Abstract
Strategies have been developed for labeling cells with micron sized iron oxide particles (MPIOs) for in vivo visualization of cells by MRI. Although this approach is well established and has a variety of applications, current protocols employ long labeling times. It has been previously demonstrated that incubation of dextran coated iron oxide nanoparticles with positively charged transfection agents, such as poly-l-lysine (PLL) increases labeling efficiency. Therefore, we sought to ascertain whether pre-incubating MPIOs with various quantities of PLL would similarly enhance the rate of magnetic cell labeling. This was also tested against an NH2 functionalized, commercially available MPIO. Indeed, we demonstrate significantly increased rate of magnetic cell labeling with MPIOs previously incubated with varying amounts of PLL, with robust intracellular labeling at 2 hours. Yet the most robust labeling was achieved with the MPIO-NH2. Interestingly, even for particle formulations which still had negative zeta potential, enhancement of magnetic cell labeling was achieved.
Keywords: MRI, iron, cells, poly-l-lysine, microparticles
INTRODUCTION
Cellular MRI offers the potential to noninvasively track cells in vivo using innovative approaches to cell labeling and image acquisition. The basics of cellular MRI involve labeling cells with MR contrast agents. Incorporation of contrast agents into cells distinguishes their magnetic properties from the surrounding tissue and allows the in vivo identification and tracking of labeled cells by MRI. Most cellular MRI strategies are based on labeling with superparamagnetic iron oxide nanoparticles (SPIOs). SPIO are negative contrast agents that are typically composed of an iron oxide crystal core surrounded by a polymer or polysaccharide shell. Although SPIOs are widely used for cell imaging, large numbers of nanoparticles must be incorporated into the targeted cells to enable their detection by MRI, and cell division can dilute the label beyond detectability (1). To overcome these limitations, micron-sized iron oxide particles (MPIO) were introduced to cellular imaging (2). There are two distinct advantages of MPIOs over smaller particles for cell-specific imaging by MRI.
First, MPIOs have increased iron content per particle. This allows for cells to be labeled with higher amounts of iron. Also, because of the clustered nature of the iron cores within the large particles, the r2* molar relaxivity is higher for MPIOs than SPIOs. Both of these phenomena provide better contrast enhancement for cells labeled with MPIOs. With the smaller SPIO particles, a cell must ingest millions of particles to be detectable by MRI (3). In contrast, the loading of only one or a few MPIO particles per cell is sufficient to produce detectable signal attenuation within one imaging voxel. This significant advantage has lead to the ability to label endogenous neural progenitor cells directly, in vivo (4). The use of MPIOs has also provided for MRI detection of single magnetically labeled cells, in vivo (5, 6).
Second, due to their inert nature, MPIOs allow detection of cells over a longer period. Because smaller particles can be diluted beyond MRI detection, long term MRI tracking of highly proliferative cells is challenging with SPIOs. With MPIOs, it is possible to inject MPIO labeled cells into an area of interest and follow their migration over a period of several days (5, 7). It has been shown that the label in dividing bone marrow-derived stem cells heavily labeled with MPIOs distributes evenly between daughter cells (8). Even after MPIOs are diluted to a single particle per cell, the fate of the daughter cell that still retains the particle can be followed (3). This was demonstrated by injecting MPIOs into a single cell mouse embryo, and performing MRI on the labeled embryo at E11.5. Clearly visible were several dark contrast areas, even after cell division from a single cell to form an embryo, which were identified as single MPIOs on matched histological sections.
Various strategies have been developed for labeling cells with MPIOs and many cell types have been labeled in vitro with these particles, including hepatocytes, macrophages, T cells, and different human tumor cell lines (2, 9–11). Although this approach is well established and has a variety of applications, current protocols employ long labeling times, often overnight incubation, the exception being highly phagocytic cells such as macrophages (12). This is problematic for cells that are difficult to culture such as certain immune cells and hepatocytes. Various modification strategies of USPIOs and SPIOs have been shown to enhance internalization (13, 14). One of the more robust methods is the use of transfection agents (TAs) to coat the surface of SPIOs prior to cell labeling. When the complexes are added to the cell culture, the TA effectively shuttles the (U)SPIO into the cell. It has been shown that mammalian stem cells and other cells from various species (ie, mice, rats, and humans) can be labeled efficiently by simply combining commercially available (FDA-approved) ferumoxides with a polyamine, poly-l-lysine (PLL) (14). Therefore, it was hypothesized that pre-incubating MPIOs with various quantities of PLL would similarly enhance the rate of magnetic cell labeling. This was assayed against an NH2 functionalized commercial MPIO.
