Abstract
The primary cilium has recently stepped into the spotlight, as a flood of data demonstrate that this organelle has crucial roles in vertebrate development and human genetic diseases. Cilia are required for the response to developmental signals, and evidence is accumulating that the primary cilium is specialized for Hedgehog (Hh) signal transduction. Formation of cilia, in turn, is regulated by other signaling pathways, possibly including the planar cell polarity pathway. The cilium therefore represents a nexus for signaling pathways during development. The connections between cilia and developmental signaling have begun to clarify the basis of human diseases associated with ciliary dysfunction.
The primary cilium, a slim microtubule-based organelle that projects from the surface of vertebrate cells, has been the focus of intensive studies transforming it from a poorly understood curiosity into a structure recognized for its importance in development, inherited human disease and cancer. Cilia and flagella are ancient structures present in organisms as diverse as single celled eukaryotes and humans. The evolutionarily conserved mechanism of Intraflagellar Transport (IFT), first described in the alga Chlamydomonas, is essential for the construction and maintenance of these structures in all species1, 2.
In the past decade, the function of mammalian primary cilia has been revealed by both developmental genetic analyses and human genetic studies. Disruptions of the primary cilium have been associated with the common disorder human cystic kidney disease3–6. In addition, rare recessive human disorders known as ciliopathies, with complex syndromes that include cystic kidneys, obesity, mental retardation, blindness and various developmental malformations, have been shown to be caused by mutations in proteins localized to cilia and ciliary basal bodies (reviewed in7–10). In parallel, genetic studies in the mouse demonstrated that cilia are essential for signaling through the Hh pathway, a crucial signaling pathway for organizing the body plan, organogenesis and tumorigenesis11.
The importance of primary cilia in vertebrate development was first revealed in genetic experiments that demonstrated that cilia are required for survival and patterning of the mouse embryo11. Phenotypic, genetic and biochemical analysis then showed that embryonic phenotypes of the cilia mutants were caused by disruption of Hh signal transduction. This unexpected finding raised many questions, including why the cilium is a good locale for signal transduction, why cilia are required for vertebrate but not invertebrate Hh signaling, and whether primary cilia are important in regulating other developmental signaling pathways.
Other recent experiments have suggested that additional developmental signaling pathways help regulate the formation of cilia. The most complete studies have implicated components of the planar cell polarity (PCP) pathway in the regulation of the position and formation of cilia. These processes, which could indirectly regulate the activity of Hh signaling, appear to be particularly important during organogenesis.
Here we review the relationships between primary cilia and signaling pathways during vertebrate embryonic development. After describing the evolutionarily conserved mechanism of IFT, we review the evidence that Hh signaling requires IFT and cilia. We then describe recent work suggesting that the primary cilium in vertebrate embryos is specialized such that Hh signaling is restricted to the cilium. After considering whether additional developmental signaling pathways require cilia, we discuss the evidence that other signaling pathways regulate ciliogenesis. We conclude with a discussion of how the findings on the relationship between cilia and developmental signals are beginning to explain the syndromes seen in cilia-related human diseases, focusing on the formation of kidney cysts, a hallmark of disorders caused by abnormal primary cilia.
Intraflagellar Transport
The cilium is extended and maintained by the transport of particles along the axoneme mediated by the IFT machinery (Fig. 1). IFT trafficking from the base to the tip of the cilium depends on the microtubule plus-end directed motor Kinesin-2, which associates with two IFT protein complexes, IFT-A and IFT-B. IFT-B is essential for anterograde trafficking, while IFT-A and the minus-end directed motor cytoplasmic Dynein-2 (Dync2h1) are required for retrograde trafficking1. In all organisms studied, disruption of the Kinesin-2 motor or the IFT-B complex blocks cilia formation. Perturbation of retrograde trafficking by disruption of the Dynein or the IFT-A complex results in short, bulged cilia1, 12–15 (Table1).
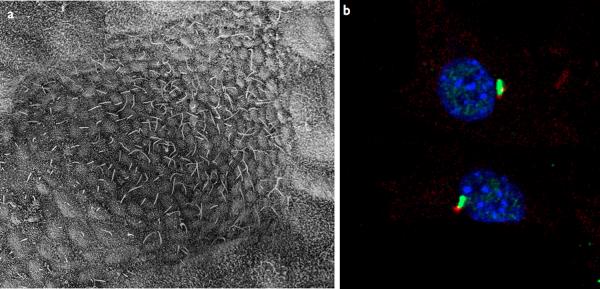
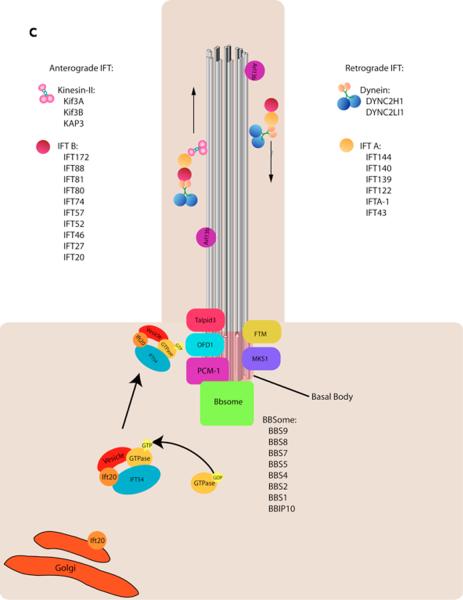
Figure 1. Cilia structure and IFT.
a–b | Examples of types of mammalian cilia. a | The long cilia of the node at E7.5 are required for left-right asymmetry. b | Primary cilia in embryonic fibroblasts in green with the basal body in red.
c | Cargo is transported from the base to the tip of the cilium along the microtubule axoneme by Kinesin-2 together with the IFT-A and IFT-B complexes. Dynein mediates the return of IFT cargo to the base of the cilium1, 14. IFT-B proteins IFT-20 and IFT-54 may also participate in the trafficking of membrane vesicles from the Golgi to the ciliary membrane together with small GTPases128. Other small GTPases, including Arl13b also localize to cilia. While its precise trafficking role is not known, Arl13b is required for axoneme structure129. Certain basal body proteins also influence ciliary trafficking. Among these are components of the Bbsome, named their association with Bardet-Biedl syndrome (BBS). The precise functions of BBS proteins in cilia formation are unclear as they are not individually required for primary cilia formation, however they may function to promote loading of cargo to the ciliary axoneme17. Other basal body associated protein such as MKS1, FTM, OFD1, and Talpid3 are required for cilia formation, though how they regulate ciliogenesis has not been defined (For a review, see10).
Table 1.
Roles of Ciliary and Basal Body Genes in Development and Disease
| Mouse Gene | Function | Mutant Phenotype | Primary Cilia Phenotype | Human Disorder |
|---|---|---|---|---|
| Arl13b | Small GTPase | Hh signaling defects129 | Abnormal microtubule structure129 | Joubert syndrome143 |
| Bbs1 | Basal body protein, Bbsome component | Sensory defects, obesity138 | Defects in specialized cilia only138 | Bardet-Biedl syndrome (BBS)144 |
| Bbs10 | Chaperonin-like | ND | ND | BBS145 |
| Bbs11 | E3 ubiquitin ligase | Muscle defects146 | ND | BBS147, Muscular dystrophy148 |
| Bbs12 | Chaperonin-like | ND | ND | BBS149 |
| Bbs2 | Basal body protein, Bbsome component | Sensory defects, obesity140 | Defects in specialized cilia only140 | BBS150 |
| Bbs3 | Small GTPase | ND | ND | BBS151 |
| Bbs4 | Basal body protein, Bbsome component | Sensory defects, male infertility, obesity138, 139 | Defects in specialized cilia only138, 139 | BBS152 |
| Bbs5 | Basal body protein, Bbsome component | ND | ND | BBS153 |
| Bbs6 | Chaperonin-like | Sensory defects, male infertility, obesity | Defects in specialized cilia only | BBS, McKusick-Kaufman syndrome |
| Bbs7 | Basal body protein, Bbsome component | ND | ND | BBS154 |
| Bbs8 | Basal body protein, Bbsome component | ND | ND | BBS155 |
| Bbs9 | Basal body protein, Bbsome component | ND | ND | BBS156 |
| Dync2h1 | Dynein retrograde motor subunit | Reduced Hh signaling18, 19 | Bulged18 | Jeune asphyxiating thorasic dystrophy (JATD)157 |
| Evc | Basal body protein- skeletal specific | Ihh signaling defects136 | Normal136 | Ellis-van Creveld syndrome33 |
| Ftm/Rgrip1 | Basal body protein | Reduced Hh signaling31 | Short31 | Joubert syndrome type B28 Meckel syndrome158 |
| Fuz | PCP effector | Hh signaling defects103, 104 | Short cilia103, 104 | ND |
| Ift122 | IFT Complex A | Increased Hh signaling45 | Bulged45 | ND |
| Ift139 | IFT Complex A | Increased Hh signaling15 | Bulged, short15 | ND |
| Ift172 | IFT Complex B | Reduced Hh signaling11 | Absent11 | ND |
| Ift52 | IFT Complex B | Reduced Hh signaling21 | ND | ND |
| Ift57 | IFT Complex B | Reduced Hh signaling43 | Absent43 | ND |
| Ift80 | IFT Complex B | ND | ND | JATD159 |
| Ift88 | IFT Complex B | Reduced Hh signaling11 | Absent160 | ND |
| Inturned | PCP effector | Hh signaling defects105 | Short cilia105 | ND |
| Kif3a | Kinesin2 subunit-anterograde | Reduced Hh signaling11 | Absent161 | ND |
| Kif3b | Kinesin2 subunit | ND | Absent162 | ND |
| Kif7 | Kinesin-like; Cos2 homolog | Hh signaling defects40, 55, 56 | Normal40, 55 | ND |
| Mks1 | Basal body protein | Hh defects, skeletal defects, cystic kidneys32 | Sparse, short32 | Meckel syndrome163 |
| Ofd1 | Basal body protein | Skeletal defects, reduces Hh signaling29 | Short29 | Oral-facial-digital syndrome30 |
| Stil | Centrosomal protein | Hh signaling defects164 | ND | Primary microcephaly165 |
ND, Not determined
Cilia are nucleated by the basal body, which is made up of the mother centriole and associated pericentriolar proteins. Some basal body proteins are required for cilia formation; evidence suggests some may recruit cargo from the Golgi to the nascent ciliary membrane and others may promote loading of cargo into the axoneme16, 17 (Fig. 1).
