Abstract
Background & Aims
Liver disease is a major cause of morbidity and mortality among HIV-infected persons. We evaluated the prevalence, etiology, and factors associated with liver dysfunction in patients during the highly active antiretroviral therapy (HAART) era.
Methods
We performed tests for liver function (baseline and after a 6-month follow-up period) in HIV-infected patients treated at a large clinic. Comprehensive laboratory and ultrasound analyses were performed. Factors associated with liver test abnormalities were assessed using multivariate logistic regression models.
Results
Eighty of 299 HIV-positive patients (27%) had abnormal liver test results during the 6-month study period. The majority of abnormalities were grade 1. Of those with liver test abnormalities, the most common diagnosis was nonalcoholic fatty liver disease (NAFLD, 30%), followed by excessive alcohol use (13%), chronic hepatitis B (9%), chronic active hepatitis C (5%), and other (hemochromatosis and autoimmune hepatitis, 2%); 8 participants (10%) had more than 1 diagnosis. In total, 39 HIV patients with abnormal liver tests (49%) had a defined underlying liver disease. Despite laboratory tests and ultrasound examination, 41 abnormal liver test results (51%) were unexplained. Multivariate analyses of this group found that increased total cholesterol levels (odds ratio 1.6 per 40 mg/dl increase, p=0.01) were associated with liver abnormalities.
Conclusions
Liver test abnormalities are common among HIV patients during the HAART era. The most common diagnosis was NAFLD. Despite laboratory and radiologic investigations into the cause of liver dysfunction, 51% were unexplained, but might be related to unrecognized fatty liver disease.
Keywords: HIV, liver test abnormalities, liver disease, NAFLD, antiretroviral medications
BACKGROUND & AIMS
Liver disease is among the leading causes of death in the general population and has recently been described as a major cause of morbidity and mortality among HIV-infected persons.1 As HIV patients are experiencing longer life expectancies,2 liver disease has been recognized as a major issue, and in some HIV populations, it is now the leading cause of death.1,3–5
Liver test abnormalities are useful surrogates of the burden of liver disease in the population6 and are typically part of routine care for HIV-infected persons. Previous studies have shown that HIV-infected persons frequently have elevated aminotransferase levels.7 Often, laboratory liver test abnormalities in this population are attributed to chronic viral hepatitis (B and C) co-infections due to shared routes of transmission. Other causes of liver disease among HIV patients may include opportunistic pathogens (e.g., cytomegalovirus, Mycobacteria) or tumors (e.g., lymphoma and Kaposi’s sarcoma), although these etiologies have become less common in the era of highly active antiretroviral therapy (HAART).7,8 Ingestion of substances including alcohol, drugs or prescribed medications may also lead to liver abnormalities. With the rising obesity epidemic, reports have suggested that nonalcoholic fatty liver disease (NAFLD) may be an important cause of liver disease in the general population, but data among HIV-infected persons are more limited.6,9,10
No recent study has evaluated the burden and causes of liver disease among HIV patients in the clinical setting. Our aims were to: (1) determine the prevalence of liver enzyme abnormalities, (2) determine the potential cause(s), and (3) identify factors associated with liver test elevations among HIV-infected patients.
METHODS
Demographics of study population
We conducted a cross-sectional study to determine the prevalence and factors associated with liver test abnormalities among HIV-infected patients. Study subjects had confirmed HIV infection by enzyme-linked immunosorbent assay (ELISA) and Western Blot testing, and were receiving care at the Naval Medical Center San Diego (NMCSD), San Diego, California. HIV-infected patients (n=450) attending the clinic were military active duty members, retirees, and dependents. Active duty service members routinely undergo periodic HIV screening (approximately every two years) and mandatory drug testing; service members found positive for illicit drugs are discharged from the military. All HIV-infected persons were invited to participate in this study, excluding those who were <18 years of age or who had a positive pregnancy test. All participants provided written informed consent, and the study was approved by the Institutional Review Board at NMCSD.
