any shunt lesion. Shunt lesions were defined as any cardiac shunt or patent ductus arteriosus. Estimated sPAP was calculated with no allowance for the right atrial pressure (RAP), by using the modified Bernoulli equation (TRJV2 × 4). Systemic systolic blood pressure (sBP) was recorded via blood pressure cuff unless the patient had an existing arterial catheter. Qualitative measures of PH and right-sided stress, as determined by the cardiologist interpreting the study, were also recorded, including right atrial enlargement, right ventricular dilation, right ventricular hypertrophy, ventricular septal flattening, and PA dilation.
Cardiac Catheterization
Patients received general anesthesia or were sedated with midazolam and fentanyl, at the discretion of the cardiologist or anesthesiologist performing the procedure. Vascular access was obtained through the femoral approach with the standard Seldinger technique. Cardiac output was measured with thermodilution for patients without intracardiac shunts. For patients with intracardiac shunts, the Fick method was used.
Data for echocardiographic variables were collected as available from the medical chart, including sPAP, mean PA pressure (mPAP), sBP, mean aortic pressure/mean systemic arterial pressure, mean RAP, cardiac index, pulmonary vascular resistance index, systemic vascular resistance index, and pulmonary/systemic vascular resistance ratio. Pulmonary and systemic vascular resistances were indexed for body surface area and expressed as pulmonary vascular resistance index and systemic vascular resistance index, respectively, in Wood units. The presence or absence of any shunt lesion, the pulmonary flow/systemic flow ratio, and any interventional procedures were recorded.
Study Design
Doppler echocardiographic and cardiac catheterization measurements were performed when clinically indicated and were analyzed under conditions similar to the patients' baseline state. The baseline state was defined as the cardiopulmonary support, including PH medications, required by the patient and prescribed by the primary care team at the time the measurements were recorded. Many patients who did not require mechanical ventilation at baseline were assessed with cardiac catheterization under general anesthesia, and sedation level and mechanical ventilatory and oxygen support were not consistent between echocardiography and cardiac catheterization for those patients (n = 12). In those cases, an effort was made to maintain preexisting goals for oxygenation and ventilation for baseline measurements with cardiac catheterization. Systemic saturation under baseline conditions was >85% for all except 4 patients, with the lowest recorded saturation being 77% and the other 3 values being recorded at 84%. PH was defined by an estimated sPAP of ≥40 mm Hg with echocardiography and by a mPAP of >25 mm Hg with cardiac catheterization under baseline conditions. Mild/moderate PH was defined as PH with sPAP/sBP estimated with echocardiography and mPAP/mean systemic arterial pressure measured with cardiac catheterization of <0.67. Severe PH was defined by sPAP/sBP estimated with echocardiography and mPAP/mean systemic arterial pressure measured with cardiac catheterization of ≥0.67. Measurements with cardiac catheterization served as the standards. All patients were evaluated in Denver, Colorado (altitude: 1600 m).
Statistical Analyses
The capacity of Doppler echocardiography to estimate sPAP and to diagnose PH was evaluated in 3 different ways. First, to examine the overall relationship of echocardiography-estimated sPAP to cardiac catheterization measurements, we compared the results as continuous variables by using Pearson correlation methods. We repeated the correlation analysis for the subset of patients for whom the interval between echocardiography and cardiac catheterization was <10 days, to minimize the impact of time on the measurements. Second, we determined the ability of echocardiography-estimated sPAP/sBP to diagnose accurately and to determine the severity of PH, as measured with subsequent cardiac catheterization. Third, by using cardiac catheterization measurements as the standards, we calculated the sensitivity, specificity, and positive and negative predictive values of diagnosing PH on the basis of an echocardiography-estimated sPAP of ≥40 mm Hg and the presence of qualitative right ventricular structural or functional abnormalities. Posthoc adjustments to the estimated sPAP for the estimated RAP were also made, to determine whether accounting for RAP improved the performance statistics of the measurement. The presence of any one of the following findings was considered a positive study result: right atrial enlargement, right ventricular hypertrophy, right ventricular dilation, PA dilation, or ventricular septal flattening. This analysis was also performed separately for the subsets of patients with and without an estimated sPAP.
Descriptive statistics for patient characteristics are reported as median and range or mean ± SD. Results are expressed as mean ± SD or percentage. Comparisons of group means were made with Student's t test. Proportions were tested with Fisher's exact test. Confidence intervals (CIs) for proportions were calculated by using the score interval described by Wilson.13 In all analyses, P values of ≤.05 were considered significant. Statistical analyses were conducted by using SAS 8.2 (SAS Institute, Cary, NC).
Results
Twenty-nine patients with CLD who underwent cardiac catheterization for evaluation of PH met the initial criteria for this study; 3 patients with BPD and 1 patient with CDH were excluded because of the presence of complex anatomic congenital heart disease. The remaining 25 patients underwent a total of 31 catheterizations.