Evidence Linking Hh Signaling to Cilia
Vertebrate Hh signaling requires IFT
The first evidence that vertebrate Hh signaling depends on cilia came from a phenotype-based screen for mutations that alter patterning of the mouse embryo. This screen identified several mutants displaying morphological and patterning phenotypes consistent with altered Hh signaling, including loss of the ventral cell types in the neural tube specified by high levels of Sonic hedgehog (Shh)11. The genes disrupted in these mutants encode several components of the IFT machinery, including the IFT-B complex components Ift172 and Ift88, as well as Dync2h1, the IFT-dedicated retrograde motor11, 18, 19 (Fig. 1). Disruption of the Kinesin-2 motor in Kif3a null embryos also caused similar defects in Shh-dependent neural patterning (Fig. 2)5. Genetic studies showed that IFT proteins act at the heart of the Shh pathway, downstream of the membrane proteins Patched (Ptch) and Smoothened (Smo) and upstream of the Gli transcription factors that implement the pathway11, 18 (Fig. 3, Table 1).
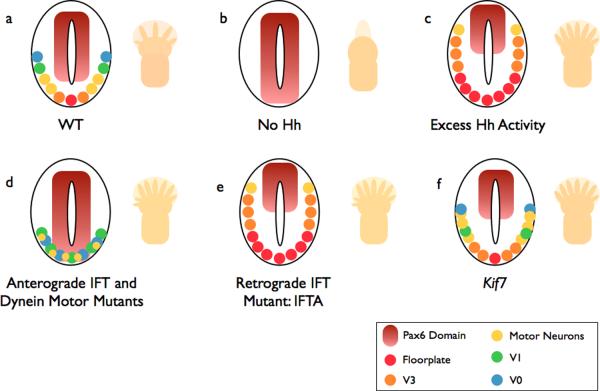
Figure 2. Summary of neural and limb patterning phenotypes in Hh pathway and cilia mutants.
a | In wild-type (WT) embryos, ventral neural cell fates are specified by a gradient of Shh. The number and identity of digits in the limb is established by a Shh gradient from the posterior limb bud opposed by anterior Gli3 repressor (Gli3R). b | In the absence of Hh, (e.g. Smo −/−), ventral neural cell fates are lost. The limbs of Shh mutants lack digits. c | If the pathway is hyperactive (e.g. Ptch −/−), ventral cell types expand in the neural tube. Activation of the pathway within the limb, such as in Gli3 mutants (which lack Gli3R), causes the formation of extra digits. d | Anterograde IFT mutants (e.g. the IFT-B mutants Ift88 or Ift172) lack cilia: Hh signaling is reduced, and the neural tube is dorsalized. This phenotype is milder than in (b) because cilia are also required for cilia Gli3R processing, and cell types that require low levels of Hh signaling are specified. Reduced Gli3R results in polydactyly. Dynein mutants display a similar phenotype, however they retain motor neurons in the caudal neural tube. e | IFT-A mutants (ie Ift139) exhibit phenotypes consistent with excess Hh signaling. f | Mutations that disrupt the kinesin Kif7 cause a partial activation of the Hh pathway, with a modest expansion of cells that require intermediate levels of Hh.
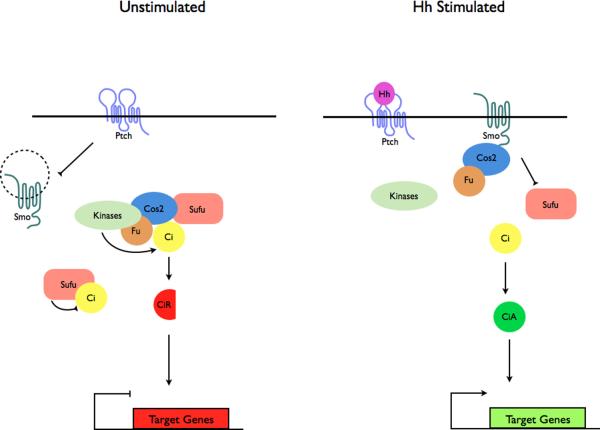
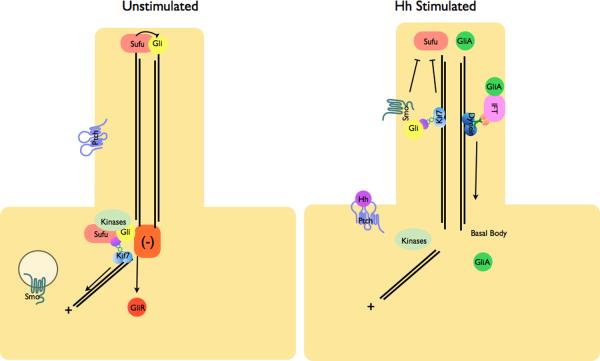
Figure 3. Localization of Hh pathway complexes in Drosophila and mammals.
a | Modulation of protein complex structure and localization in Drosophila by Hh. In the absence of ligand, Ptch prevents translocation of Smo to the plasma membrane. A microtubule-associated complex including Cos2, Fu, Sufu and Ci recruits kinases including PKA, CK1, GSK3β that promote the processing of Ci into its repressor form (CiR)130–133. Sufu may also associate with full length Ci to prevent its translocation to the nucleus130. Upon activation of the pathway, Smo moves to the plasma membrane, and the Fu-Cos2 complex associates with the C-terminal tail of Smo resulting in the release of Ci. Pathway activation also inactivates the negative regulator130–133. b | In vertebrate Hh signaling, signal transduction takes place within cilia, but the behavior of protein complexes may parallel that in Drosophila. In the absence of ligand, Ptch localizes to the cilium, and is thought to block the entry of Smo into cilia35. Kif7 (the Cos2 homologue) localizes to the base of the cilium55 where it may form a complex Gli proteins and other pathway components. Kif7 at the cilium base prevents Gli enrichment within the cilium and promotes processing of GliR. Upon activation of the pathway, Smo moves to the ciliary membrane and Kif7 translocates into the cilium thereby promoting Gli2 accumulation at the cilium tip40, 55. Kif7 at the cilia tip may also block the function of Sufu. Activated Gli is transported out of the cilium by Dynein and IFT particles.
The role of IFT proteins in Hh signaling is complex, partly because of the complex output of the Hh pathway. In the absence of Hh ligand, Gli transcription factors, which function as effectors of the pathway, are proteolytically processed to Gli repressor forms (GliR) that keep Hh target genes off (Fig. 3). In response to Hh ligand, processing of GliR is blocked and activated Gli transcription factors (GliA) activate the expression of Hh target genes. IFT is required both for production of GliA and Gli3R18–21; as a result, IFT mutants show loss of Hh phenotypes in some cell types and gain of Hh phenotypes in others. For example, GliA plays a central role in neural patterning, and IFT mutant embryos show a loss of Hh signaling in the neural tube. In contrast, GliR plays a central role in limb development and IFT mutants that survive to later stages of embryogenesis show pre-axial polydactyly, characteristic of loss of GliR (Fig. 2)18, 19, 21.
Recent experiments demonstrated that IFT is also required for Hh signaling in zebrafish. Zebrafish lacking both maternal and zygotic Ift88 exhibit Hh signaling defects in the neural tube and somites22, however the patterning defects caused by loss of IFT in zebrafish are slightly different than those seen in mammals. Mouse Ift88 mutants lack Shh-dependent ventral neural cell fates11, and zebrafish maternal/zygotic Ift88 mutants lack cell fates requiring the highest levels of Hh, such as V3 interneuron progenitors and muscle pioneer cells. However, cell types within both the neural tube and somites normally specified by lower levels of Hh are expanded22. This difference may be due to a different balance of Gli activators and repressors in the fish compared to the mouse22.