MATERIALS AND METHODS
The labeling efficiency and toxicity of different MPIO-PLL formulations, COOH modified (MPIO-COOH) and NH2 modified MPIOs (MPIO-NH2) were compared in mouse embryonic fibroblasts (STOs), rat mesenchymal stem cells (MSCs), and rat bone marrow derived macrophages (BMMs). MPIOs used were 1.63 μm diameter, polystyrene/divinyl benzene coated (Bangs Laboratories). Stock solution of MPIOs is 10 mg/mL. The transfection agent used in this study is poly-L-lysine (Sigma, St Louis, Mo), a 150–300 kDa polyamine. Stock solution of PLL is 1.5 mg/ml. Different formulations of MPIO-PLL were made by incubating MPIO-COOH with different amounts of PLL in dH2O for 3 hours at room temperature with gentle agitation every half hour. MPIO-PLL formulations are expressed as a ratio of mg of iron to mg of PLL. Particle electrophoretic mobilities were measured in pH 7 water using Phase Analysis Light Scattering and zeta potentials determined using the Smoluchowski equation (Zeta PALS, Brookhaven Instruments).
All cells were co-cultured for 1–4 hours in media that contained either commercial green fluorescent MPIO-COOH or the MPIO-PLL complexes in 24-well tissue culture plates. MPIOs were added at a concentration optimized for maximal cell uptake (20 MPIOs per cell), with MPIOs being 3 × 109 MPIOs/ml in the stock solution. Cells were washed with PBS three times to remove free MPIOs. Cells were released from the plate by incubation with trypsin for 3 minutes. Media was then added to inactivate the trypsin and cells were centrifuged at 1200 RPM to remove residual trypsin and resuspended in PBS. Cells were then stained with Sytox Blue Dead Cell Stain (Invitrogen) for viability and immediately analyzed via flow cytometry. Cells were gated on labeled versus non-labeled and live versus dead. Fluorescence microscopy was used to confirm data from flow cytometry.
High resolution confocal fluorescence microscopy was used to determine whether MPIOs were actually internalized or associated on the exterior of the cell membrane. STOs were co-cultured for 1–8 hours in media containing red fluorescent MPIO-COOH or MPIO-PLL complexes on 2 chamber polystyrene tissue culture treated glass slides (BD Biosciences), washed with PBS three times, and then stained with green fluorescent CFSE dye for the cell body and DAPI for the cell nucleus. Slides were imaged using confocal microscopy and number of cells with internalized MPIOs and number of cells with membrane associated MPIOs were counted. For each time point, at least 50 cells were counted. Cells were classified as ‘labeled’ when one or more internalized MPIOs were confirmed, even if there were other non-internalized MPIOs on the same cell. The rationale for a single MPIO being the minimal criteria is that only single MPIOs are required to generate significant contrast by MRI (3). Z-stack images were acquired using Leica SP5 Confocal with a 0.25 μm step size at 512 × 512 resolution, yielding an in-plane pixel resolution of 240 nm. Images were analyzed using Leica LAS AF Lite.
RESULTS
MPIO characterization
Zeta potential of MPIO-COOH was highly negative (Table 1). Notably, the NH2 coated MPIO also had negative zeta potential. All formulations of MPIO-PLL had positive zeta potential, except for the most dilute sample. As expected, increased amounts of PLL resulted in more positive charge.
Table 1.