Clinical characteristics of the study patients are presented in Table 1. The median interval between echocardiography and cardiac catheterization was 4 days (range: 0–57 days), and all except 4 studies were performed within 30 days. No patients were studied more than twice. Patients in 23 (74%) of the studies had a history of shunt lesions (including patent ductus arteriosus), 3 of whom had undergone atrial septal defect closure and 5 surgical patent ductus arteriosus closure. In 16 (52%) of the paired studies, patients were receiving specific PH therapy at the time of the studies.
Table 1. Clinical Characteristics of Study Patients.
| Patients, n | 25 |
| Paired studies, n | 31 |
| Age at catheterization, median (range), mo | 10.2 (0.4–22.4) |
| Gender, male/female, n | 11/14 |
| Gestational age at birth, median (range), wk | 28 (23–41) |
| Disease, n (%) | |
| BPD (n = 17) | 22 (70) |
| CDH (n = 4) | 5 (16) |
| Pulmonary hypoplasia (n = 4) | 4 (13) |
| Hospitalized at time of cardiac catheterization, n (%) | 21 (68) |
| Mechanical ventilation at study, n (%) | 16 (52) |
| Cardiac shunts present at study, n (%) | 18 (58) |
| Atrial shunt | 16 (52) |
| Ventricular septal defect | 2 (6) |
| Aortopulmonary collateral vessels, n (%) | 14 (45) |
| Coil-treated at time of study | 3 (10) |
| Medications at study, n (%) | 27 (87) |
| Oxygen | 27 (87) |
| Calcium channel blocker | 2 (6) |
| Sildenafil | 3 (10) |
| Bosentan | 2 (6) |
| Nitric oxide | 10 (32) |
| Epoprostenol | 2 (6) |
| Sedation for cardiac catheterization, conscious/general anesthesia, n | 6/25 |
The major hemodynamic findings from the echocardiographic and cardiac catheterization studies are presented in Table 2. The TRJV was detectable in only 19 of the 31 echocardiographic studies, allowing estimation of sPAP for 61% of the patients. The cutoff value of 40 mm Hg for echocardiography-estimated sPAP predicted correctly the presence or absence of PH, as determined with cardiac catheterization (mPAP of >25 mm Hg), in 15 of 19 studies. Cardiac catheterization diagnosed PH in 23 (74%) of the 31 studies. The mPAP determined with cardiac catheterization for patients in whom a TRJV was detected was 38 ± 13 mm Hg, which was greater than the mPAP (30 ± 6 mm Hg) for patients without a detectable TRJV (P = .03).
Table 2. Echocardiographic and Cardiac Catheterization Results (n = 31).
| Interval between echocardiography and cardiac catheterization, mean ± SD, d | 10.8 ± 14.6 |
| Echocardiography | |
| Estimated sPAP, mean ± SD, mm Hg (n = 19) | 65 ± 24 |
| Estimated sPAP/sBP, mean ± SD | 0.74 ± 0.3 |
| PH determined with echocardiography (sPAP of >40 mm Hg), n (%) | 16 (52) |
| Right atrial enlargement, n (%) | 23 (74) |
| Right ventricular dilation, n (%) | 24 (77) |
| Right ventricular hypertrophy, n (%) | 19 (61) |
| Septal flattening, n (%) | 26 (84) |
| PA dilation, n (%) | 16 (52) |
| Cardiac catheterization | |
| sPAP, mean ± SD, mm Hg | 51 ± 16 |
| sPAP/sBP, mean ± SD | 0.63 ± 0.22 |
| mPAP, mean ± SD, mm Hg | 35 ± 12 |
| In studies with echocardiography-estimated sPAP (n = 19) | 38 ± 13a |
| In studies without echocardiography-estimated sPAP (n = 12) | 30 ± 6a |
| PH determined with cardiac catheterization (mPAP of >25 mm Hg),n (%) | 23 (74) |
| mPAP/MAP, mean ± SD | 0.58 ± 0.21 |
| RAP, mean ± SD, mm Hg | 8 ± 2 |
| PCWP, mean ± SD, mm Hg | 10 ± 3 |
| Cardiac index (Qs), mean ± SD, L/min per m2 | 4.6 ± 2.1 |
| PVRI,mean ± SD,Wood units × m2 | 5.9 ± 3.1 |
| PVR/SVR, mean ± SD | 0.48 ± 0.37 |
| Shunts with Qp/Qs ratio of > 1.2:1 (n = 18), n (%) | 5 (28) |
MAP indicates mean systemic arterial pressure; PCWP, pulmonary capillary wedge pressure; Qp, pulmonary flow;Qs, systemic flow; PVRI, pulmonary vascular resistance index; PVR/SVR, pulmonary vascular resistance/systemic vascular resistance ratio.
Comparison by t test, P = .03
PH was diagnosed with cardiac catheterization in 16 (84%) of 19 studies in which a TRJV was detected and in 7 (58%) of 12 studies in which TRJV was not detected (P = .10). The ability to estimate sPAP with echocardiography was similar for patients who were receiving PH therapy (10 of 15 patients) and those who were not (9 of 16 patients). Estimations of sPAP with echocardiography were possible for 10 of 18 patients who had shunt lesions. A measurable TRJV was detected in 3 of 7 studies in which the shunt flow was reported as bidirectional, in 1 of 2 studies with right-to-left shunts, and in 6 of 9 studies with left-to-right shunts. All cardiac shunts detected with echocardiography were verified with cardiac catheterization. The pulmonary flow/systemic flow ratio was >1.2 (median: 1.00; range: 0.54–2.23) in 5 of the 18 studies in which a shunt was detected. Aortopulmonary collateral vessels were found in 14 (45%) of the cardiac catheterization studies, 3 of which were deemed clinically significant and large enough to require mechanical occlusion with coils during cardiac catheterization.