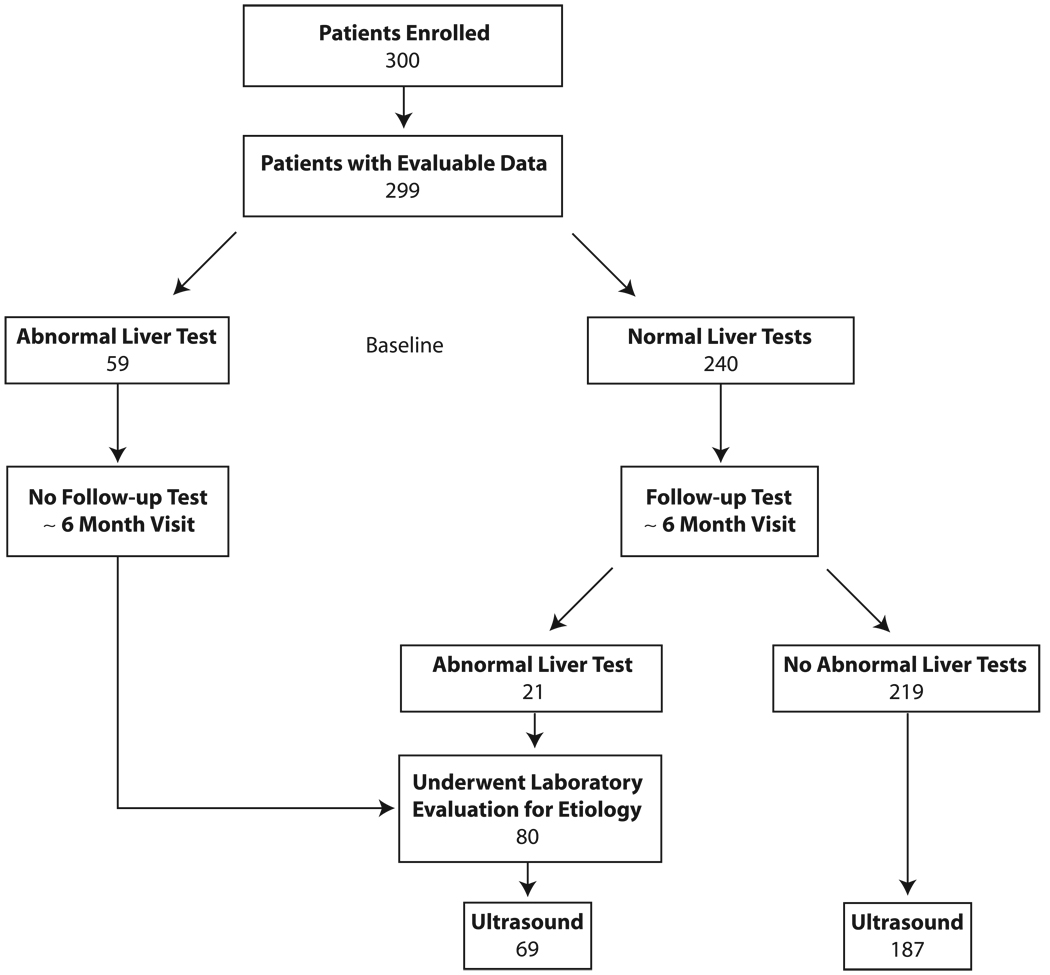
All HIV patients meeting the inclusion/exclusion criteria were asked to join the study during their regular clinic visits, and enrollment continued until 300 participants signed informed consent (January 2006 through June 2007). Of the 300 participants who provided consent, 299 (99.7%) underwent blood testing for alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels, and alkaline phosphatase (Figure 1). Bilirubin was not considered, since it can be elevated in hemolysis and with certain HIV medications (e.g., indinavir, atazanavir), nor was γ-glutamiltranspeptidase (GGT), due to the impact of medications on this test.11 Those with normal liver tests underwent repeat testing at a median time of 6 months (range 3–10 months); patients with abnormal baseline liver tests did not undergo repeat testing. Liver tests were performed at the NMCSD laboratory, which is certified by Clinical Laboratory Improvement Amendments (CLIA). Abnormal values were defined as greater than the upper limits of normal by the local laboratory standards: ALT >63 IU/L, AST >41 IU/L and alkaline phosphatase>126 mg/dl. Liver test abnormalities were graded as follows: 1.25–2.5× the upper limit of normal (ULN) (grade 1), 2.6–5× ULN (grade 2), 5.1–10× ULN (grade 3), and > 10× ULN (grade 4).7
Figure 1.
Flow Diagram of Study of Liver Enzyme Abnormalities among HIV-Infected Persons
In order to detect underlying liver disease, we reviewed the hepatitis panel (hepatitis B surface antigen, hepatitis B core antibody, and hepatitis C antibody tests) drawn as part of routine care; these tests occurred as part of clinical care per the providers discretion and were obtained a median time of 18 months from time of enrollment. In addition, all participants with any abnormal liver tests during the study had a repeat hepatitis panel. All patients with a positive hepatitis B core antibody and negative surface antigen had a hepatitis B DNA (Qiagen, lower limit of detection 40 copies/ml) drawn. In addition, those with abnormal liver tests and a CD4 cell count of <200 cells/mm3 also underwent both hepatitis B DNA and hepatitis C RNA (Applied Bio-System thermocyler sequencer, lower limit of detection of 1,000 RNA copies/ml) viral load testing. Those with a liver test abnormality also underwent blood tests to include iron panel (iron, total iron-binding capacity, ferritin; Beckman Coulter), ceruloplasmin (Behring nephelometry, lower limit of detection 18 mg/dl), alpha-1 antitrypsin phenotype (immunofixation electrophoresis), antinuclear antibody (Bio-Rad immunofluorescence test, detects ≥1:80), and antimitochondrial antibody (Bio-Rad immunofluorescence test, detects ≥1:20). All tests were performed at the NMCSD laboratory, except the hepatitis B and C viral load testing which was performed at ARUP laboratories (Salt Lake City, UT), and the ceruloplasmin and alpha-1 antitrypsin at Quest Diagnostics (San Diego, CA). All study participants were offered an ultrasound evaluation of the liver, regardless of liver test results; 256 (86%) participants had the ultrasound done, while 43 did not complete the test due to work issues, loss of military benefits, or relocation out of the area (Figure 1).