Basal body proteins required for Hh signaling
Additional evidence that cilia are required for Hh signaling came from analysis of basal body protein mutations. Dozens of proteins are localized to centrosomes and the pericentriolar material, and a subset of these proteins has been shown to be required for cilia formation (Table 1). In every case examined thus far, these proteins are required for Hh signaling. For example, the chick talpid3 mutation was first identified based on polydactyly23, 24 and causes developmental defects consistent with disrupted Hh signaling25, 26. Talpid3 mutant embryos fail to form cilia, and the affected gene was shown to encode a centrosomal protein27.
Mutations disrupting other basal body proteins, including OFD1, FTM, MKS1 and EVC, cause human ciliopathies and affect mammalian Hh signaling. Mice mutant for Ofd1, Ftm/Rpgrip1l, or Mks1 have abnormal or absent cilia, and exhibit Hh signaling defects corresponding to the severity of the cilia disruption28–32 (Table 1). EVC also localizes to the basal body and is mutated in the human skeletal disorder Ellis-van Creveld syndrome33, 34. Unlike other basal body proteins, expression of Evc in the mouse is limited to developing skeletal structures, and Evc does not appear to be required for the formation of cilia in chondrocytes. Nevertheless, Evc−/− mice exhibit reduced Indian hedgehog (Ihh) signaling specifically within skeletal structures. Thus Evc apparently does not affect ciliogenesis, but is required for Hh signaling in a specific cell type.
Enrichment of Hh pathway components in cilia
Based on the genetic studies associated Hh signaling with cilia, several groups have tested whether the proteins that mediate Hh signal transduction are localized to cilia. Remarkably, all the key components of the Hh pathway are enriched in cilia (Fig. 3b).
Two transmembrane proteins, Ptch1 (the Hh receptor) and Smo (which acts downstream of Ptch1), show dynamic, Hh-dependent trafficking in cilia35, 36. In the absence of Hh ligand, Ptch1 is localized to the base of the cilium and Smo is not associated with cilia. Upon exposure to ligand, Ptch1 exits and Smo moves into the cilium35, 36. Activating mutations in Smo, as well as pathway agonists, cause Smo to localize constitutively to the cilium36. Despite the enrichment of Smo in the cilium in response to Shh, Smo also accumulates in cilia in cells that lack the Dync2h1 retrograde IFT motor in the absence of ligand37, 38, suggesting that Smo trafficks through the cilium in the absence of ligand, and that Shh increases ciliary accumulation of Smo.
Gli transcription factors are also enriched in cilia. Both Gli2, which functions primarily as a transcriptional activator in mammalian Hh signaling, and Gli3, which can be processed into a repressor, localize to the tips of cilia39, and recent reports indicate that pathway activation increases the amount of Gli2 and Gli3 at the tips of cilia in fibroblasts37, 40. Ciliary enrichment of Gli2 depends on the presence of activated Smo37. Like Smo, Gli2 accumulates at high levels in Dync2h1 mutant cilia37, suggesting that Gli2 trafficks continuously through the cilium, and activated Smo increases the accumulation of Gli2 at the tip.
Sufu, an important negative regulator of mammalian Hh signaling, also localizes to the primary cilia tip39,40. Genetic and biochemical data have shown that Sufu can inhibit Hh signaling even in the absence of cilia,41, 42 however partial knockdown of Sufu results in pathway activation only if cilia are present, suggesting a complex role for Sufu in the cilium20 (M. Tuson and K. Anderson, unpub. data). While the relationship between Sufu and cilia remains to be defined, the data are consistent with a model in which Smo activates the pathway at the cilia tip by antagonizing the activity of Sufu, thereby promoting activation of Gli transcription factors40 (Fig. 3b). Thus, Gli2 activation requires cilia, however the precise mechanism by which this occurs is not defined. In addition to suppression of Sufu, it may require post-translational modifications to Gli2 and the presence of yet-unidentified Hh pathway components within cilia.
Trafficking within the Cilium Regulates Hh Signaling
The finding that vertebrate Hh signaling requires primary cilia has raised the question of why this organelle is particularly suited to this critical pathway. The simplest explanation is that the cilium provides an environment where pathway components are enriched to facilitate their interactions. However, the dynamic relocalization of pathway components in response to ligand suggests that trafficking of Hh pathway proteins is crucial for pathway activation, and it is likely that IFT proteins are important in this trafficking.
IFT depends on two protein complexes, IFT-A and IFT-B, that form large platforms transporting cargo between the base and tip of the cilium13 (Fig. 1). Mutants lacking components of the IFT-B protein complex (Ift172, Ift88, Ift52 and Ift57)11, 21, 43 lack cilia and all response to Hh ligands, precluding analysis of the role of IFT-mediated transport within the cilium. In contrast, mutations in IFT-A proteins allow the formation of cilia (with abnormal morphology) and cause very different developmental phenotypes from mutants that prevent cilia formation: Hh signaling is activated rather than decreased (Fig. 2, Table 1). Studies in Chlamydomonas argue that the IFT-A complex cooperates with dynein to mediate retrograde transport1, as the rate of anterograde IFT is normal in these mutants, while retrograde trafficking is slowed12, 14, 44. Mutants in two mouse IFT-A complex proteins have been characterized, THM-1 (aka Ttc1b; IFT-139) and IFT-122. These mutants show an expansion of Hh-dependent neural cell types, as well as increased expression of direct Hh target genes15, 45, 46.
The opposing phenotypes of IFT-A and IFT-B mouse mutants are surprising, as IFT-A and -B were originally identified as subcomplexes of a single large complex47, and appear to move coordinately in the same particle48, 49. Both IFT-A and Dync2h1 are important for retrograde IFT, but mutations in IFT-A proteins increased Hh pathway activity15, 45 while mutations in Dync2h1 block the response to Hh ligands18, 19. These findings suggest that disruption of IFT-A may differentially disrupt trafficking of Hh pathway components, thereby causing phenotypes distinct from those observed in mutants in which cilia or absent or the dynein motor is disrupted. Recent data suggests that Smo may be trafficked laterally from the plasma membrane into the cilium, presumably an IFT-independent mechanism50. Given that the IFT machinery functions downstream of Smo but upstream of the Gli transcription factors, it will be particularly informative to examine trafficking of Smo and the Gli proteins in IFT-A mutants.
Why is Hh Signaling Tied to Cilia?
Kif7 as a link between Hh signaling and cilia
Despite the evolutionary conservation of the Hh pathway and the importance of primary cilia in vertebrate Hh signaling, cilia are not required for Hh signaling in Drosophila. This raises the question of why vertebrate Hh signaling is coupled to cilia. Recent data suggest that Kif7, a kinesin that is the vertebrate homolog of Drosophila Costal2 (Cos2), may tether the vertebrate Hh pathway to cilia.
Cos2, a key component of the Drosophila Hh pathway, is a kinesin-related protein that serves as a scaffold for Hh signaling complexes. Cos2 has dual functions in the pathway: it promotes formation of the repressor form of Ci (the Gli homolog) in the absence of Hh ligand by recruiting kinases that prime Ci for processing; and it permits high levels of pathway activation upon Hh stimulation by antagonizing Sufu51–53 (Fig. 3). Although Cos2 can bind microtubules, amino acids in its motor domain have diverged from those of other kinesins such that its ability to bind ATP is disrupted54.
Several recent papers demonstrated that zebrafish and mouse Kif7 proteins, like Drosophila Cos2, both positively and negatively regulate the Shh pathway40, 55–57. Unlike Cos2, the vertebrate Kif7 motor domain retains all the motifs typical of kinesin motors, suggesting that it should act as a motor protein. In the absence of ligand, Kif7 localizes to the base of the primary cilium and moves to the tip of the cilium in response to pathway activation40, 55 (Fig. 3). This translocation depends on the Kif7 motor domain, suggesting that Kif7, like Kinesin-II, acts as an anterograde motor within the cilium55.
Conventional kinesins, such as Kif7, carry cargo towards the plus end of microtubules, and the minus ends of both axonemal and cytoplasmic microtubules are located at the base of the cilium. Because Kif7-eGFP is enriched at the base of the cilium, we proposed that Kif7 may traffic Gli2 protein away from the cilium in the absence of ligand to prevent Gli activation55 (Fig. 3b). The positive role of Kif7 is presumably coupled to its movement to the cilia tip after pathway activation, where Sufu and the Gli transcription factors are enriched. Kif7 may promote Gli activation at the tip, perhaps by antagonizing the activity of Sufu55. Kif7 appears to be required for the increase of both Gli2 and Gli3 at the cilia tip after exposure to ligand40, suggesting both Gli proteins may be cargos of Kif7 within the cilium. Thus, the dual roles of Kif7 as a Shh pathway component and ciliary motor could explain why mammalian Shh signaling depends on the primary cilium (Fig. 3b).