Zeta potential measurements for the various particle formulations and percentage of live STOs after 4 hours incubation with particles. MPIO-PLL formulations are expressed as a ratio of mg of iron (Fe) to mg of PLL.
| Particle Formulation | Zeta Potential (mV) | % Live Cells |
|---|---|---|
| MPIO-COOH | −19.1 ± 4.9 | 98.9 |
| MPIO-NH2 | −7.97 ± 4.1 | 99.0 |
| 41:1 Fe:PLL | 13.6 ± 2.6 | 96.0 |
| 82:1 Fe:PLL | 14.8 ± 2.5 | 98.7 |
| 409:1 Fe:PLL | 7.08 ± 2.5 | 99.1 |
| 819:1 Fe:PLL | −2.46 ± 4.0 | 99.2 |
Cell labeling with MPIOs
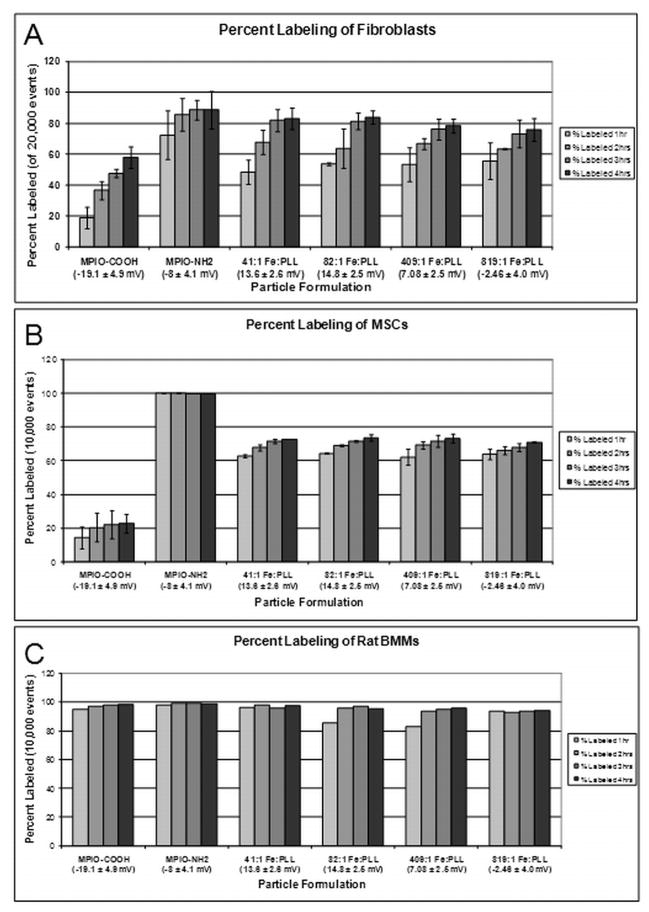
Cells were labeled with the different MPIOs by simple incubation in culture for 1–4 hours. For STOs, a significant amount of MPIO uptake was observed under light microscopy for the 41(mg Fe):1(mg PLL) MPIO-PLL formulation after only 2 hours, whereas the MPIO-COOH displayed very low labeling (Figures 1). Flow cytometry data of labeling showed higher labeling percentages with all formulations of MPIO-PLL particles than with MPIO-COOH, for all time points (Figure 2A). At 3 hours, labeling percentages for all MPIO/PLL formulations plateaued at ~80% of cells, with very little increase in labeling percentages at 4 hours. MPIO-COOH particles had the worst labeling performance, labeling up to ~20% of cells by 4 hours. MPIO-NH2 particles had higher labeling percentages than both MPIO-COOH and MPIO-PLL particles for all time points. With the exception of MPIO-NH2 and 819:1 MPIO-PLL, particles with positive zeta potentials had higher labeling efficiencies. Toxicity assessment of each particle formulation shows at least 96% viable cells for all particle formulations (Table 1).
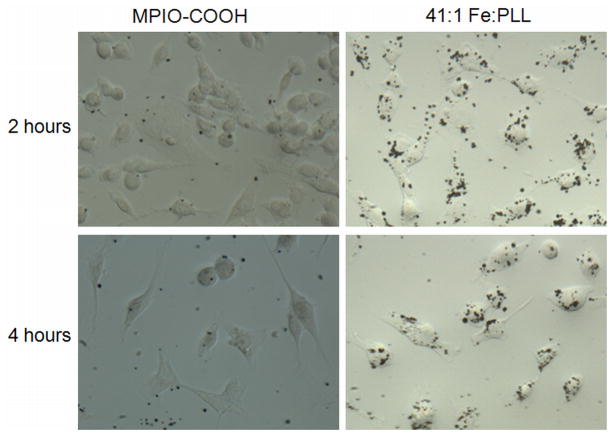
Figure 1.
A comparison between STOs incubated with MPIO-COOH and 41:1 MPIO-PLL at 2 and 4 hour incubation times. More 41:1 MPIO-PLL are internalized within cells than MPIO-COOH particles, at even 2 hours incubation time.