Participants completed a questionnaire regarding alcohol, drug use, and medical history. Study coordinators collected from the participants’ medical records: medical diagnoses; receipt of antiretroviral, antidiabetic, antihypertensive, and lipid-lowering medications; medications that may cause hepatotoxicity; and acetaminophen use. Fasting lipid data were collected including total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides. In addition, the most recent and nadir CD4 cell counts, as well as the current HIV viral load, were recorded at enrollment; an undetectable viral load was defined as <50 copies/ml. Finally, as part of our study, all participants had their body mass index (BMI) and waist circumference measured. These data were entered onto study-specific case report forms.
Chronic active hepatitis B infection was defined as having a positive surface antigen or having core antibody positive with a detectable hepatitis B DNA viral load. Likewise, a chronic hepatitis C infection was defined as having a positive RNA viral load. NAFLD was defined by an ultrasound showing steatosis, described as diffusely increased hepatic echogenicity, according to Rumack et al.,13 and no reported excessive alcohol use. Excessive alcohol use was defined as >140g ethanol/week for men and >70g ethanol/week for women.14 Duration of HIV was defined at the date of enrollment minus the midpoint between the date of last HIV seronegative and first seropositive (median time of seroconversion of one year); for those without a documented HIV seronegative test (n=81, 27%), the first seropositive date was utilized in this calculation. BMI was categorized using NIH criteria; obesity was defined as ≥30 kg/m2.15 Metabolic syndrome was defined by the Adult Treatment Panel (ATP) III guidelines by the presence of ≥3 of the following abnormalities: 1) abdominal obesity (abdominal circumference >102 cm for men and >88 cm for women), 2) elevated triglyceride level (≥150 mg/dl), 3) decreased HDL level (<40 mg/dl for men and <50 mg/dl for women), 4) elevated blood pressure, and 5) elevated fasting glucose (≥110 mg/dl).16
Statistical analysis
Logistic regression models were utilized to evaluate factors associated with liver enzyme abnormalities among participants with no known cause of liver disease. We evaluated the following variables as potential factors associated with liver enzyme abnormalities: demographics; fasting lipid levels; BMI; waist circumference; use of antilipid, antihypertensive, and antidiabetic medications; metabolic syndrome; acetaminophen use; illicit drug use; HIV duration; CD4 cell count (nadir and current); HIV viral load; and antiretroviral medication use. Univariate logistic regression models were used to examine each variable with the presence of any abnormal liver test. Each variable for which the regression coefficient was significant at a p-value <0.10 was included in a final multivariate models. If two variables were significant in the univariate models, but highly correlated and clinically related (e.g., both being lipid levels), only the most significant variable was included in the final model. Correlations between variables were computed using Pearson’s correlation coefficient. Logistic regression models were also utilized to compare those with and without liver disease, and to compare factors between participants with different types of liver disease. P-values of <0.05 were considered to be statistically significant. Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Baseline Demographic and Clinical Characteristics
The median age of the study population (n=299) was 39 years (interquartile range, IQR 30, 46); 279 (93%) were male; 146 (49%) reported being Caucasian, 81 (27%) African American, 42 (14%) Latino/Hispanic, and 30 (10%) other race/ethnicity (Table 1). Eight (3%) participants in the study reported the use of illicit substances. The median duration of HIV infection was 10 years (IQR 4, 17); the median current CD4 cell count was 496 cells/mm3 (IQR 370, 672), and the median viral load was 1.7 log10 copies/ml (IQR 1.7, 3.8), with 52% having an undetectable HIV viral load. At the time of the study, 67% of the participants were receiving antiretroviral therapy; 66% were on a nucleoside reverse transcriptase inhibitor (NRTI); 38%, a protease inhibitor (PI); and 30%, a non-nucleoside reverse transcriptase inhibitor (NNRTI). The median BMI was 25.8 kg/m2 (IQR 23.2, 28.5), and 14% were obese. Seventy-three percent had dyslipidemia, defined as an abnormality in any element of the lipid panel. Twenty-eight percent of participants were receiving lipid-lowering medications; 25%, anti-hypertensive medications; and 4%, medications for the treatment of diabetes.
Table 1.
