Abstract
TOR (target of rapamycin) signaling regulates lifespan in many organisms, but the mechanism behind the effect is unknown. In this issue of Cell Metabolism, Pan and colleagues find that reduced TORC1 activity promotes yeast lifespan via a mechanism that, paradoxically, relies upon the production of normally deleterious reactive oxygen species.
The budding yeast Saccharomyces cerevisiae has proven to be a remarkably fruitful model for aging researchers. S. cerevisiae undergoes two distinct types of aging; replicative aging, in which the number of daughter cells that bud from a single mother cell is tallied, and chronological aging, which is defined by the length of time that a yeast culture can maintain viability in stationary phase. Yeast replicative aging is mediated, at least in part, by translation, and longevity can be promoted by deletion of ribosomal subunits (reviewed in (Kaeberlein and Kennedy, 2010)). Yeast chronological lifespan, however, is thought to be heavily dependent on resistance to oxidative stress (Fabrizio et al., 2003).
Inhibition of TOR (target of rapamycin) signaling in yeast, either by deletion of TOR1 or by treatment with rapamycin, an FDA-approved inhibitor of mTOR signaling, extends both chronological and replicative lifespan ((Ha and Huh, 2010; Medvedik et al., 2007) and reviewed in (Kaeberlein and Kennedy, 2010)). Studies in yeast, C. elegans and D. melanogaster have linked TOR-mediated lifespan extension to inhibition of translation, and suggest that calorie restriction (CR), an intervention that extends the lifespan of many organisms including mammals, works to some extent through similar mechanisms (reviewed in (Kaeberlein and Kennedy, 2010)). Recent work demonstrating that rapamycin can extend the lifespan of mice has generated significant interest in understanding the mechanism by which inhibition of mTOR signaling promotes lifespan extension (Harrison et al., 2009).
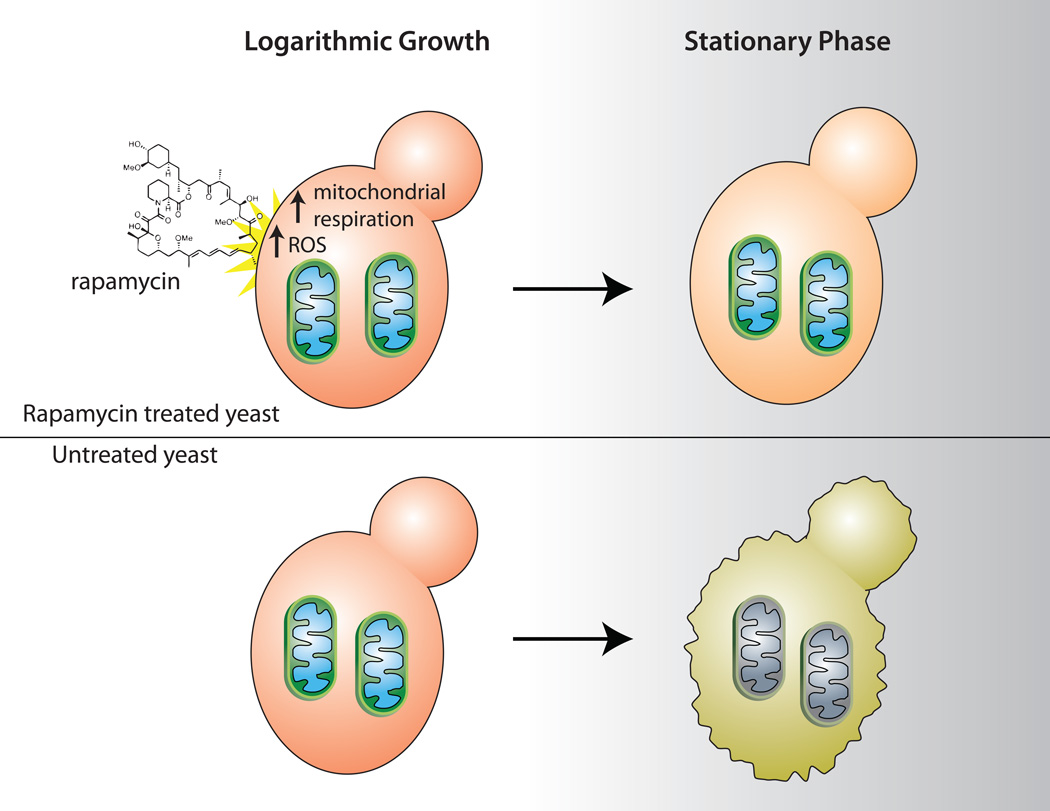
Recently, the validity of using yeast chronological lifespan as a longevity model has been questioned with the discovery that yeast chronological lifespan is significantly inhibited by the buildup of acetic acid and ethanol in the growth media (Burtner et al., 2009; Fabrizio et al., 2005). In this issue of Cell Metabolism, Pan and colleagues show that the effect of TOR on lifespan in yeast is cell-intrinsic and demonstrate that reduced TORC1 signaling leads to increased mitochondrial respiration during logarithmic phase growth, with increased generation of reactive oxygen species (ROS) (Figure 1). Blocking this increase in ROS by overexpressing SOD2, the yeast mitochondrial superoxide dismutase, significantly decreases the ability of TOR inhibition to extend lifespan. This shows that the superoxide signal is the key to yeast chronological lifespan extension.