Figure 2.
Flow cytometry data of labeling efficiency versus particle formulation for A) STOs, B) MSCs, C) BMMs. Zeta potential is shown in mV.
For MSCs, a nearly four fold increase in labeled cells was observed after only 1 hour for all MPIO-PLL formulations over the negatively charged MPIO-COOH (Figure 2B). Even at 4 hours incubation time, the MPIO-COOH labeled cell percentage remained at ~20%, whereas all MPIO-PLL labeled cell percentages increased to ~70%. The MPIO-NH2 particles showed a similar trend here as with the STOs, labeling nearly 100% of the MSCs after only 1 hour. For BMMs, all particle types had labeling percentages >80% at only 1 hour, and continued to saturate to nearly 100% at 4 hours (Figure 2C).
MPIO internalization
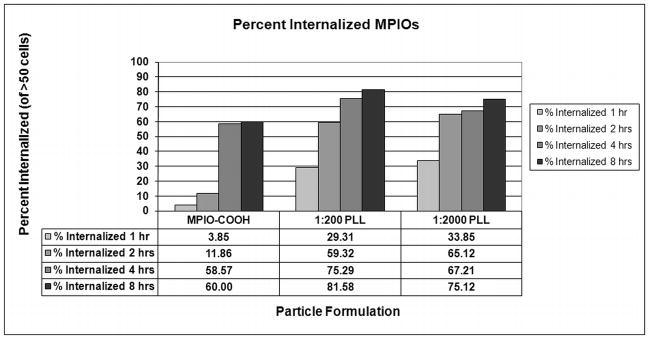
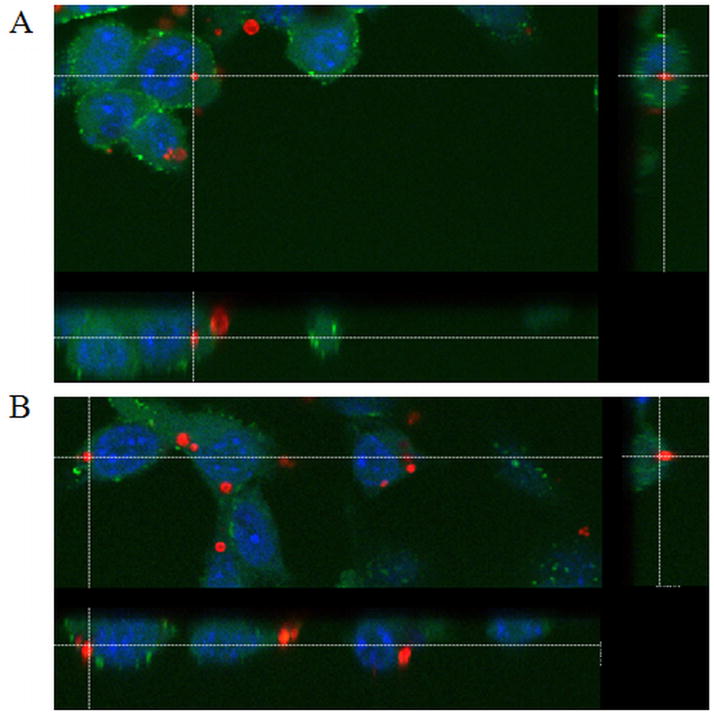
Confocal microscopy analysis showed that at 2 hours, approximately 12 % of STOs contain one or more internalized MPIO-COOH, whereas greater than 50% of the cells incubated with MPIO-PLL formulations had internalized MPIOs (Figure 3). At 4 hours, there was a sharp increase in % internalization for MPIO-COOH to nearly 60%, while the MPIO-PLL percentages also increased to 67–75%. At 8 hours, % internalization for MPIO-COOH remained at 60%, while the MPIO-PLL percentage continued to increase to 75–80%. Figure 4 shows the difference between an internalized (Figure 4A) and a membrane associated MPIO (Figure 4B) as determined by high resolution confocal fluorescent microscopy.
Figure 3.
Percent internalized MPIOs by STOs determined by confocal microscopy. Percentage of labeled cells with one or more internalized MPIOs.
Figure 4.