Fused is a cilia-associated protein in vertebrates
Fused (Fu) is an important component of the Drosophila Hh pathway: it is a serine/threonine kinase that phosphorylates Cos2, Sufu and perhaps other components of the pathway. and is required for activation of Ci in response to Hh ligand53. Fu is also important for Hh signaling in zebrafish,58 but Hh signaling is normal in mice lacking Fu59,60. Recent work has shown that mammalian and zebrafish Fu are required for the construction of specialized motile cilia61, and Fu mutant mice die postnatally with hydrocephalus, presumably due to dysfunction of motile cilia in brain ventricles59,60. Thus Fu, like Kif7/Cos2, links Hh signaling with cilia, although this connection appears to have been lost in mammals. It has been proposed that another unidentified kinase may substitute for Fu in mammalian Hh signaling, and several human kinome screens have been undertaken to identify kinases required for Hh signaling62, 63. While the kinases identified in these screens have yet to be characterized in vivo, it will be interesting to determine whether a protein that is functionally homologous to Fu also links Shh signaling to cilia in mammals.
The evolution of Kif7 and Fused
Recent work in planaria supports the view that some conserved components of the Hh pathway were associated with cilia before they were associated with Hh signaling. Planaria homologues of the Hh pathway components Kif7/Cos2, Fu, and Iguana are required in planaria for formation of motile cilia but not Hh signaling64, 65. Planaria represent a lineage of animals distinct from both that of insects and of vertebrates. Thus, the finding that Kif7 and Fu function in cilia in two independent metazoan lineages suggests the ancestral role of these proteins was in cilia. The requirement for these cilia-associated proteins in Drosophila Hh signaling suggests that Hh signaling was associated with cilia in the common ancestor of Drosophila and vertebrates.
Are Cilia Dedicated to Hh Signaling?
Most cells in the mouse embryo have primary cilia, while a relatively small number of cells respond to Hh at any particular stage. This has raised interest in the possibility that other developmental signaling pathways may also depend on cilia. However, the disruption of other developmental signaling pathways, including canonical and non-canonical Wnt, TGFβ, Notch and FGF signaling, causes developmental abnormalities that do not overlap with the IFT mutant phenotypes. Nevertheless, cilia could have more subtle roles in other signaling pathways or might be important for signaling at later embryonic stages, after IFT mutants arrest.
Wnt signaling
Most attention has focused on the relationship between cilia and Wnt signaling. Several groups reported that knockdown of cilia-associated proteins in cultured cells or zebrafish embryos elevates canonical Wnt signaling and/or disrupts processes that depend on non-canonical Wnt signaling, such as convergent extension66–72. The primary cilium was therefore proposed to act as a switch between canonical and non-canonical Wnt signaling pathways68, 69.
However, this connection between cilia and Wnt signaling is controversial. Mouse IFT mutants do not show the phenotypes characteristic of Wnt pathway mutants. For example, reduced canonical Wnt signaling disrupts gastrulation and early patterning73 and inappropriate activation of the Wnt pathway can cause axis duplications and failure to form anterior structures74–77. Although mammalian non-canonical Wnt pathway mutants fail to close the entire neural tube caudal to the forebrain, neural patterning in these mutants is relatively normal78, 79. Similarly, zebrafish mutants that lack both maternal and zygotic activity of the Ift88 gene have defects in Hh signaling, but do not show the defects in convergent extension defects associated with disruption of non-canonical Wnt signaling22.
Recent work examined the expression of canonical Wnt reporters in vivo in Kif3a, Ift88, Ift172 and Dync2h1 mutant mice and failed to find any alteration in either the domain or levels of Wnt activity38. Similarly, mouse embryos homozygous for a mutation in the IFTA protein Tct21b (IFT-139) have a neural patterning phenotype consistent with activation of Hh signaling15, 46, but do not show altered canonical Wnt signaling46. Thus it appears that cilia are not required for canonical or noncanonical Wnt signaling in the first half of vertebrate embryogenesis.
Pdgfra signaling
Cilia have been found to be important for signaling by PDGF receptor alpha (Pdgfrα) in cultured fibroblasts80, as well as for PDGF-dependent directed cell migration in these cells81. Additionally, the receptor is localized to primary cilia in vivo in neural stem cells of the adult rat subventricular zone (SVZ)82. Loss of Pdgfrα signaling does not produce any striking phenotypes in early mouse embryos, but is critical for development of later tissues, including oligodendrocytes and neural crest-derived craniofacial structures83, 84. It will be important to test whether loss of cilia in the second half of embryogenesis affects Pdgfrα signaling in these cell types in vivo.
Hedgehog signaling in adult tissues
After birth, Shh signaling continues to play important roles in the growth of the brain and the maintenance of neural progenitors85. Conditional deletion of Ift88 or Kif3a within the brain results in severe hypoplasia of the cerebellum due to a failure of granule cell progenitor proliferation86, a process that depends on Shh signaling85, 87. Primary cilia are also required to modulate the Shh-dependent formation and maintenance of hippocampal granule neuron precursors, important for maintaining neurogenesis in the adult88. Based on the tight association between IFT/cilia mutants and specific defects on Hh signaling, we propose that cilia are essential for Hedgehog signaling in all cell types and that, at least in early development, primary cilia in vertebrate embryos are dedicated to Hh signal transduction. The data do not rule out the possibility that cilia may have important roles in other signaling pathways later in development or in specific cell types. For example, although a number of the defects observed in human ciliopathies can be attributed to abnormal Hh signaling, the molecular bases of other features of these diseases remain unknown (Box 1).
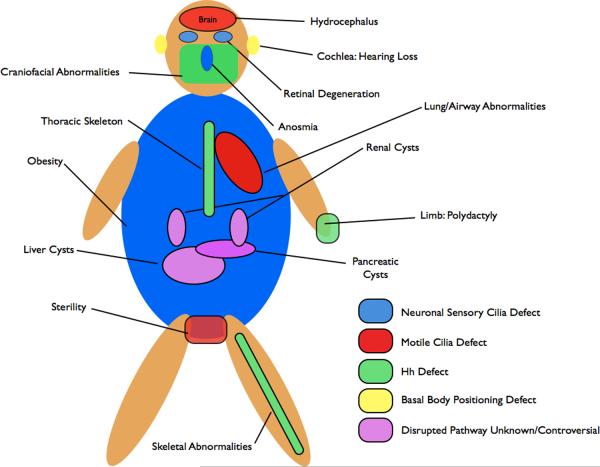
Box 1. Organs affected in human ciliopathies.
Numerous pleiotropic human disorders have been attributed to defects in cilia formation10, 135. Some aspects of these syndromes, such as the polydactyly in patients with Bardet-Biedl syndrome (BBS) and Meckel Syndrome (MKS) and the skeletal abnormalities affecting the limbs in Ellis-van Creveld are attributed to defective Hh signaling. Polydactyly results from a loss of Gli3R and skeletal abnormalities resemble those observed in mutants that lack Ihh signaling136. Shh signaling is also required for craniofacial development, and defects in craniofacial structures, such as those observed in Mks1 mutant mice, are also likely due to mis-regulated Hh signaling32. In addition, patients with a Joubert syndrome-like disorder exhibit ataxia due to cerebellar hypoplasia137. Growth of this tissue is Hh dependent85. Other attributes of human disorders result from defective specialized cilia. Retinal degeneration results from defects to photoreceptor cilia, which connect the outer light-responsive segment to the cell body. Detection of odorants depends on the primary cilia of sensory neurons in the olfactory epithelium and BBS patients often exhibit anosmia138–140. Another sensory deficit, hearing loss, is due to a requirement for the specialized primary cilia of the cochlea downstream of the PCP pathway in establishing the correct polarity of sensory hair cells99. Infertility observed in patients with ciliopathies is the result of defective motile cilia of spermatids or oviducts139.
For some of the most severe and common abnormalities associated with ciliopathies, such as cyst formation in the kidneys, liver, biliary duct and pancreas, the underlying molecular causes downstream of the cilium remain unclear. Cyst formation is thought to result from defects in cell proliferation or mis-orientation of the mitotic spindle, however, whether and how these processes are regulated by cilia remain the subject of active investigation124. In addition, BBS patients often exhibit obesity and cognative impairments thought to be due to neuronal defects, however the specific pathways responsible for these attributes in BBS patients have not been clearly identified141, 142.
Signaling Pathways that Regulate Ciliary Development
Because of the importance of primary cilia in embryonic patterning, there is considerable interest in identifying signaling pathways that regulate cilia formation. Several transcription factors are known to be required for formation of motile cilia and node cilia (reviewed by89). However, only recently has evidence emerged about signaling pathways that regulate the formation and position of primary cilia.
Fgf and inositol signaling
Recent evidence from zebrafish implicates FGF signaling in the regulation of cilia length. Knockdown of Fgfr1 or Fgf ligands results in shortened cilia in Kupffer's vesicle and randomized organ laterality90–92. The expression of ciliogenic transcription factors and Ift88 is reduced in these embryos 91. It will be interesting to test whether Fgf pathway mutations in the mouse also affect ciliogenesis, and if these effects on ciliogenesis alter Hh signal transduction.
Components of the phosphatidylinositol signaling cascade also appear to regulate cilia length. In zebrafish, morpholino knockdown of the inositol kinase Ipk1 reduces the frequency of cilia beating and decreased cilia length93. In humans, the inositol phosphatase INPP5E is mutated in one form of Joubert syndrome, a ciliopathy. INPP5E is enriched in the ciliary axoneme, and in fibroblasts from patients with Joubert syndrome, cilia were more labile than wild type94.