Figure 1. Rapamycin promotes yeast chronological lifespan.
Rapamycin treatment of a yeast culture (top) during the logarithmic growth phase increases mitochondrial respiration and the generation of reactive oxygen species, resulting in the induction of cellular defense mechanisms that protect the yeast and will preserve viability during stationary phase. In contrast, an untreated yeast culture (bottom) experiences less oxidative stress during logarithmic growth, and is unprepared for the harsh environment of stationary phase. Figure by Tom DiCesare, Whitehead Institute.
Pan and colleagues’ work supports the validity of yeast chronological lifespan as a model for aging, and not simply as an assay for resistance to the detrimental effects of acetic acid as previously suggested (Burtner et al., 2009). The authors find that neutralizing the media, which was previously shown to extend yeast chronological lifespan, significantly alters mitochondrial metabolism. In a series of elegant media-exchange experiments, the authors demonstrate that the effect of TOR on chronological lifespan is not affected by a differential accumulation of acetic acid or other metabolites in the growth media of tor1Δ yeast. While yeast replicative aging genes more closely overlap genes that extend lifespan in C. elegans than yeast chronological lifespan genes (Burtner et al., 2011), homologues of yeast chronological lifespan genes may still play a role in lifespan in higher organisms, and it seems likely that yeast chronological lifespan still has more to teach us about aging.
The surprising concept that mitochondrial superoxide plays a key, positive role in the regulation of lifespan has emerged in the past few years. In 2007, Michael Ristow’s group found that mitochondrial superoxide generation was required for glucose restriction to extend the lifespan of C. elegans (Schulz et al., 2007). More recently, it was demonstrated that a superoxide-based signal was required for CR to extend yeast chronological lifespan (Mesquita et al., 2010). When combined with the present work, these studies provide significant support for the concept that while ROS may contribute to the aging process, the generation of a low level of superoxide in the mitochondria can prime cellular defenses against ROS and other stressors, with a net positive effect on cellular defenses and organismal lifespan. This effect has been named mitochondrial hormesis (mitohormesis). As we continue to learn more about the fundamental mechanisms of aging in mammals, it will not be surprising if mitohormesis, like other key mechanisms of aging, is conserved in humans, mice, flies, worms and yeasts.
It remains an open question if mitohormesis will promote lifespan in mammals. If so, it is possible that antioxidants, which have generally been thought of as beneficial, may actually have negative consequences. . Understanding the mechanism by which increased mitochondrial ROS production leads to extended chronological lifespan may eventually pave the way for the development of small molecules which can activate this pathway without directly inducing oxidative stress. Efforts to find small molecule mimetics of CR will have to deal with the paradoxical conclusion that in order to obtain beneficial effects on lifespan and organismal health, we may have to, first, do a small amount of harm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burtner CR, Murakami CJ, Kennedy BK, Kaeberlein M. A molecular mechanism of chronological aging in yeast. Cell Cycle. 2009;8:1256–1270. doi: 10.4161/cc.8.8.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtner CR, Murakami CJ, Olsen B, Kennedy BK, Kaeberlein M. A genomic analysis of chronological longevity factors in budding yeast. Cell Cycle. 2011:10. doi: 10.4161/cc.10.9.15464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Liou LL, Moy VN, Diaspro A, SelverstoneValentine J, Gralla EB, Longo VD. SOD2 Functions Downstream of Sch9 to Extend Longevity in Yeast. Genetics. 2003;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CW, Huh WK. Rapamycin increases rDNA stability by enhancing association of Sir2 with rDNA in Saccharomyces cerevisiae. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Kennedy BK. Hot topics in aging research: protein translation and TOR signaling, 2010. Aging Cell. 2010;10:185–190. doi: 10.1111/j.1474-9726.2010.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 Link Calorie Restriction and TOR to Sirtuin-Mediated Lifespan Extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5:e261. doi: 10.1371/journal.pbio.0050261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita A, Weinberger M, Silva A, Sampaio-Marques B, Almeida B, Leao C, Costa V, Rodrigues F, Burhans WC, Ludovico P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc Natl Acad Sci U S A. 2010;107:15123–15128. doi: 10.1073/pnas.1004432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose Restriction Extends Caenorhabditis elegans Life Span by Inducing Mitochondrial Respiration and Increasing Oxidative Stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]