Confocal z-stack image of A) an internalized MPIO, and B) a surface associated MPIO. Particles are red, CFSE cell tracer dye is green, nucleus is blue. Cells are STOs.
DISCUSSION
Prior uses of MPIOs for magnetic cell labeling have employed long incubation times, usually 16–24 hours. An exception is the work of Van Tiel, et al (15), which achieved robust labeling of HUVEC in 4 hours. This work tested whether, similar to the successes achieved by using PLL to enhance magnetic cell labeling with SPIOs, PLL could enhance labeling with MPIOs and whether this might be better than a commercially available NH2 functionalized MPIO. Three cell types were tested: STOs, MSCs, and BMMs. Similar results were predicted for STOs and MSCs as both cell types grow elongated and adherent, whereas no difference in labeling efficiency among particle types was expected in the highly phagocytic macrophage. Indeed, preincubation of MPIO-COOH with PLL resulted in significantly faster cell labeling than native MPIO-COOH for STOs and MSCs and no difference for BMMs. Interestingly, this was the case for both formulations which had positive and negative zeta potential, in particular, the negatively charged MPIO-NH2, which yielded the fastest labeling rates for both STOs and MSCs. The rapid labeling of BMMs with all particle formulations is in agreement with previous reports of MPIO labeling of macrophages (12).
All MPIO-PLL formulations had a greater than two fold improvement for STOs and greater than three fold improvement for MSCs after only 1 hour. STO images obtained under light microscopy are consistent with the STO flow cytometry data. The lower labeling percentages at 1 hour for MPIO-PLL formulations were due to particles only associating with cells at the cell surface, which was clear in the microscope images too. This suggests that minimum labeling time for MPIO-PLL is between 1 and 2 hours. The labeling efficiency of MPIO-NH2 is also higher than that of the MPIO-COOH for both STOs and MSCs, suggesting that the NH2 groups may have a positive influence on cellular uptake despite the net negative charge on the particle. While 819:1 MPIO-PLL has a small net negative charge, it is clear that the positive charged transfection agent PLL still enhances labeling efficiency just as well in low concentrations. This may be due to the fact that zeta potentials are commonly measured in water, and labeling occurs in cell culture media.
Confocal microscopy data revealed that MPIO-PLL formulations are internalized by STOs over two fold faster than MPIO-COOH at 1–2 hours incubation times. At 4 hours incubation time, there is a marked increase in number of internalized MPIO-COOH (~60%), however the number is still less than the amount of internalized MPIO-PLL formulations at this time point (~70%). It should be noted that even at 8 hours incubation time, there are still some membrane associated extracellular particles, both MPIO-COOH and MPIO-PLL, which are not internalized into cells. There are two possible explanations for this. One is that the cells have reached their maximal capacity for particle uptake. Two is that without trypsinizing the cells off the chamber surface, it is difficult to completely remove unbound particles with just PBS washing. This must be evaluated while taking into account the resolution limits of confocal microscopy. As such, internalized MPIOs close to the surface of the cells have been conservatively discounted as extracellular. However, the presence of extracellular particles, even following extensive washing, is also the case for SPIO complexed with various transfection agents (16).
The principal finding in this study is that complexation of PLL with MPIOs prior to magnetic cell labeling or the use of MPIOs directly functionalized with surface NH2 groups, produces better labeling efficiency than COOH functionalized MPIOs. It also shows that charge and coating of particles does not affect uptake rate of natural phagocytes such as macrophages, which is already fast. Complexation of MPIOs with PLL does not require novel synthesis of iron oxide particles or synthetic modification of the particle coating, and therefore provides a simple, fast method of magnetically labeling cells for in vivo MRI cell tracking. Indeed, there is evidence that complexation of MPIOs with PLL enhances in vivo cell labeling as well (17). Furthermore, MPIOs with NH2 surface functionality are commercially available. While in this case an NH2 functionalized MPIO was available and proved to be optimal, often times other commercial beads or particles synthesized in laboratories do not have nascent NH2 surfaces. In these cases, PLL would likely be useful in enhancing magnetic cell labeling with these particles. This enhancement in cell labeling rate and efficiency is potentially useful for cells that are difficult to maintain in culture, in particular immune cells. In MRI based cell tracking, shortening the required in vitro labeling time will reduce the chances of unintentional cell activation and greatly increase the amount of viable cells available for transfection.