The mechanisms by which the Fgf and phosphatidylinositol pathways regulate cilia formation or maintenance remain to be elucidated. It will be informative to investigate whether these pathways have a general role in primary cilia formation or act in a subset of specialized cilia.
Planar cell polarity signaling and cilia
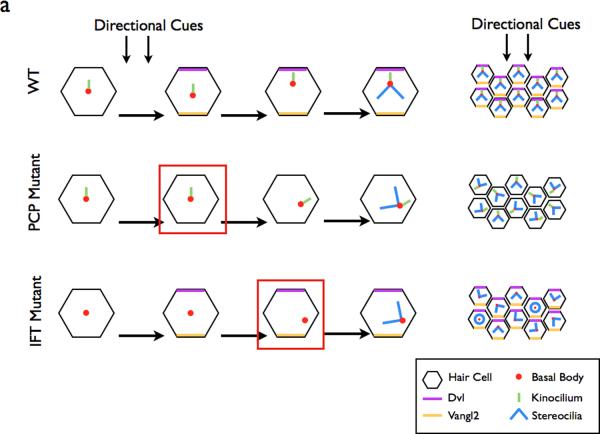
Recent experiments argue that there is a close connection between components of planar cell polarity (PCP) signaling and cilia positioning. An excellent example of this connection is found in the mechanosensory hair cells in the organ of Corti in the cochlea. The primary cilium of the hair cell, called the kinocilium, is always oriented on the lateral side of the developing cell. The position of the kinocilium determines the polarity of the chevron of stereocilia (actin-based sensory organelles) on the hair cell. Core components of the non-canonical Wnt pathway, a PCP pathway, are required for the polarity of these hair cells95–97,98.
When primary cilia are removed by conditional deletion of Ift88, the polarity of the hair cells is disrupted99, similar to the phenotype seen in non-canonical Wnt mutants97. This finding indicated that the presence of the kinocilium is important for the correct orientation of the stereocilia and the organization of the hair cells, and raised the possibility that the primary cilium might regulate the non-canonical Wnt pathway in this tissue; however the relationship between the kinocilium and planar polarity is more complex. As in Drosophila, components of the non-canonical Wnt pathway are planar polarized in hair cells, and that polarity is both required for PCP signaling and provides a readout of effective PCP signaling97, 100. In the hair cells of Ift88 conditional mutants, the polarity of PCP proteins is not disrupted. This indicates that IFT88, and presumably cilia, are not required for the activity of the core PCP pathway in this tissue, as in early embryos. Instead, it appears that one output of the non-canonical Wnt signaling is to control the position of the basal body and thereby cilia position99 (Fig. 4a). In addition, the findings indicate that IFT88 itself must be required to reposition the basal body to a polarized position. The mechanisms by which the position of the basal body is regulated by IFT88 are not known.
Figure 4. The role of the planar cell polarity pathway in cilia formation.
a | In the WT cochlea, directional cues establish localization of core PCP pathway components. The kinocilium then directs the basal body towards the medial side of the cells. This in turn directs the orientation of the stereocilia bundles, resulting in the correct orientation of the bundles within the cochlea. In PCP mutants, the initial cell polarity is never established, resulting in the improper positioning of the basal body and stereocilia. In IFT mutants, planar polarity is established, however the basal body is not repositioned in the absence of the IFT-dependent kinocilium99. Red boxes depict the step in the establishment of ppolarity that is defective in each category of mutants.
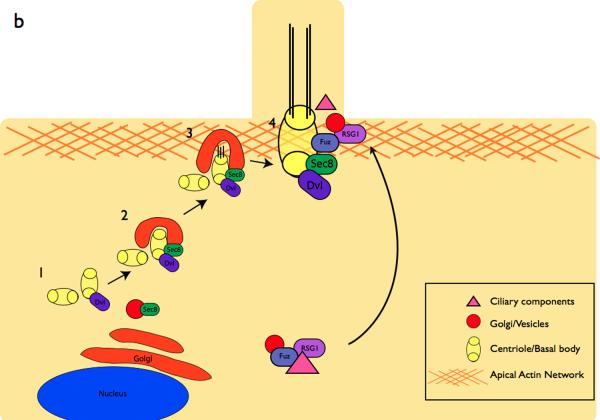
b | Components of the PCP pathway are implicated in cilia formation. The centrioles are initially located away from the cell surface (1)1 where they associate with Dvl, a PCP protein. Sec8, a component of vesicle trafficking machinery is recruited to the basal body in (2)101. At the cell surface in (3), Dvl mediates the fusion of the basal body-associated membrane with the cell membrane101, 134. The axoneme of the cilium extends in (4). In vivo mouse experiments indicate that the PCP effector Fuzzy, together with the small GTPase RGS1, also plays a role in trafficking membrane vesicles and ciliary components to the basal body103.
Studies on the motile cilia on the epidermis of Xenopus embryos support the hypothesis that components of the planar polarity pathway control polarized organization of cilia. These cells are multiciliated and the cilia on each cell share a common polarity101. Disruption of the activity of the PCP protein Disheveled (Dvl) disrupts the polarity of the cilia on these cells. Dvl localizes to the basal bodies of epidermal cells and Dvl morphants have a reduced number of short cilia101; this finding indicates that apical docking of basal bodies, and therefore the ability to form cilia, depends on Dvl and possibly other components of the PCP pathway (Fig. 4b).
While changes in the position of cilia are unlikely to influence their ability to transduce Hh signals, some PCP components do affect Hh signaling. Inturned and Fuzzy are downstream effectors of the non-canonical Wnt pathway in Drosophila, and morpholino knockdown of Xenopus Inturned or Fuzzy disrupts both the apical actin network and cilia formation102. These morphants fail to undergo normal convergent extension due to defects in PCP and also display defects consistent with a loss of Shh signaling102. Similarly, mouse Fuz and Inturned mutants have short cilia and disrupted Hh signaling103–105. Thus, components of the planar polarity pathway can be important for both formation and polarity of cilia (Fig. 4b). There is no evidence, however, that other components of the non-canonical Wnt pathway are required for ciliogenesis, suggesting that the roles of Inturned and Fuzzy in cilia formation may be unrelated to non-canonical Wnt signaling.
Cilia, signaling and disease
Numerous human disorders have now been linked to defects in cilia structure or in cilia localized proteins. These include autosomal dominant polycystic kidney disease (PKD), and recessive pleiotropic disorders such as Bardet-Biedl syndrome, Joubert syndrome, Meckel syndrome, and Ellis-van Creveld syndrome. Some aspects of these disorders, such as polydactyly and skeletal abnormalities, are likely due to misregulated Hh signaling, but the molecular basis of other defects, such as cystic kidneys, are not well understood (Box 1). The dysregulated Hh signaling associated with several types of human cancers also depends on cilia. Thus studies on the relationship between cilia and signaling during development have direct implications for human disease.
Hedgehog Signaling in Tumors
Inappropriate activation of Shh signaling can cause medulloblastoma and rhabdomyosarcoma, pediatric tumors of the cerebellum and muscle, and is found in all cases of basal cell carcinoma106–108. In addition, growing evidence indicates that Shh signals promote the growth of other types of tumors109, 110. Recent studies demonstrate that cilia regulate Hh signaling in tumors, and that the role of cilia in tumors depends on how the pathway is activated. Expression of activated Smo in the postnatal mouse brain can cause medulloblastoma, but removal of cilia prevents tumor formation111, consistent with the earlier genetic experiments indicating that cilia are required for activity of the pathway at a step downstream of Smo. Constitutively active Gli2 can also cause in medulloblastoma in mice, but only when cilia are removed111. This suggests that Gli2 alone cannot activate tumorigenesis in this cellular context when cilia-dependent Gli3 repressor is also present. Similar results were observed in basal cell carcinomas in mice harboring similar activating Hh pathway mutations: activated Smo caused tumors only in the presence of cilia whereas removal of cilia enhanced tumorigenesis caused by expression of activated Gli2112. Thus, in tumors as in development, cilia have both positive and negative effects on the Hh pathway.
Cilia and polycystic kidney disease
A hallmark of many human ciliopathies is the formation of kidney cysts, which often begins during fetal life, and is a developmental, rather than physiological, defect3, 113. This raises the question of whether kidney cysts result from a disruption of cilia-dependent developmental signals. Hh signaling is required for normal kidney development114, but kidney cysts have not been reported in mutants that lack either positive or negative Hh regulators115, 116. Although a role for Hh signaling in PKD cannot be ruled out, some data suggest that cilia might modulate Wnt signaling in this tissue117.
The connection between cilia, cystic kidneys and Wnt signaling was first raised by analysis of the inversin (inv) gene. Inv binds microtubules, and localizes to the basal body118 and cilium119. Mutations in inv cause kidney disease in humans120, and renal cysts in mice118. Inv interacts with Dvl, targeting membrane-bound Dvl for destruction. Inv has been proposed to act as a switch between canonical and non-canonical Wnt signaling, based on cell culture and morpholino knock-down experiments68. However, altered canonical Wnt signaling has not been reported in the kidneys of inv−/− mice.