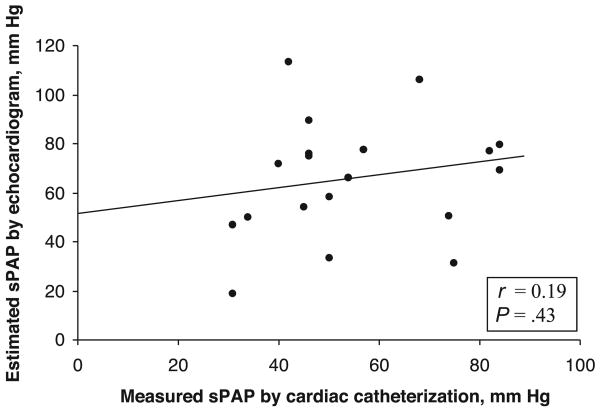
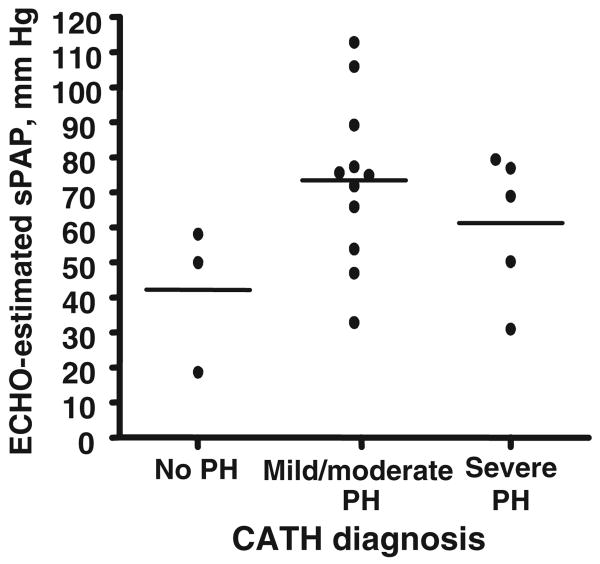
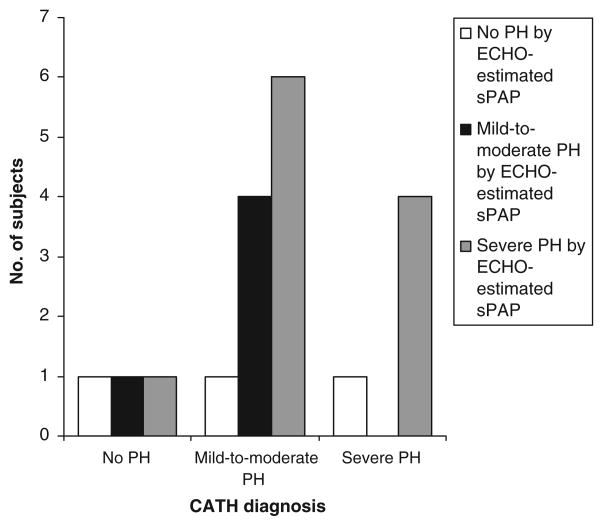
There was poor correlation between sPAP values estimated with echocardiography and those measured with cardiac catheterization (r = 0.19; P = .43) (Fig 1). To study the potential effect on the data of the length of time between echocardiographic and cardiac catheterization studies, the subset of patients for whom the interval between echocardiography and cardiac catheterization was ≤10 days (n = 12) was analyzed separately. Despite the shorter time interval, the correlation between sPAP values measured with echocardiography and cardiac catheterization remained poor (r = −0.26; P = .42). The use of other measurements, such as sPAP/sBP, yielded similarly poor correlations between echocardiography and cardiac catheterization (r = 0.18; P = .5). Mean sPAP estimated with echocardiography (65 ± 24 mm Hg) was not statistically different from mean sPAP measured during cardiac catheterization (51 ± 16 mm Hg; P = .13) (Table 2). When echocardiographic estimates of sPAP were grouped according to the severity of PH, as determined with cardiac catheterization, there was no difference in mean sPAP determined with echocardiography between the groups (Fig 2). Echocardiography-estimated sPAP overestimated and underestimated the severity of PH, as determined with cardiac catheterization, correctly identifying PH severity in only 9 of the 19 studies (Fig 3). Of the 3 studies that did not show PH with echocardiography, 1 patient had mild/moderate PH and 1 had severe PH with cardiac catheterization. Both of the children with PH for whom echocardiography indicated no PH had been diagnosed as having PH during previous cardiac catheterization. One child had BPD and was hospitalized with mechanical ventilation and nitric oxide therapy. The other (severe PH) had CDH and was not hospitalized but was being treated chronically with epoprostenol and nasally administered nitric oxide. The intervals between echocardiography and cardiac catheterization for these patients were 3 and 2 days, respectively. Both patients had all of the assessed qualitative echocardiographic findings of PH. Estimated sPAP misclassified 6 patients with mild/moderate PH, as determined with cardiac catheterization, as having severe PH and misclassified 2 patients (1 as having mild/moderate PH and 1 severe PH) who did not have PH, as determined with cardiac catheterization; the time intervals between echocardiography and cardiac catheterization for the latter 2 patients were 1 and 11 days, respectively. Both patients were hospitalized and undergoing mechanical ventilation. Other than sedation level (both received general anesthesia during cardiac catheterization), there were no significant differences in their condition or support at the times of the 2 studies. Both had qualitative findings of PH with echocardiography.