Selected Characteristics of HIV-Infected Persons by Presence of Liver Test Abnormalities
| Characteristic | Total Cohort | Liver Test Abnormality at Baseline or Follow- up Visit |
Normal Liver Tests |
P-value |
|---|---|---|---|---|
| N=299 | N=80 | N=219 | ||
| Demographics and Behaviors | ||||
| Age, years1 | 39.0 (30.0, 46.0) | 41.0 (33.5, 48.0) | 38.0 (29.0, 46.0) | 0.19 |
| Female | 20 (6.7%) | 3 (3.8%) | 17 (7.8%) | 0.30 |
| Race | 0.87 | |||
| White | 146 (48.8%) | 36 (45.0%) | 110 (50.2%) | |
| African American | 81 (27.1%) | 22 (27.5%) | 59 (26.9%) | |
| Latino/Hispanic | 42 (14.0%) | 14 (17.5%) | 28 (12.8%) | |
| Other race | 30 (10.0%) | 8 (10.0%) | 22 (10.0%) | |
| Illicit drug use | 8 (2.7%) | 2 (2.5%) | 6 (2.7%) | 1.00 |
| HIV Related Factors | ||||
| Duration of HIV Infection1, years | 10 (4, 17) | 14 (6, 20) | 9 (4, 15) | 0.001 |
| Nadir CD4 count1, cells/mm3 | 286 (150, 390) | 252 (76, 325) | 296 (170, 398) | 0.006 |
| CD4 count1, cells/mm3 | 496 (370, 672) | 472 (350, 612) | 520 (380, 680) | 0.20 |
| HIV-RNA1, log10 copies/mL | 1.7 (1.7, 3.8) | 1.8 (1.7, 3.8) | 1.7 (1.7, 3.8) | 0.88 |
| HIV-RNA ≤ 50 | 154 (51.5%) | 39 (48.8%) | 115 (52.5%) | 0.57 |
| On antiretrovirals | 200 (66.9%) | 58 (72.5%) | 142 (64.8%) | 0.21 |
| On NRTI | 196 (65.6%) | 55 (68.8%) | 141 (64.4%) | 0.48 |
| On NNRTI | 90 (30.1%) | 21 (26.3%) | 69 (31.5%) | 0.38 |
| On PI | 114 (38.1%) | 38 (47.5%) | 76 (34.7%) | 0.04 |
| Metabolic Factors | ||||
| Body mass index1, kg/m2 | 25.8 (23.2, 28.5) | 25.8 (23.7, 29.6) | 25.9 (23.1, 28.4) | 0.42 |
| Body mass index > 30 (kg/m2) | 41 (13.7%) | 15 (18.8%) | 26 (11.9%) | 0.13 |
| Waist size1, cm | 89.0 (84.0, 97.0) | 89.5 (84.0, 98.0) | 89.0 (83.0, 97.0) | 0.46 |
| Total cholesterol1, mg/dl | 181 (160, 210) | 194 (172, 227) | 177 (157, 206) | <0.001 |
| Total cholesterol > 200 mg/dl | 96 (32.8%) | 32 (41.6%) | 64 (29.6%) | 0.06 |
| HDL1, mg/dl | 39 (33, 47) | 40 (34, 50) | 39 (32, 46) | 0.29 |
| HDL < 35 mg/dl | 104 (35.5%) | 24 (31.2%) | 80 (37.0%) | 0.36 |
| LDL1, mg/dl | 111 (93, 128) | 116 (95, 140) | 110 (91, 127) | 0.15 |
| LDL > 130 mg/dl | 64 (21.8%) | 18 (23.4%) | 46 (21.3%) | 0.70 |
| Triglyceride1, mg/dl | 128 (80, 208) | 150 (99, 275) | 118 (78, 192) | 0.002 |
| Triglyceride > 150 mg/dl | 125 (42.7%) | 38 (49.4%) | 87 (40.3%) | 0.17 |
| Dyslipidemia2 | 215 (73.4%) | 55 (71.4%) | 160 (74.1%) | 0.65 |
| Metabolic syndrome | 55 (18.4%) | 17 (21.3%) | 38 (17.4%) | 0.44 |
| Other Medication Use | ||||
| On lipid-lowering medication | 84 (28.1%) | 24 (30.0%) | 60 (27.4%) | 0.66 |
| On diabetes medication | 12 (4.0%) | 4 (5.0%) | 8 (3.7%) | 0.74 |
| On antihypertension medication | 74 (24.7%) | 27 (33.8%) | 47 (21.5%) | 0.03 |
| Daily acetaminophen | 14 (4.7%) | 4 (5.0%) | 10 (4.6%) | 1.00 |
| On a potentially hepatotoxic drug | 108 (36.1%) | 28 (35.0%) | 80 (36.5%) | 0.81 |
| Liver Related Conditions | ||||
| Chronic hepatitis B | 19 (6.4%) | 7 (8.8%) | 12 (5.5%) | 0.43 |
| Chronic hepatitis C | 4 (1.3%) | 4 (5.0%) | 0 (0%) | 0.11 |
| Alcohol consumed per week1,3, gm |
14 (0, 56) | 25 (0, 70) | 14 (0, 56) | 0.34 |
| Alcohol overuse | 27 (9.0%) | 10 (12.5%) | 17 (7.8%) | 0.21 |
| NAFLD4 | 78 (26.1%) | 24 (30.0%) | 54 (24.7%) | 0.36 |
| Other Liver Diagnoses5 | 3 (1.0%) | 2 (2.5%) | 1 (0.5%) | 0.18 |
| Any Liver Diagnosis6 | 117 (39.1%) | 39 (48.8%) | 78 (35.6%) | 0.04 |
Median (IQR)
Dyslipidemia defined as any abnormal lipid level
Maximum of baseline and follow-up
US was performed on 256 of the participants
Includes hemochromatosis, autoimmune hepatitis and schistosomiasis
Includes chronic hepatitis B or C, alcohol overuse, NAFLD and other liver diagnoses
Prevalence of Liver Enzyme Abnormalities
Overall, 80 (27%) subjects in the cohort had at least one abnormal liver test during the study period. The descriptive characteristics of HIV-infected persons with and without liver test abnormalities are shown in Table 1. Abnormal liver enzyme values for any liver test, ALT, AST, and alkaline phosphatase were present at baseline in 20%, 10%, 17%, and 4%, respectively (Table 2). Including both baseline and follow-up visits, a total of 27% of participants had at least one liver test abnormality, 13% had an abnormal ALT value, 22% had an abnormal AST, and 4% an abnormal alkaline phosphatase. The majority of liver abnormalities were grade 1, with only 2% of subjects experiencing a grade 2 elevation, and no participant had a grade 3 or 4 abnormality. ALT and AST were highly correlated (r=0.73, p<0.001).