Mouse overexpression experiments show that increased Wnt signaling can cause kidney cysts, and increased nuclear β-catenin is observed in the cystic kidney tubules of mice in which cilia have been conditionally ablated121, 122. However, reduced Wnt signaling has been observed in mice lacking Jouberin (Jbn), a cilia-localized protein mutated in a form of Joubert syndrome: mice that lack Jbn (Ahi1−/−) have cystic kidneys with reduced expression of a Wnt reporter and reduced nuclear β-catenin123. Thus additional experiments that examine Wnt signaling during kidney development will be required to reconcile the conflicting data.
Recent theories of PKD have focused on the importance of the plane of cell division, under the control of PCP signaling, as a possible underlying defect in kidney cysts124. The elongation of kidney tubules is thought to depend on oriented cell divisions, and this is disrupted in kidney tubule cells of mice with cystic kidneys117, 124. Supporting this hypothesis, mice lacking the PCP protein Fat4 exhibit polycystic kidneys beginning at E16 associated with misorientation of mitotic spindles within the renal tubules125. This cyst formation is enhanced by removal of one or both copies of a core PCP pathway component Vangl2. Moreover, Fat4 localizes to cilia within the kidney, implicating the cilium in the modulation of the kidney PCP pathway125. Drosophila Fat, however, acts in a PCP pathway that does not depend on non-canonical Wnt signaling126. Moreover, mouse mutants of other PCP pathway components such as Vangl2 and Fuzzy have not been reported to have cystic kidneys, and the polarity of PCP component localization in kidney tubule cells has not been assessed in mice lacking renal cilia. Recent results suggest that misoriented cell division is neither necessary nor sufficient for the formation of kidney cysts113. Thus the pathway (or pathways) downstream of primary cilia that are misregulated to cause renal cysts remain to be determined.
Conclusions and Perspectives
Non-motile primary cilia play vital roles in vertebrate development from early stages of embryonic patterning, when they regulate the activity of the Hh pathway, to organogenesis, when they are important in the development and homeostasis of numerous tissues. The recent resurgence in interest in primary cilia has raised many new questions about the roles of cilia.
We know very little about the events of Hh signal transduction that occur within cilia. The mechanisms that traffic Smo to cilia, Ptch out of cilia, and that modulate the trafficking of Gli proteins within cilia in response to Hh pathway activation are all unknown. The opposing effects of different IFT components upon the regulation of the Hh pathway suggest that, in addition to providing a compartment where Hh pathway components are enriched, the IFT machinery plays a more complex role in regulating the pathway, but those roles have not been defined. In order to address these questions, it will be necessary to examine the trafficking of Hh pathway components in real time as well as probe their physical associations with the IFT machinery in wild-type cells as well as in cells mutant for various IFT components.
The dual roles of Kif7 in intraciliary trafficking and in the Hh pathway suggest a reason why vertebrate Hh signaling is tied to cilia, and other proteins may also have dual roles. For example: is there a mammalian kinase that performs functions analogous to those of Drosophila Fu and, if so, does it play roles in both ciliogenesis and Hh signaling? Do other components of the Hh signaling pathway affect the dynamics of ciliary trafficking?
Based on the phenotypes of the numerous IFT mutants characterized to date, it appears that during early vertebrate development, the cilium functions as a Hh-dedicated organelle. However, this does not preclude a requirement for cilia in modulating other signaling pathways in specific tissues later in development. These may include Pdgfrα signaling, signaling through G-protein coupled receptors in specific neurons127, and PCP signaling in the kidney. A particularly interesting question is whether, in specific cell types, cilia are sites where Hh and other signaling pathways are integrated. As complex cross-talk between pathways is vital in regulating cellular responses during development and in disease states like cancer, understanding the function of the cilium as a signaling center will be critical.
Acknowledgements
This work was funded by NIH grant NS044385 to KVA. SCG is an American Cancer Society postdoctoral fellow.
References
- 1.Pedersen LB, Veland IR, Schroder JM, Christensen ST. Assembly of primary cilia. Dev Dyn. 2008;237:1993–2006. doi: 10.1002/dvdy.21521. [DOI] [PubMed] [Google Scholar]
- 2.Silverman MA, Leroux MR. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 2009;19:306–16. doi: 10.1016/j.tcb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Lancaster MA, Gleeson JG. The primary cilium as a cellular signaling center: lessons from disease. Curr Opin Genet Dev. 2009;19:220–9. doi: 10.1016/j.gde.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens. 2009;18:99–106. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- 6.Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321–37. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerdes J, Davis EE, Katsanis N. The Vertebrate Primary Cilium in Development, Homeostasis, and Disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–48. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 9.Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C:281–95. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- 10.Tobin JL, Beales PL. The nonmotile ciliopathies. Genet Med. 2009;11:386–402. doi: 10.1097/GIM.0b013e3181a02882. [DOI] [PubMed] [Google Scholar]
- 11.Huangfu D, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–7. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 12.Blacque OE, et al. The WD repeat-containing protein IFTA-1 is required for retrograde intraflagellar transport. Mol Biol Cell. 2006;17:5053–62. doi: 10.1091/mbc.E06-06-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole DG. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic. 2003;4:435–42. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- 14.Iomini C, Babaev-Khaimov V, Sassaroli M, Piperno G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J Cell Biol. 2001;153:13–24. doi: 10.1083/jcb.153.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran P, et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provided the first phenotypic description of and IFT-A mutant, and showed that disrupting IFT-A, in contrast to other IFT mutants, causes hyper activation of the Hh pathway.
- 16.Kim JC, et al. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat Genet. 2004;36:462–70. doi: 10.1038/ng1352. [DOI] [PubMed] [Google Scholar]
- 17.Nachury M, et al. A Core Complex of BBS Proteins Cooperates with the GTPase Rab8 to Promote Ciliary Membrane Biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 18.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA. 2005;102:11325–30. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May SR, et al. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Developmental Biology. 2005;287:378–89. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 20.Ocbina PJ, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev Dyn. 2008;237:2030–8. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–11. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 22.Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–98. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]; References 20 and 22 demonstrate a conserved requirement for cilia in Hh signaling, but not Wnt signaling, in vertebrates.
- 23.Ede DA, Kelly WA. Developmental Abnormalities in the Trunk and Limbs of the Talpid3 Mutant of the Fowl. J Embryol Exp Morphol. 1964;12:339–56. [PubMed] [Google Scholar]
- 24.Ede DA, Kelly WA. Developmental Abnormalities in the Head Region of the Talpid Mutant of the Fowl. J Embryol Exp Morphol. 1964;12:161–82. [PubMed] [Google Scholar]
- 25.Lewis KE, et al. Expression of ptc and gli genes in talpid3 suggests bifurcation in Shh pathway. Development. 1999;126:2397–407. doi: 10.1242/dev.126.11.2397. [DOI] [PubMed] [Google Scholar]
- 26.Davey MG, James J, Paton IR, Burt DW, Tickle C. Analysis of talpid3 and wild-type chicken embryos reveals roles for Hedgehog signalling in development of the limb bud vasculature. Dev Biol. 2007;301:155–65. doi: 10.1016/j.ydbio.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Yin Y, et al. The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development. 2009;136:655–64. doi: 10.1242/dev.028464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delous M, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–81. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 29.Ferrante M, et al. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet. 2006;38:112–117. doi: 10.1038/ng1684. [DOI] [PubMed] [Google Scholar]
- 30.Ferrante MI, et al. Identification of the gene for oral-facial-digital type I syndrome. Am J Hum Genet. 2001;68:569–76. doi: 10.1086/318802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vierkotten J, Dildrop R, Peters T, Wang B, Ruther U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134:2569–77. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- 32.Weatherbee SD, Niswander LA, Anderson KV. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum Mol Genet. 2009;18:4565–75. doi: 10.1093/hmg/ddp422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz-Perez VL, et al. Mutations in a new gene in Ellis-van Creveld syndrome and Weyers acrodental dysostosis. Nat Genet. 2000;24:283–6. doi: 10.1038/73508. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz-Perez VL, et al. Mutations in two nonhomologous genes in a head-to-head configuration cause Ellis-van Creveld syndrome. Am J Hum Genet. 2003;72:728–32. doi: 10.1086/368063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohatgi R, Milenkovic L, Scott M. Patched1 Regulates Hedgehog Signaling at the Primary Cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 36.Corbit KC, et al. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 37.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One. 2009;4:e6839. doi: 10.1371/journal.pone.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haycraft C, et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]; References 35, 36, and 39 demonstrated that components of the Hh pathway localize to cilia, providing cellular evidence of a direct connection between cilia and Hh signaling.