Figure 1.
Relationship between sPAP values estimated with echocardiography (ECHO) and directly measured with cardiac catheterization (CATH).
Figure 2.
Echocardiography (ECHO)-estimated sPAP values according to severity of PH diagnosed with cardiac catheterization (CATH). PH was defined as mPAP of >25 mm Hg, mild/moderate PH was defined as PH with a mPAP/mean systemic arterial pressure ratio of <0.67, and severe PH was defined as PH with a mPAP/mean systemic arterial pressure ratio of ≥0.67. There were no significant differences between the mean sPAP values of the 3 groups according to analysis of variance.
Figure 3.
Ability of echocardiography (ECHO)-estimated sPAPto predict the severity of PH determined with cardiac catheterization (CATH). PH was defined as estimated sPAP of >40 mm Hg in the presence of a measurable TRJV with echocardiography and as mPAP of >25 mm Hg during cardiac catheterization, mild/moderate PH was defined as PH with an estimated sPAP/sBP ratio of <0.67 with echocardiography and a mPAP/mean systemic arterial pressure ratio of <0.67 with cardiac catheterization, and severe PH was defined as PH with an estimated sPAP/sBP ratio of ≥0.67 with echocardiography and a mPAP/mean systemic arterial pressure ratio of ≥0.67 with cardiac catheterization.
To determine whether the addition of estimated values of RAP to the measured TRJV improved the accuracy of echocardiographic estimates of sPAP, sPAP values were recalculated by adding progressive estimates of RAP (5, 7.5, and 10 mm Hg). The ability of echocardiography-estimated sPAP to predict correctly the severity of PH, as determined with cardiac catheterization, decreased when any of the adjustments for RAP were used (data not shown). The mean RAP measured with cardiac catheterization for the entire study group was 8.13 ± 2.4 mm Hg; for the group in which sPAP could be estimated with echocardiography, the value was 8.16 ± 2.0 mm Hg.
Echocardiographic estimates of sPAP (without adjustment for RAP) were greater than the 40 mm Hg cutoff value for the diagnosis of PH in 16 (84%) of the 19 studies. The actual prevalence of PH in this group, based on the standard definition of mPAP of >25 mm Hg determined with cardiac catheterization, was 74%. The sensitivity, specificity, and positive and negative predictive values of estimated sPAP in diagnosing PH were 88% (95% CI: 64%–97%), 33% (95% CI: 6%–79%), 88% (95% CI: 64%–97%), and 33% (95% CI: 6%–79%), respectively (Table 3).
Table 3. Sensitivity, Specificity, and Positive and Negative Predictive Values of Echocardiographic Findings for Diagnosis of PH in Children <2 Years of Age With CLD.
| Finding | Mean (95% CI), % | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | |
| Estimated sPAP of >40 mm Hg (n = 19) | 88 (64–97) | 33 (6–79) | 88 (64–97) | 33 (6–79) |
| Right atrial enlargement | ||||
| All (n = 31) | 80 (61–91) | 50 (19–81) | 87 (68–94) | 38 (14–69) |
| With measurable TRJV (n = 19) | 94 (72–99) | 67 (21–94) | 94 (72–99) | 67 (21–94) |
| Without measurable TRJV (n = 12) | 56 (27–81) | 33 (6–79) | 71 (36–92) | 20 (4–62) |
| Right ventricular dilation | ||||
| All | 84 (66–93) | 50 (19–81) | 88 (69–96) | 43 (16–75) |
| With measurable TRJV | 94 (72–99) | 67 (21–94) | 94 (72–99) | 67 (21–94) |
| Without measurable TRJV | 67 (37–86) | 33 (6–79) | 75 (41–93) | 25 (5–70) |
| Right ventricular hypertrophy | ||||
| All | 72 (52–86) | 83 (44–97) | 95 (75–99) | 42 (19–68) |
| With measurable TRJV | 81 (57–93) | 100 (44–100) | 100 (77–100) | 50 (19–81) |
| Without measurable TRJV | 56 (27–81) | 67 (21–94) | 83 (44–97) | 33 (10–70) |
| PA dilation | ||||
| All | 52 (33–70) | 50 (19–81) | 81 (57–93) | 20 (7–45) |
| With measurable TRJV | 50 (28–72) | 33 (6–79) | 80 (49–94) | 11 (2–44) |
| Without measurable TRJV | 56 (27–81) | 67 (21–94) | 83 (44–97) | 33 (10–70) |
| Septal flattening | ||||
| All | 88 (70–96) | 33 (10–70) | 85 (66–94) | 40 (12–77) |