References
- 1.Walczak P, Kedziorek DA, Gilad AA, Barnett BP, Bulte JW. Applicability and limitations of MR tracking of neural stem cells with asymmetric cell division and rapid turnover: the case of the shiverer dysmyelinated mouse brain. Magn Reson Med. 2007;58(2):261–269. doi: 10.1002/mrm.21280. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro EM, Skrtic S, Koretsky AP. Sizing it up: cellular MRI using micron-sized iron oxide particles. Magn Reson Med. 2005;53(2):329–338. doi: 10.1002/mrm.20342. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci U S A. 2004;101(30):10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro EM, Gonzalez-Perez O, Garcia-Verdugo JM, Alvarez-Buylla A, Koretsky AP. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. Neuroimage. 2006;32(3):1150–1157. doi: 10.1016/j.neuroimage.2006.04.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55(2):242–249. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 6.Wu YL, Ye Q, Foley LM, Hitchens TK, Sato K, Williams JB, Ho C. In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc Natl Acad Sci U S A. 2006;103(6):1852–1857. doi: 10.1073/pnas.0507198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill JM, Dick AJ, Raman VK, Thompson RB, Yu ZX, Hinds KA, Pessanha BS, Guttman MA, Varney TR, Martin BJ, Dunbar CE, McVeigh ER, Lederman RJ. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108(8):1009–1014. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinds KA, Hill JM, Shapiro EM, Laukkanen MO, Silva AC, Combs CA, Varney TR, Balaban RS, Koretsky AP, Dunbar CE. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood. 2003;102(3):867–872. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez O, Fricke S, Chien C, Dettin L, Vanmeter J, Shapiro E, Dai HN, Casimiro M, Ileva L, Dagata J, Johnson MD, Lisanti MP, Koretsky A, Albanese C. Contrast-enhanced in vivo imaging of breast and prostate cancer cells by MRI. Cell Cycle. 2006;5(1):113–119. doi: 10.4161/cc.5.1.2295. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro EM, Medford-Davis LN, Fahmy TM, Dunbar CE, Koretsky AP. Antibody-mediated cell labeling of peripheral T cells with micron-sized iron oxide particles (MPIOs) allows single cell detection by MRI. Contrast Media Mol Imaging. 2007;2(3):147–153. doi: 10.1002/cmmi.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juanyin J, Tracy K, Zhang L, Munasinghe J, Shapiro E, Koretsky A, Kelly K. Noninvasive imaging of the functional effects of anti-VEGF therapy on tumor cell extravasation and regional blood volume in an experimental brain metastasis model. Clin Exp Metastasis. 2009;26(5):403–414. doi: 10.1007/s10585-009-9238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAteer MA, Schneider JE, Ali ZA, Warrick N, Bursill CA, von zur MC, Greaves DR, Neubauer S, Channon KM, Choudhury RP. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28(1):77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arbab AS, Bashaw LA, Miller BR, Jordan EK, Bulte JW, Frank JA. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation. 2003;76(7):1123–1130. doi: 10.1097/01.TP.0000089237.39220.83. [DOI] [PubMed] [Google Scholar]
- 14.Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, Bryant LH, Jr, Bulte JW. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228(2):480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 15.van Tiel ST, Wielopolski PA, Houston GC, Krestin GP, Bernsen MR. Variations in labeling protocol influence incorporation, distribution and retention of iron oxide nanoparticles into human umbilical vein endothelial cells. Contrast Media Mol Imaging. 2010;5(5):247–257. doi: 10.1002/cmmi.379. [DOI] [PubMed] [Google Scholar]
- 16.Janic B, Rad AM, Jordan EK, Iskander AS, Ali MM, Varma NR, Frank JA, Arbab AS. Optimization and validation of FePro cell labeling method. PLoS One. 2009;4(6):e5873. doi: 10.1371/journal.pone.0005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vreys R, Velde GV, Krylychkina O, Vellema M, Verhoye M, Timmermans JP, Baekelandt V, Van Der Linden A. MRI visualization of endogenous neural progenitor cell migration along the RMS in the adult mouse brain: Validation of various MPIO labeling strategies. Neuroimage. 2010;49(3):2094–2103. doi: 10.1016/j.neuroimage.2009.10.034. [DOI] [PubMed] [Google Scholar]