Table 2.
Baseline and Follow-up Liver Enzyme Test Results
| All Patients | Without a Liver Diagnosis1 | |||||
|---|---|---|---|---|---|---|
| N | Median (IQR) | Abnormal (%)2 | N | Median (IQR) | Abnormal (%)2 | |
| Baseline | 299 | 182 | ||||
| ALT (IU/L) | 29 (22, 44) | 30 (10.0%) | 27 (21, 39) | 14 (7.7%) | ||
| AST (IU/L) | 28 (24, 36) | 50 (16.7%) | 27 (23, 34) | 24 (13.2%) | ||
| Alkaline phosphatase (IU/L) | 74 (59, 89) | 11 (3.7%) | 74 (59, 90) | 10 (5.5%) | ||
| Any of the above | 59 (19.7%) | 31 (17.0%) | ||||
| Follow-up (median 6 months) | 215 | 134 | ||||
| ALT (IU/L) | 27 (21, 35) | 9 (4.2%) | 26 (21, 32) | 3 (2.2%) | ||
| AST (IU/L) | 28 (24, 33) | 16 (7.4%) | 27 (23, 33) | 8 (6.0%) | ||
| Alkaline phosphatase (IU/L) | 72 (59, 85) | 2 (0.9%) | 72 (58, 85) | 0 (0.0%) | ||
| Any of the above | 21 (9.8%) | 10 (7.5%) | ||||
| Maximum of both visits | 299 | 182 | ||||
| ALT (IU/L) | 39 (13.0%) | 17 (9.3%) | ||||
| AST (IU/L) | 66 (22.1%) | 32 (17.6%) | ||||
| Alkaline phosphatase (IU/L) | 13 (4.3%) | 10 (5.5%) | ||||
| Any of the above | 80 (26.8%) | 41 (22.5%) | ||||
| Grade 1 | 77 (25.8%) | 39 (21.4%) | ||||
| Grade 23 | 5 (1.7%) | 3 (1.6%) | ||||
Excludes patients with hepatitis B or C, excessive alcohol use, NAFLD, or other known liver disease.
Abnormal liver enzyme tests were defined as follows: ALT > 63 IU/L; AST > 41 IU/L; alkaline phosphatase > 126.
There were no grade 3 or 4 abnormalities.
Presence of Underlying Liver Disease
Among the 80 participants with abnormal liver enzymes, the most common diagnoses were NAFLD (n=24 or 30% of those with an ultrasound) and excessive alcohol use (n=10 or 13%). Chronic active hepatitis B was found in seven participants (9%), while chronic hepatitis C was noted in four (5%) (Table 1). Two other cases of liver disease were newly diagnosed during our investigation: hemochromatosis (elevated transferrin iron binding capacity confirmed by HFE gene with homozygosity for the C282Y mutation) and autoimmune hepatitis (positive antinuclear antibodies); both cases were definitively diagnosed by liver biopsy. Eight participants (10%) had more than one type of liver disease. Overall, 39 (49%) of those with abnormal liver tests had a defined underlying liver disease.
Among all study participants, regardless of liver test results, the most common diagnoses were NAFLD (n=78 or 30% of those with an ultrasound) and excessive alcohol use (n=27 or 9%). Chronic hepatitis B and C was noted in 19 (6%) and 4 (1%) participants, respectively. Sixteen patients (84%) with chronic hepatitis were receiving therapy with anti-hepatitis B activity during the study including tenofovir in combination with lamivudine or emtricitabine (n=11), lamivudine alone (n=4), or entecavir (n=1); no patient with hepatitis C was receiving interferon/ribavirin. Three other cases of liver disease were noted, including hemochromatosis, autoimmune hepatitis, and schistosomiasis. Fourteen participants had more than one liver diagnosis. Overall, 117 (39%) of HIV-infected persons had a potential underlying cause for liver disease.
As noted, not all cases of potential liver disease had elevated liver tests. Participants with concurrent chronic hepatitis C were the most likely to present with abnormal liver tests (100%), followed by those with chronic hepatitis B (37%), NAFLD (31%), and excessive alcohol use (30%). The two participants diagnosed with hemochromatosis and autoimmune hepatitis had abnormal liver tests.
Comparisons of HIV-Infected Persons with and without Known Liver Disease
Characteristics of HIV patients with a documented liver diagnosis (NAFLD, alcohol overuse, and chronic hepatitis B/C) were compared to those with no known liver disease (Table 3). HIV patients with NAFLD compared to those without liver disease were more likely to be Caucasian, obese, have lower HDL levels and higher triglyceride levels, have the metabolic syndrome, use anti-lipid and potentially hepatotoxic medications (all p<0.05). Of note, liver test abnormalities were not significantly different between those with and without NAFLD (p=0.19). Participants with chronic hepatitis B or C were significantly more likely to have elevated liver tests than those without liver disease (p=0.009). Finally, those reporting alcohol overuse did not differ significantly by demographics, HIV related factors, or liver results from those without liver disease. We also compared the three most common causes of liver disease (NAFLD, alcohol overuse, and hepatitis B/C) with each other and found that those with NAFLD had lower HDL levels, higher triglyceride levels, and more likely to have the metabolic syndrome than those with other two liver diagnoses (all with p<0.05) and greater waist circumference (p=0.05) (data not shown).