| With measurable TRJV | 100 (81–100) | 33 (6–79) | 89 (67–97) | 100 (21–100) |
| Without measurable TRJV | 67 (35–88) | 33 (6–79) | 75 (41–93) | 25 (5–70) |
| ≥1 abnormality | ||||
| All | 96 (81–99) | 33 (10–70) | 86 (69–94) | 67 (21–94) |
| With measurable TRJV | 100 (81–100) | 33 (6–79) | 89 (67–97) | 100 (21–100) |
| Without measurable TRJV | 86 (50–96) | 20 (4–62) | 60 (31–83) | 50 (9–91) |
| ≥2 abnormalities | ||||
| All | 88 (70–96) | 33 (10–70) | 85 (66–94) | 40 (12–77) |
| With measurable TRJV | 94 (72–99) | 33 (6–79) | 88 (66–97) | 50 (9–91) |
| Without measurable TRJV | 71 (38–90) | 20 (4–62) | 56 (27–81) | 33 (6–79) |
| ≥3 abnormalities | ||||
| All | 84 (66–94) | 50 (19–81) | 88 (69–96) | 43 (16–75) |
| With measurable TRJV | 94 (72–99) | 67 (21–94) | 94 (72–99) | 67 (21–94) |
| Without measurable TRJV | 57 (27–82) | 20 (4–62) | 50 (22–78) | 25 (5–70) |
The standard comparison for diagnosis of PH was mPAP of >25 mm Hg measured with cardiac catheterization.
The performance characteristics of qualitative echocardiographic abnormalities as surrogate markers of PH were also examined (Table 3). Septal flattening was the most commonly identified abnormality (84% studies) and also yielded the highest sensitivity (88%) of any single abnormality. PA dilation had the worst performance for diagnosis of PH. Having ≥ 1 abnormality suggesting PH yielded sensitivity, specificity, and positive and negative predictive values of 96%, 33%, 86%, and 67%, respectively for the diagnosis of PH, as determined with cardiac catheterization. Performance characteristics were not substantially different for patients with ≥2 qualitative abnormalities. We also evaluated the performance characteristics for the subgroups of patients for whom estimates of sPAP could (n = 19) and could not (n = 12) be obtained. Overall, the performance statistics of the qualitative findings were better in studies with a measurable TRJV, compared with those without a measurable TRJV (Table 3).
Discussion
In this study, we investigated the clinical utility of Doppler echocardiography to estimate sPAP and to determine the presence and severity of PH in young children with neonatal CLD. We found that estimation of sPAP with echocardiography on the basis of the presence of adequate TRJV measurements was possible for 61% of infants with CLD. As used in typical clinical practice, however, echocardiographic estimates of sPAP correlated poorly with measurements of sPAP obtained with subsequent cardiac catheterization. Estimates of sPAP diagnosed the presence or absence of PH correctly in 79% of the studies in which a TRJV was detected. However, echocardiography was able to determine the severity of PH correctly in only 47% of those studies. Estimations of presence of adequate TRJV measurements was possible for 61% of infants with CLD. As used in typical clinical practice, however, echocardiographic estimates of sPAP correlated poorly with measurements of sPAP obtained with subsequent cardiac catheterization. Estimates of sPAP diagnosed the presence or absence of PH correctly in 79% of the studies in which a TRJV was detected. However, echocardiography was able to determine the severity of PH correctly in only 47% of those studies. Estimations of sPAP with echocardiography produced errors in both directions, failing to diagnose PH in 11% of studies in which PH was diagnosed with cardiac catheterization and inaccurately diagnosing PH in 11% of studies in which no PH was determined with cardiac catheterization. In the absence of a measurable TRJV, qualitative echocardiographic findings, including right atrial enlargement, right ventricular hypertrophy, right ventricular dilation, PA dilation, and septal flattening, either alone or in combination, have relatively poor predictive value. We found that, when the TRJV can be measured, echocardiography may be a useful screening tool for PH detection, but assessments of PH severity are unreliable in young children with CLD.