- 40.Endoh-Yamagami S, et al. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr Biol. 2009;19:1320–6. doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 41.Jia J, et al. Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Developmental Biology. 2009:1–31. doi: 10.1016/j.ydbio.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen MH, et al. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 2009;23:1910–28. doi: 10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houde C, et al. Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Dev Biol. 2006;300:523–33. doi: 10.1016/j.ydbio.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iomini C, Li L, Esparza JM, Dutcher SK. Retrograde IFT Mutants Identify Complex A Proteins With Multiple Genetic Interactions in Chlamydomonas reinhardtii. Genetics. 2009 doi: 10.1534/genetics.109.101915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cortellino S, et al. Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Developmental Biology. 2009;325:225–37. doi: 10.1016/j.ydbio.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stottmann RW, Tran PV, Turbe-Doan A, Beier DR. Ttc21b is required to restrict sonic hedgehog activity in the developing mouse forebrain. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole DG, et al. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ou G, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–7. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 49.Qin H, et al. Intraflagellar Transport Is Required for the Vectorial Movement of TRPV Channels in the Ciliary Membrane. Current Biology. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 50.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187:365–74. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sisson JC, Ho KS, Suyama K, Scott MP. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell. 1997;90:235–45. doi: 10.1016/s0092-8674(00)80332-3. [DOI] [PubMed] [Google Scholar]
- 52.Wang G, Amanai K, Wang B, Jiang J. Interactions with Costal2 and suppressor of fused regulate nuclear translocation and activity of cubitus interruptus. Genes Dev. 2000;14:2893–905. doi: 10.1101/gad.843900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aikin RA, Ayers KL, Therond PP. The role of kinases in the Hedgehog signalling pathway. EMBO Rep. 2008;9:330–6. doi: 10.1038/embor.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farzan SF, et al. Costal2 functions as a kinesin-like protein in the hedgehog signal transduction pathway. Curr Biol. 2008;18:1215–20. doi: 10.1016/j.cub.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liem KF, Jr., He M, Ocbina PJ, Anderson KV. Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2009;106:13377–82. doi: 10.1073/pnas.0906944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheung HO, et al. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Sci Signal. 2009;2:ra29. doi: 10.1126/scisignal.2000405. [DOI] [PubMed] [Google Scholar]
- 57.Tay SY, Ingham PW, Roy S. A homologue of the Drosophila kinesin-like protein Costal2 regulates Hedgehog signal transduction in the vertebrate embryo. Development. 2005;132:625–34. doi: 10.1242/dev.01606. [DOI] [PubMed] [Google Scholar]; References 55–57 together with reference 40 demonstrate that the Cos2 homolog Kif7 is required for Hh signaling in vertebrate development, contrasting with previous reports that its role in Hh signaling was not evolutionarily conserved.References 36 and 63 showed that Kif7-GFP fusion proteins localize to cilia, and reference 63 further demonstrated that this localization is ligand-dependent, proposing that Kif7 may act as accessory anterograde motor linking cilia trafficking with the Hh pathway.
- 58.Wolff C, Roy S, Ingham PW. Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr Biol. 2003;13:1169–81. doi: 10.1016/s0960-9822(03)00461-5. [DOI] [PubMed] [Google Scholar]
- 59.Chen MH, Gao N, Kawakami T, Chuang PT. Mice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic development. Mol Cell Biol. 2005;25:7042–53. doi: 10.1128/MCB.25.16.7042-7053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merchant M, et al. Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus. Mol Cell Biol. 2005;25:7054–68. doi: 10.1128/MCB.25.16.7054-7068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson CW, et al. Fused has evolved divergent roles in vertebrate Hedgehog signalling and motile ciliogenesis. Nature. 2009;459:98–102. doi: 10.1038/nature07883. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides establishes Fused as a link between the Hh pathway and cilia in vertebrates, demonstrating that it is required both for cilia structure and Hh signaling in zebrafish, and for motile cilia formation in mammals, although the link with Hh appears to have been lost in mammals.
- 62.Evangelista M, et al. Kinome siRNA Screen Identifies Regulators of Ciliogenesis and Hedgehog Signal Transduction. Science Signaling. 2008;1:ra7–ra7. doi: 10.1126/scisignal.1162925. [DOI] [PubMed] [Google Scholar]
- 63.Varjosalo M, et al. Application of active and kinase-deficient kinome collection for identification of kinases regulating hedgehog signaling. Cell. 2008;133:537–48. doi: 10.1016/j.cell.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 64.Glazer AM, et al. The Zn Finger protein Iguana impacts Hedgehog signaling by promoting ciliogenesis. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rink JC, Gurley KA, Elliott SA, Alvarado AS. Planarian Hh Signaling Regulates Regeneration Polarity and Links Hh Pathway Evolution to Cilia. Science. 2009 doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]; References 64 and 65 show that several components of the Hh pathway in Drosophila, Iguana, Fu, and Cos2, are required in a separate invertebrate lineage for cilia formation. This provided evidence that the connection between the Hh pathway and cilia might be evolutionarily ancient.
- 66.Ross AJ, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–40. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 67.Gerdes J, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 68.Simons M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–43. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corbit K, et al. Kif3a constrains β-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- 70.Bergmann C, et al. Loss of Nephrocystin-3 Function Can Cause Embryonic Lethality, Meckel-Gruber-like Syndrome, Situs Inversus, and Renal-Hepatic-Pancreatic Dysplasia. The American Journal of Human Genetics. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jonassen JA, San Agustin J, Follit JA, Pazour GJ. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol. 2008;183:377–84. doi: 10.1083/jcb.200808137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiens CJ, et al. The Bardet-Biedl syndrome-associated small GTPase ARL6 (BBS3) functions at or near the ciliary gate and modulates Wnt signalling. J Biol Chem. doi: 10.1074/jbc.M109.070953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barrow J, et al. Wnt3 signaling in the epiblast is required for proper orientation of the anteroposterior axis. Developmental Biology. 2007;312:312–320. doi: 10.1016/j.ydbio.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 74.Chazaud C, Rossant J. Disruption of early proximodistal patterning and AVE formation in Apc mutants. Development. 2006;133:3379–87. doi: 10.1242/dev.02523. [DOI] [PubMed] [Google Scholar]
- 75.Zeng L, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–92. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 76.Mukhopadhyay M, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–34. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 77.Ishikawa TO, Tamai Y, Li Q, Oshima M, Taketo MM. Requirement for tumor suppressor Apc in the morphogenesis of anterior and ventral mouse embryo. Dev Biol. 2003;253:230–46. doi: 10.1016/s0012-1606(02)00020-9. [DOI] [PubMed] [Google Scholar]
- 78.Greene ND, Gerrelli D, Van Straaten HW, Copp AJ. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech Dev. 1998;73:59–72. doi: 10.1016/s0925-4773(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 79.Curtin JA, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–33. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 80.Schneider L, et al. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–6. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 81.Schneider L, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 25:279–92. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Danilov AI, et al. Ultrastructural and antigenic properties of neural stem cells and their progeny in adult rat subventricular zone. Glia. 2009;57:136–52. doi: 10.1002/glia.20741. [DOI] [PubMed] [Google Scholar]
- 83.Klinghoffer RA, Hamilton TG, Hoch R, Soriano P. An allelic series at the PDGFalphaR locus indicates unequal contributions of distinct signaling pathways during development. Dev Cell. 2002;2:103–13. doi: 10.1016/s1534-5807(01)00103-4. [DOI] [PubMed] [Google Scholar]
- 84.Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- 85.Ruiz i Altaba A, Palma V, Dahmane N. Hedgehog-Gli signalling and the growth of the brain. Nat Rev Neurosci. 2002;3:24–33. doi: 10.1038/nrn704. [DOI] [PubMed] [Google Scholar]
- 86.Chizhikov V, et al. Cilia Proteins Control Cerebellar Morphogenesis by Promoting Expansion of the Granule Progenitor Pool. Journal of Neuroscience. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vaillant C, Monard D. SHH pathway and cerebellar development. Cerebellum. 2009;8:291–301. doi: 10.1007/s12311-009-0094-8. [DOI] [PubMed] [Google Scholar]
- 88.Han Y, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 89.Lee JD, Anderson KV. Morphogenesis of the node and notochord: the cellular basis for the establishment and maintenance of left-right asymmetry in the mouse. Dev Dyn. 2008;237:3464–76. doi: 10.1002/dvdy.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong SK, Dawid IB. FGF-dependent left-right asymmetry patterning in zebrafish is mediated by Ier2 and Fibp1. Proc Natl Acad Sci U S A. 2009;106:2230–5. doi: 10.1073/pnas.0812880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neugebauer J, Amack J, Peterson A, Bisgrove B, Yost H. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature. 5:2009. doi: 10.1038/nature07753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yamauchi H, Miyakawa N, Miyake A, Itoh N. Fgf4 is required for left-right patterning of visceral organs in zebrafish. Dev Biol. 2009;332:177–85. doi: 10.1016/j.ydbio.2009.05.568. [DOI] [PubMed] [Google Scholar]
- 93.Sarmah B, Winfrey VP, Olson GE, Appel B, Wente SR. A role for the inositol kinase Ipk1 in ciliary beating and length maintenance. Proc Natl Acad Sci U S A. 2007;104:19843–8. doi: 10.1073/pnas.0706934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bielas SL, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–6. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Montcouquiol M, et al. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–7. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 97.Wang J, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–5. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qian D, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–33. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones C, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]; This study revealed a complex link between cilia and the PCP pathway, wherein PCP is required for the polarized orientation of the hair cell primary cilium, which in turn acts in parallel with the PCP pathway to promote correct orientation of stereocilia in the cochlea.