Table 3.
Selected Characteristics of HIV-Infected Persons by Liver Diagnosis
| Liver Diagnosis Category | |||||||
|---|---|---|---|---|---|---|---|
| NAFLD2 n=78 |
ETOH2 n=27 |
Hepatitis B or C2 n=23 |
None N=182 |
P-value3 | P-value4 | P-value5 | |
| Age (years)1 | 40.5 (32.0, 46.0) | 36.0 (24.0, 42.0) | 45.0 (37.0, 48.0) | 38.5 (29.0, 47.0) | .51 | .13 | .97 |
| Female (%) | 2 (2.6%) | 4 (14.8%) | 1 (4.3%) | 14 (7.7%) | .16 | .26 | 1.00 |
| White (%) | 43 (55.1%) | 13 (48.1%) | 14 (60.9%) | 84 (46.2%) | .04 | .78 | .84 |
| African American (%) | 11 (14.1%) | 7 (25.9%) | 6 (26.1%) | 59 (32.4%) | |||
| Latino/Hispanic (%) | 12 (15.4%) | 6 (22.2%) | 2 (8.7%) | 23 (12.6%) | |||
| Other race (%) | 12 (15.4%) | 1 (3.7%) | 1 (4.3%) | 16 (8.8%) | |||
| Duration of HIV infection, years1 | 10 (5, 19) | 6 (3, 16) | 16 (12, 20) | 9 (4, 16) | .08 | .57 | .32 |
| Nadir CD4 count (cells/mm3)1 | 259 (144, 358) | 320 (160, 433) | 169 (96, 406) | 294 (150, 390) | .32 | .61 | .82 |
| Current CD4 count (cells/mm3)1 | 470 (346, 608) | 462 (328, 644) | 494 (410, 766) | 504 (380, 678) | .25 | .23 | .40 |
| HIV-RNA (log copies/mL)1 | 2.1 (1.7, 4.1) | 2.6 (1.7, 4.1) | 1.7 (1.7, 3.3) | 1.7 (1.7, 3.8) | .25 | .28 | .81 |
| HIV-RNA ≤ 50 (%) | 37 (47.4%) | 11 (40.7%) | 15 (65.2%) | 97 (53.3%) | .39 | .22 | .28 |
| On antiretrovirals (%) | 52 (66.7%) | 15 (55.6%) | 17 (73.9%) | 124 (68.1%) | .82 | .20 | .57 |
| On NRTI (%) | 51 (65.4%) | 15 (55.6%) | 16 (69.6%) | 122 (67.0%) | .80 | .24 | .81 |
| On PI (%) | 31 (39.7%) | 9 (33.3%) | 12 (52.2%) | 68 (37.4%) | .72 | .69 | .17 |
| On NNRTI (%) | 22 (28.2%) | 6 (22.2%) | 5 (21.7%) | 58 (31.9%) | .56 | .31 | .32 |
| Body mass index (kg/m2)1 | 27.9 (24.4, 30.0) | 25.9 (24.4, 29.8) | 27.2 (23.1, 29.0) | 25.2 (23.1, 27.8) | < .001 | .14 | .12 |
| Body mass index > 30 (kg/m2) (%) | 19 (24.4%) | 6 (22.2%) | 2 (8.7%) | 18 (9.9%) | .002 | .06 | .86 |
| Waist size (cm)1 | 93.8 (87.0, 103.5) | 89.5 (85.0, 98.0) | 92.0 (85.0, 98.0) | 88.5 (82.0, 94.0) | < .001 | .21 | .96 |
| Total cholesterol (mg/dl)1 | 184.0 (166.0, 206.0) | 186.0 (162.0, 205.0) | 177.0 (162.0, 196.0) | 179.0 (160.0, 211.0) | .50 | .68 | .45 |
| Total cholesterol > 200 mg/dl (%) | 26 (33.8%) | 7 (25.9%) | 3 (13.0%) | 61 (34.5%) | .91 | .38 | .04 |
| HDL (mg/dl)1 | 33.0 (30.0, 41.0) | 44.0 (34.0, 55.0) | 39.0 (32.0, 48.0) | 40.0 (34.0, 47.5) | < .001 | .14 | .17 |
| HDL < 35 mg/dl (%) | 43 (55.8%) | 8 (29.6%) | 8 (34.8%) | 50 (28.2%) | < .001 | .88 | .52 |
| LDL (mg/dl)1 | 110.0 (89.0, 126.0) | 104.5 (86.0, 148.0) | 107.0 (103.0, 121.0) | 111.5 (92.0, 130.5) | .39 | .91 | .76 |
| LDL > 130 mg/dl (%) | 11 (14.3%) | 8 (29.6%) | 2 (8.7%) | 43 (24.3%) | .07 | .55 | .09 |
| Triglycerides (mg/dl)1 | 177.0 (110.0, 301.0) | 126.0 (78.0, 232.0) | 139.0 (81.0, 179.0) | 109.0 (75.5, 184.5) | < .001 | .61 | < .001 |
| Triglycerides > 150 mg/dl (%) | 48 (62.3%) | 11 (40.7%) | 9 (39.1%) | 64 (36.2%) | < .001 | .65 | .78 |
| Dyslipidemia (%) | 64 (83.1%) | 19 (70.4%) | 13 (56.5%) | 125 (70.6%) | .04 | .98 | .17 |
| Metabolic syndrome (%) | 27 (34.6%) | 5 (18.5%) | 4 (17.4%) | 24 (13.2%) | < .001 | .46 | .58 |
| On lipid-lowering medications (%) | 32 (41.0%) | 5 (18.5%) | 9 (39.1%) | 43 (23.6%) | .005 | .56 | .11 |
| On diabetes medications (%) | 5 (6.4%) | 0 (0.0%) | 1 (4.3%) | 7 (3.8%) | .35 | .60 | 1.00 |
| On antihypertension medications (%) | 23 (29.5%) | 7 (25.9%) | 8 (34.8%) | 43 (23.6%) | .32 | .79 | .24 |
| On hepatotoxic drugs (%) | 40 (51.3%) | 11 (40.7%) | 9 (39.1%) | 56 (30.8%) | .002 | .30 | .42 |
| LFT abnormality (%) | 24 (30.8%) | 10 (37.0%) | 11 (47.8%) | 41 (22.5%) | .16 | .10 | .009 |
Median (IQR)
The three liver-diagnosis categories are not mutually exclusive.
For comparison of those with NAFLD with those with no liver-related diagnosis