As applied in clinical practice, echocardiography is used to determine the presence and severity of PH or to monitor patients with known PH. For older patients with PH, serial determinations of functional class and exercise capacity and routine right heart catheterization are recommended to guide therapy.14 The inability to assess functional class and exercise capacity in young children and the reluctance of many physicians to perform right heart catheterizations in these children further complicate decision-making regarding the management of PH in CLD. Therefore, practitioners have relied more on echocardiographic findings in this population, not only to screen and to diagnose PH but also to monitor disease progression and to assess responses to therapy. Confusing this issue further is the lack of a data-derived definition of PH and a known basal level of PA pressure above which predictable consequences occur. Therefore, defining the levels of PA pressure to identify the presence and severity of PH and to guide therapy remains uncertain.14 We chose to use the commonly accepted cutoff value of mPAP of >25 mm Hg, as determined with cardiac catheterization, to define PH.15 Although echocardiography-estimated sPAP of >35 mm Hg has been used to define PH, we used the more-conservative cutoff value of >40 mm Hg to eliminate possible false-positive results.16
Estimated sPAP derived from the TRJV has become one of the most often used echocardiographic findings for evaluation of PH in adults with heart disease or idiopathic PA hypertension.5–8 Those studies showed excellent correlation coefficients (r = 0.93–0.97), in comparison with the standard cardiac catheterization measurements. Such studies are extremely limited for children <2 years of age and have been performed only in patients with congenital heart disease.7, 12 Those studies evaluated echocardiography and cardiac catheterization performed simultaneously under the same hemodynamic conditions, eliminating differences in sedation level, oxygenation, and ventilator support and significant time intervals between studies. Although previous studies of children with PH showed better correlation between echocardiography-estimated sPAP and the cardiac catheterization measurement, previous studies represent the best possible performance of echocardiography under ideal conditions, not as applied in routine clinical practice as in the present study.
Although the time interval between echocardiography and cardiac catheterization can be considered a limitation of this study, a strength of the current study is evaluation of these tests as actually applied in clinical practice. Patients included in this study received similar levels of support and treatment at the times of echocardiography and cardiac catheterization. There were no detectable differences in medications or fluid status between the studies. In comparison with echocardiography, however, patients were treated longer by the time cardiac catheterization was performed. Whether the additional treatment time could alter hemodynamic factors to a significant degree is unclear. Although the time interval between studies ranged from 0 days to 57 days, when the data were reanalyzed to reduce the interval between echocardiography and cardiac catheterization to ≤10 days, the correlation and accuracy between the measurements did not improve. Another factor that might have contributed to the differing results is that fact that most patients who did not require mechanical ventilatory support at baseline were evaluated with cardiac catheterization under general anesthesia. Although an effort was made to maintain the preexisting goals for oxygenation and ventilation, subtle changes in gas exchange during anesthesia with mechanical ventilation and differing levels of oxygen supplementation could have led to changes in the assessed hemodynamic variables. Differences in the sedation levels of patients undergoing echocardiography and cardiac catheterization might further limit the comparability of the assessed hemodynamic values. In clinical decision-making, however, echocardiography and cardiac catheterization are generally interpreted with little regard to the level of sedation and its subsequent effect on hemodynamic factors. We chose to define the severity of PH on the basis of the pulmonary/systemic pressure ratio, rather than absolute PA pressure, to limit the potential impact of sedation on hemodynamic factors.
A few studies specifically evaluated echocardiography-estimated sPAP in adults with CLD, most with chronic obstructive pulmonary disease.17–19 Those studies reported smaller proportions of patients in whom a TRJV could be measured and lower correlation coefficients between echocardiography-estimated sPAP and cardiac catheterization measurements, compared with adults with primary PH or heart disease. Echocardiography-estimated sPAP in that population also had poor accuracy.18,20 Echocardiography was able to detect a measurable TRJV in 61% of our patients, but estimates of sPAP had both poor correlation and poor accuracy for determining PH severity, compared with values measured with subsequent cardiac catheterization. The ability to estimate sPAP in this study was greater than that reported in a study of premature infants with established CLD (31%), in which 79% of studies revealed PH.21 It has been postulated that factors associated with CLD, specifically marked pulmonary hyperinflation, expansion of the thoracic cage, and alteration of the position of the heart, adversely affect the ability to detect and to measure TRJV.18 These mechanisms may also apply to children with CLD, especially infants with BPD and those requiring mechanical ventilation. It was also reported that tricuspid regurgitation is not always present, even in neonates with systemic pressure-level PA pressures,22 which was confirmed in this study.
The ability to estimate sPAP accurately through echocardiography depends on the quality of the tricuspid regurgitant jet. Doppler recording of the frequency spectrum of a tricuspid regurgitation jet optimally shows a smooth, sharply demarcated envelope. In some patients, however, this frequency spectrum is incomplete and its envelope is poorly demarcated. Such inadequate signals may not allow reliable measurement of the spectrum's peak velocity, yielding imprecise estimates of sPAP. Clinical documentation of the quality of the envelope from which the TRJV is measured may be lacking or may not be well understood by noncardiologists, limiting proper interpretation of the estimated sPAP. We recommend close communication with the consulting cardiologist to determine the reliability of the tricuspid regurgitant jet envelope and the subsequently estimated sPAP.
Because the tricuspid regurgitant jet is not always present or measurable, qualitative echocardiographic measures of PH, such as right atrial enlargement, right ventricular hypertrophy, right ventricular dilation, PA dilation, and septal flattening, have been used as non-invasive screening tools. These measurements, with the exception of PA dilation, seem to have good sensitivity and positive predictive value for diagnosing PH in children with CLD, but specificity and negative predictive value are poor. Furthermore, for patients in whom a tricuspid regurgitant jet was not measurable, qualitative measures were less reliable, which suggests that the ability to estimate sPAP may influence the subjective assessment of these parameters. Several studies have used right ventricular outflow patterns or time intervals obtained with echocardiography to estimate PA pressures and to diagnose PH in children,11,23–28 with limited success, especially in children with CLD.26,28
Interestingly, 68% of children in this study had a history of a shunt lesion, and shunt lesions, primarily atrial shunts, were detected in 58% of the patients. Whether the presence of shunt lesions contributed to the decision to evaluate these patients with cardiac catheterization, accelerated changes of pulmonary vascular disease, or both, is unclear. Although there is limited evidence to describe accurately the prevalence of shunt lesions in neonatal patients with CLD,29 the observations of this study raise the possibility that patients with CLD who have shunt lesions may be at increased risk for pulmonary vascular disease and PH. Additional studies on the impact of left-to-right shunt lesions in children with CLD, as well as early treatment for pulmonary overcirculation, should be evaluated.