- 100.Montcouquiol M, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26:5265–75. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park T, Mitchell B, Abitua P, Kintner C, Wallingford J. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–11. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 103.Gray RS, et al. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol. 2009;11:1225–32. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heydeck W, Zeng H, Liu A. Planar cell polarity effector gene Fuzzy regulates cilia formation and Hedgehog signal transduction in mouse. Dev Dyn. 2009;238:3035–42. doi: 10.1002/dvdy.22130. [DOI] [PubMed] [Google Scholar]
- 105.Zeng H, Hoover AN, Liu A. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev Biol. 339:418–28. doi: 10.1016/j.ydbio.2010.01.003. [DOI] [PubMed] [Google Scholar]; References 101–105 demonstrate that components of the PCP pathway can function upstream of cilia in certain contexts by regulating the apical docking of basal bodies in conjuction with vesical trafficking components.
- 106.Hahn H, et al. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med. 1998;4:619–22. doi: 10.1038/nm0598-619. [DOI] [PubMed] [Google Scholar]
- 107.Daya-Grosjean L, Couve-Privat S. Sonic hedgehog signaling in basal cell carcinomas. Cancer Lett. 2005;225:181–92. doi: 10.1016/j.canlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 108.Marino S. Medulloblastoma: developmental mechanisms out of control. Trends Mol Med. 2005;11:17–22. doi: 10.1016/j.molmed.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 109.Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 110.Theunissen JW, de Sauvage FJ. Paracrine Hedgehog signaling in cancer. Cancer Res. 2009;69:6007–10. doi: 10.1158/0008-5472.CAN-09-0756. [DOI] [PubMed] [Google Scholar]
- 111.Han YG, et al. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15:1062–5. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong SY, et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–61. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; References 111 and 112 demonstrate that cilia are important to modulate Hh signaling in two types of tumors- medulloblastoma and basal cell carcinoma. Furthermore, while ablation of cilia in cells with activated Smo suppresses tumor formation, both studies found that activation of Gli2 results in tumors only when cilia are absent. Thus these results underscore that cilia have both positive and negative roles in Hh signaling.
- 113.Nishio S, et al. Loss of Oriented Cell Division Does not Initiate Cyst Formation. J Am Soc Nephrol. 2009 doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gill PS, Rosenblum ND. Control of murine kidney development by sonic hedgehog and its GLI effectors. Cell Cycle. 2006;5:1426–30. doi: 10.4161/cc.5.13.2928. [DOI] [PubMed] [Google Scholar]
- 115.Hu M. GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development. 2006;133:569–578. doi: 10.1242/dev.02220. [DOI] [PubMed] [Google Scholar]
- 116.Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–12. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- 117.Benzing T, Simons M, Walz G. Wnt signaling in polycystic kidney disease. J Am Soc Nephrol. 2007;18:1389–98. doi: 10.1681/ASN.2006121355. [DOI] [PubMed] [Google Scholar]
- 118.Phillips CL, et al. Renal cysts of inv/inv mice resemble early infantile nephronophthisis. J Am Soc Nephrol. 2004;15:1744–55. doi: 10.1097/01.asn.0000131520.07008.b3. [DOI] [PubMed] [Google Scholar]
- 119.Shiba D, et al. Localization of Inv in a distinctive intraciliary compartment requires the C-terminal ninein-homolog-containing region. J Cell Sci. 2009;122:44–54. doi: 10.1242/jcs.037408. [DOI] [PubMed] [Google Scholar]
- 120.Otto EA, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–20. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin F, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003;100:5286–91. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saadi-Kheddouci S, et al. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene. 2001;20:5972–81. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- 123.Lancaster MA, et al. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med. 2009;15:1046–54. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fischer E, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–3. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 125.Saburi S, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–5. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 126.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–72. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA. 2008;105:4242–6. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Omori Y, et al. elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- 129.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–78. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 130.Jia J, Tong C, Jiang J. Smoothened transduces Hedgehog signal by physically interacting with Costal2/Fused complex through its C-terminal tail. Genes Dev. 2003;17:2709–20. doi: 10.1101/gad.1136603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lefers MA, Wang QT, Holmgren RA. Genetic dissection of the Drosophila Cubitus interruptus signaling complex. Dev Biol. 2001;236:411–20. doi: 10.1006/dbio.2001.0345. [DOI] [PubMed] [Google Scholar]
- 132.Lum L, et al. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol Cell. 2003;12:1261–74. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- 133.Osterlund T, Kogerman P. Hedgehog signalling: how to get from Smo to Ci and Gli. Trends Cell Biol. 2006;16:176–80. doi: 10.1016/j.tcb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 134.Pan J, You Y, Huang T, Brody S. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. Journal of Cell Science. 2007;120:1868–1876. doi: 10.1242/jcs.005306. [DOI] [PubMed] [Google Scholar]
- 135.Toriello HV, Parisi MA. Cilia and the ciliopathies: an introduction. Am J Med Genet C Semin Med Genet. 2009;151C:261–2. doi: 10.1002/ajmg.c.30230. [DOI] [PubMed] [Google Scholar]
- 136.Ruiz-Perez VL, et al. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134:2903–12. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- 137.Brancati F, et al. MKS3/TMEM67 mutations are a major cause of COACH Syndrome, a Joubert Syndrome related disorder with liver involvement. Hum Mutat. 2009;30:E432–42. doi: 10.1002/humu.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kulaga H, et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36:994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- 139.Mykytyn K, et al. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci U S A. 2004;101:8664–9. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nishimura DY, et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci U S A. 2004;101:16588–93. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee JH, Gleeson JG. The role of primary cilia in neuronal function. Neurobiol Dis. doi: 10.1016/j.nbd.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Davenport J, et al. Disruption of Intraflagellar Transport in Adult Mice Leads to Obesity and Slow-Onset Cystic Kidney Disease. Current Biology. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cantagrel V, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–9. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mykytyn K, et al. Evaluation of complex inheritance involving the most common Bardet-Biedl syndrome locus (BBS1) Am J Hum Genet. 2003;72:429–37. doi: 10.1086/346172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stoetzel C, et al. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet. 2006;38:521–4. doi: 10.1038/ng1771. [DOI] [PubMed] [Google Scholar]
- 146.Kudryashova E, Wu J, Havton LA, Spencer MJ. Deficiency of the E3 ubiquitin ligase TRIM32 in mice leads to a myopathy with a neurogenic component. Hum Mol Genet. 2009;18:1353–67. doi: 10.1093/hmg/ddp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chiang AP, et al. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet-Biedl syndrome gene (BBS11) Proc Natl Acad Sci U S A. 2006;103:6287–92. doi: 10.1073/pnas.0600158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cossee M, et al. Use of SNP array analysis to identify a novel TRIM32 mutation in limb-girdle muscular dystrophy type 2H. Neuromuscul Disord. 2009;19:255–60. doi: 10.1016/j.nmd.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 149.Stoetzel C, et al. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet-Biedl syndrome. Am J Hum Genet. 2007;80:1–11. doi: 10.1086/510256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nishimura DY, et al. Positional cloning of a novel gene on chromosome 16q causing Bardet-Biedl syndrome (BBS2) Hum Mol Genet. 2001;10:865–74. doi: 10.1093/hmg/10.8.865. [DOI] [PubMed] [Google Scholar]
- 151.Chiang AP, et al. Comparative genomic analysis identifies an ADP-ribosylation factor-like gene as the cause of Bardet-Biedl syndrome (BBS3) Am J Hum Genet. 2004;75:475–84. doi: 10.1086/423903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mykytyn K, et al. Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nat Genet. 2001;28:188–91. doi: 10.1038/88925. [DOI] [PubMed] [Google Scholar]
- 153.Li JB, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–52. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 154.Badano JL, et al. Identification of a novel Bardet-Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. Am J Hum Genet. 2003;72:650–8. doi: 10.1086/368204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ansley SJ, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–33. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 156.Nishimura DY, et al. Comparative genomics and gene expression analysis identifies BBS9, a new Bardet-Biedl syndrome gene. Am J Hum Genet. 2005;77:1021–33. doi: 10.1086/498323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dagoneau N, et al. DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. Am J Hum Genet. 2009;84:706–11. doi: 10.1016/j.ajhg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arts HH, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–8. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- 159.Beales PL, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–9. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 160.Murcia NS, et al. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–55. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- 161.Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96:5043–8. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nonaka S, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 163.Kyttälä M, et al. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet. 2006;38:155–157. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- 164.Izraeli S, et al. Genetic evidence that Sil is required for the Sonic Hedgehog response pathway. Genesis. 2001;31:72–7. doi: 10.1002/gene.10004. [DOI] [PubMed] [Google Scholar]
- 165.Kumar A, Girimaji SC, Duvvari MR, Blanton SH. Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am J Hum Genet. 2009;84:286–90. doi: 10.1016/j.ajhg.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]