For comparison of those with excessive alcohol use with no liver-related diagnosis
For comparison of those with hepatitis B or C with those with no liver-related diagnosis
Factors Associated with Abnormal Liver Enzyme Tests among HIV-Infected Persons with No Known Cause of Liver Disease
Of the 80 subjects in our study population with abnormal liver tests, 41 (51%) were among participants without known liver disease by laboratory testing and imaging. Among those with no defined cause of liver disease, we performed logistic regression models to evaluate for factors associated with liver test abnormalities (Table 4). In the univariate models, low CD4 nadir counts (OR 0.85 per 50 cells/mm3, p=0.003), longer duration of HIV infection (OR 1.52 per year, p=0.009), protease inhibitor use (OR 2.39, p=0.02), and higher LDL (OR 1.38 per 30 mg/dl, p=0.04) and cholesterol levels (OR 1.72 per 40 mg/dl, p=0.003) were associated with abnormal liver tests. Since HIV duration and CD4 nadir were highly correlated as were cholesterol and LDL levels, HIV duration and total cholesterol were used in the final multivariate model. In the multivariate model, elevated total cholesterol levels (OR 1.62, p=0.01) remained significantly associated with abnormal liver tests. Of note, protease inhibitor use and total cholesterol levels were correlated (r=0.2, p<0.01). We repeated the logistic regression model limited to those with an abnormal ALT or AST with similar findings, although the p-values were less significant.
Table 4.
Logistic Regression of Presence of Liver Enzyme Abnormalities (Baseline or Follow-up) among HIV-Infected Persons without a Known Cause of Liver Disease
| LFT Abnormality | Univariate logistic regression |
Multivariate logistic regression1 |
||||
|---|---|---|---|---|---|---|
| Characteristic | Yes | No | Odds ratio |
P- value |
Odds ratio |
P- value |
| Age2,3 | 41.0 (35.0, 48.0) | 38.0 (28.0, 46.0) | 1.26 | .14 | ||
| Female | 3 (7.3%) | 11 (7.8%) | 0.93 | .92 | ||
| White | 17 (41.5%) | 67 (47.5%) | ||||
| African American | 13 (31.7%) | 46 (32.6%) | 1.11 | .80 | ||
| Latino/Hispanic | 7 (17.1%) | 16 (11.3%) | 1.72 | .30 | ||
| Other race | 4 (9.8%) | 12 (8.5%) | 1.31 | .67 | ||
| Illicit drug use | 2 (4.9%) | 3 (2.1%) | 2.36 | .36 | ||
| Daily acetaminophen | 2 (4.9%) | 7 (5.0%) | 0.98 | .98 | ||
| Duration of HIV Infection (years)2 | 14 (5, 20) | 8 (3, 13) | 1.52 | .009 | ||
| Nadir CD4 count (cells/mm3)2,4 | 225 (48, 316) | 318 (187, 398) | 0.85 | .003 | 0.91 | .15 |
| Current CD4 count (cells/mm3)2,5 | 474 (354, 616) | 520 (388, 680) | 0.75 | .38 | ||
| HIV-RNA (log10 copies/mL)2 | 1.7 (1.7, 3.5) | 1.7 (1.7, 3.8) | 0.93 | .64 | ||
| On NRTI | 31 (75.6%) | 91 (64.5%) | 1.70 | .19 | ||
| On PI | 22 (53.7%) | 46 (32.6%) | 2.39 | .02 | 1.65 | .26 |
| On NNRTI | 11 (26.8%) | 47 (33.3%) | 0.73 | .43 | ||
| Body mass index (kg/m2)2 | 24.4 (23.2, 28.0) | 25.4 (23.1, 27.6) | 0.93 | .74 | ||
| Waist size2,6 | 88.0 (83.0, 97.0) | 89.0 (81.8, 94.0) | 1.07 | .68 | ||
| Total cholesterol(mg/dl)2,7 | 195 (178, 234) | 175 (156, 210) | 1.72 | .003 | 1.62 | .01 |
| HDL (mg/dl)2,8 | 46 (37, 51) | 40 (33, 46) | 1.32 | .05 | ||
| LDL (mg/dl)2,9 | 122 (96, 149) | 110 (90, 128) | 1.38 | .04 | ||
| Triglycerides (mg/dl)2,10 | 125 (90, 194) | 104 (71, 178) | 2.17 | .11 | ||
| Metabolic syndrome | 4 (9.8%) | 20 (14.2%) | 0.65 | .46 | ||
| On lipid-lowering medications | 12 (29.3%) | 31 (22.0%) | 1.47 | .34 | ||
| On diabetes medication | 1 (2.4%) | 6 (4.3%) | 0.56 | .60 | ||
| On antihypertensive medication | 13 (31.7%) | 30 (21.3%) | 1.72 | .17 | ||
Odds ratios are from the multivariate logistic regression of the presence of abnormality on selected characteristics, based on results from the univariate logistic regressions.