There are several other limitations to this study. First, the patients described in this study clearly represent a group of children with severe lung disease, with more than two thirds of patients being hospitalized and more than one half of the patients requiring mechanical ventilation at the time of study. Echocardiographic findings may be more accurate in young children with less-severe lung disease but, because many such children do not undergo cardiac catheterization, it is difficult to make that assessment. The high prevalence of PH in this study also makes it difficult to assess the negative predictive value of echocardiography as used in clinical practice, where the prevalence is assumed to be lower. Because patients with normal or mildly abnormal echocardiographic findings rarely are referred for cardiac catheterization, it is difficult to evaluate the false-negative rate of echocardiography in this group.
Conclusions
As used in clinical practice, echocardiography fails to detect a measurable TRJV in a significant number of high-risk patients; more importantly, its absence does not rule out the presence of severe PH. When the TRJV can be measured to estimate sPAP with echocardiography, it has good sensitivity for the diagnosis of PH; however, the estimated sPAP predicted inadequately the severity of PH as determined with subsequent cardiac catheterization. Qualitative echocardiographic measures of PH were less predictive of PH in patients for whom a TRJV was not obtained. These results suggest that echo- cardiography is a helpful adjunctive tool for determining which patients should undergo cardiac catheterization, but they raise questions regarding reliance on echocardiography without cardiac catheterization for the diagnosis and management of PH in infants and young children with CLD. Additional studies are warranted to identify echocardiographic parameters and other noninvasive methods to improve the clinical utility of these approaches for diagnosing and quantifying PH in neonates and young children with CLD.
Acknowledgments
This publication was made possible by the Thrasher Foundation and by grant 5 K23-RR021021 and General Clinical Research Centers grant M01-RR00069 from the National Center for Research Resources, a component of the National Institutes of Health.
Abbreviations
- CLD
chronic lung disease
- PH
pulmonary hypertension
- sPAP
systolic pulmonary artery pressure
- mPAP
mean pulmonary artery pressure
- TRJV
tricuspid regurgitant jet velocity
- BPD
bronchopulmonary dysplasia
- CDH
congenital diaphragmatic hernia
- PA
pulmonary artery
- sBP
systemic systolic blood pressure
- CI
confidence interval
- RAP
right atrial pressure
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
These findings were presented in part at the Pediatric Academic Society meeting; May 5–8 2007; Toronto, Ontario, Canada.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or the National Institutes of Health.
References
- 1.Hislop AA, Haworth SG. Pulmonary vascular damage and the development of cor pulmonale following hyaline membrane disease. Pediatr Pulmonol. 1990;9(3):152–161. doi: 10.1002/ppul.1950090306. [DOI] [PubMed] [Google Scholar]
- 2.Abman S, Sondheimer H. Pulmonary circulation and cardiovascular sequelae of BPD. In: Weir EK, Archer SL, Reeves JT, editors. Diagnosis and Treatment of Pulmonary Hypertension. New York, NY: Futura; 1992. pp. 155–180. [Google Scholar]
- 3.Goodman G, Perkin RM, Anas NG, Sperling DR, Hicks DA, Rowen M. Pulmonary hypertension in infants with bronchopulmonary dysplasia. J Pediatr. 1988;112(1):67–72. doi: 10.1016/s0022-3476(88)80125-2. [DOI] [PubMed] [Google Scholar]
- 4.Dillon PW, Cilley RE, Mauger D, Zachary C, Meier A. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg. 2004;39(3):307–312. doi: 10.1016/j.jpedsurg.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70(4):657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 6.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6(2):359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 7.Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6(4):750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 8.Skjaerpe T, Hatle L. Noninvasive estimation of systolic pressure in the right ventricle in patients with tricuspid regurgitation. Eur Heart J. 1986;7(8):704–710. doi: 10.1093/oxfordjournals.eurheartj.a062126. [DOI] [PubMed] [Google Scholar]
- 9.Chan KL, Currie PJ, Seward JB, Hagler DJ, Mair DD, Tajik AJ. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J Am Coll Cardiol. 1987;9(3):549–554. doi: 10.1016/s0735-1097(87)80047-5. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson JG. Comparison of several noninvasive methods for estimation of pulmonary artery pressure. J Am Soc Echocardiogr. 1989;2(3):157–171. doi: 10.1016/s0894-7317(89)80053-7. [DOI] [PubMed] [Google Scholar]
- 11.Kosturakis D, Goldberg SJ, Allen HD, Loeber C. Doppler echocardiographic prediction of pulmonary arterial hypertension in congenital heart disease. Am J Cardiol. 1984;53(8):1110–1115. doi: 10.1016/0002-9149(84)90646-5. [DOI] [PubMed] [Google Scholar]
- 12.Skinner JR, Stuart AG, O'Sullivan J, Heads A, Boys RJ, Hunter S. Right heart pressure determination by Doppler in infants with tricuspid regurgitation. Arch Dis Child. 1993;69(2):216–220. doi: 10.1136/adc.69.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. [Google Scholar]
- 14.McGoon MD. The assessment of pulmonary hypertension. Clin Chest Med. 2001;22(3):493–508. doi: 10.1016/s0272-5231(05)70286-0. [DOI] [PubMed] [Google Scholar]
- 15.Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 suppl S):40S–47S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 16.McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104(23):2797–2802. doi: 10.1161/hc4801.100076. [DOI] [PubMed] [Google Scholar]
- 17.Laaban JP, Diebold B, Zelinski R, Lafay M, Raffoul H, Rochemaure J. Noninvasive estimation of systolic pulmonary artery pressure using Doppler echocardiography in patients with chronic obstructive pulmonary disease. Chest. 1989;96(6):1258–1262. doi: 10.1378/chest.96.6.1258. [DOI] [PubMed] [Google Scholar]
- 18.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167(5):735–740. doi: 10.1164/rccm.200210-1130OC. [DOI] [PubMed] [Google Scholar]
- 19.Tramarin R, Torbicki A, Marchandise B, Laaban JP, Morpurgo M. Doppler echocardiographic evaluation of pulmonary artery pressure in chronic obstructive pulmonary disease: a European multicentre study: Working Group on Noninvasive Evaluation of Pulmonary Artery Pressure: European Office of the World Health Organization, Copenhagen. Eur Heart J. 1991;12(2):103–111. doi: 10.1093/oxfordjournals.eurheartj.a059855. [DOI] [PubMed] [Google Scholar]
- 20.Homma A, Anzueto A, Peters JI, et al. Pulmonary artery systolic pressures estimated by echocardiogram vs cardiac catheterization in patients awaiting lung transplantation. J Heart Lung Transplant. 2001;20(8):833–839. doi: 10.1016/s1053-2498(01)00274-1. [DOI] [PubMed] [Google Scholar]
- 21.Benatar A, Clarke J, Silverman M. Pulmonary hypertension in infants with chronic lung disease: non-invasive evaluation and short term effect of oxygen treatment. Arch Dis Child Fetal Neonatal Ed. 1995;72(1):F14–F19. doi: 10.1136/fn.72.1.f14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skinner JR, Boys RJ, Hunter S, Hey EN. Non-invasive assessment of pulmonary arterial pressure in healthy neonates. Arch Dis Child. 1991;66(4 spec no):386–390. doi: 10.1136/adc.66.4_spec_no.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper MJ, Tyndall M, Silverman NH. Evaluation of the responsiveness of elevated pulmonary vascular resistance in children by Doppler echocardiography. J Am Coll Cardiol. 1988;12(2):470–475. doi: 10.1016/0735-1097(88)90421-4. [DOI] [PubMed] [Google Scholar]
- 24.Chotivittayatarakorn P, Pathmanand C, Thisyakorn C, Sueblinvong V. Doppler echocardiographic predictions of pulmonary artery pressure in children with congenital heart disease. J Med Assoc Thai. 1992;75(2):79–84. [PubMed] [Google Scholar]
- 25.Caceres Espejo J, Santos de Soto J, Gavilan Camacho JL, Cabello Laureano R, Grueso Montero J, Descalzo Senorans A. Doppler echocardiographic evaluation of pulmonary hypertension in children [in Spanish] Rev Esp Cardiol. 1995;48(2):122–127. [PubMed] [Google Scholar]
- 26.Newth CJ, Gow RM, Rowe RD. The assessment of pulmonary arterial pressures in bronchopulmonary dysplasia by cardiac catheterization and M-mode echocardiography. Pediatr Pulmonol. 1985;1(1):58–62. doi: 10.1002/ppul.1950010113. [DOI] [PubMed] [Google Scholar]
- 27.Liberman L, Kaufman S, Alfayyadh M, Hordof AJ, Apfel HD. Noninvasive prediction of pulmonary artery pressure in patients with isolated ventricular septal defect. Pediatr Cardiol. 2000;21(3):197–201. doi: 10.1007/s002460010039. [DOI] [PubMed] [Google Scholar]
- 28.Newth CJ, Corey ML, Fowler RS, Gilday DL, Gross D, Mitchell I. Thallium myocardial perfusion scans for the assessment of right ventricular hypertrophy in patients with cystic fibrosis: a comparison with other noninvasive techniques. Am Rev Respir Dis. 1981;124(4):463–468. doi: 10.1164/arrd.1981.124.4.463. [DOI] [PubMed] [Google Scholar]
- 29.Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2004;170(9):1006–1013. doi: 10.1164/rccm.200310-1483OC. [DOI] [PubMed] [Google Scholar]