Median (IQR).
Odds ratio based on square root.
Odds ratio based on 50 cell difference.
Odds ratio based on 10 cell difference (square root).
Odds ratio based on 10 cm difference.
Odds ratio based on 40 mg/dl difference.
Odds ratio based on 10 mg/dl difference.
Odds ratio based on 30 mg/dl difference.
Odds ratio based on 10 mg/dl difference (square root).
In addition to laboratory and ultrasound evaluation, a complete review of all medications was performed. The most common potentially hepatotoxic medications were lipid-lowering drugs (usually HMG CoA-reductase inhibitors) followed by azoles. The current use of these non-HIV medications was not significantly associated with liver test abnormalities in our multivariate models (data not shown). Even if the use of non-HIV medications accounted for the liver abnormalities in all the participants taking these medications, 35% (28/80) of liver abnormalities still had no defined etiology.
CONCLUSIONS
We sought to determine the prevalence of abnormal liver tests and their causes among HIV-infected persons during the HAART era. The prevalence of liver test abnormalities in our HIV population at the baseline visit was 20%. This percentage is higher than that found in the general population by the National Health and Nutrition Examination Survey (NHANES) (10%), but is similar to other studies among HIV-infected persons.6,7,17 Since liver tests are markers of the burden of liver disease in a population,6 these data suggest that HIV-infected persons have a higher rate of underlying liver disease compared to the general population. The reason(s) for this excess prevalence has largely been attributed to concurrent chronic hepatitis B and C infections; however, other medical conditions may play an important role.
We evaluated our HIV population for underlying liver disease among those with abnormal liver tests and found that 30% had NAFLD, 13% had excessive alcohol use, 9% had chronic hepatitis B, 5% had chronic hepatitis C, and 2% had other forms of liver diseases; some cases had multiple potential causes for liver dysfunction. Although previous studies have suggested that the leading cause of elevated liver enzymes is chronic viral hepatitis,4,7 in our HIV cohort, the two most common diagnoses were the often “overlooked conditions” of NAFLD and excessive alcohol use. These data are similar to a recent large study among the general population, which also emphasized the importance of NAFLD and alcohol excess as causes of liver dysfunction.6 Despite our study population having a lower prevalence of obesity compared to that of the U.S. general population18 (likely due to our military-based cohort), we found a considerable prevalence of NAFLD among our HIV patients. Regarding the diagnosis of alcohol overuse, we utilized a similar approach to other studies6 utilizing alcohol self-reporting; however, we acknowledge that a liver biopsy showing evidence of alcoholic hepatitis is the gold standard for this diagnosis. Finally, our rate of hepatitis C was lower than most HIV cohorts and is reflective of our military population which has a low incidence of illicit drug use.
Despite an aggressive work-up for the etiology, 51% of abnormal liver tests in our study remained “unexplained”. A recent study in the general population found that 69% of participants had “unexplained” aminotransferase levels; based on their statistical analyses, many of these cases were attributed to undiagnosed NAFLD.6 Our study had the advantage of utilizing ultrasound evaluations for NAFLD and a panel of blood tests to identify more unusual causes of liver disease. Despite these additional tests, one-half of cases had no clear etiology for the liver test elevations.
We utilized statistical models to identify factors associated with “unexplained” liver test abnormalities in our study population. After excluding known causes of liver disease (i.e., chronic viral hepatitis, alcohol use, NAFLD by ultrasound, and other liver diseases), higher cholesterol levels were significantly associated with “unexplained” liver test abnormalities. Our finding that elevated cholesterol was associated with liver test abnormalities among those with no identifiable cause of liver disease suggests that fatty liver deposition may account for even more cases of liver disease than recognized in our study. The sensitivity of ultrasonography in detecting NAFLD has been estimated by previous reports as 82–94% compared to liver biopsy,19,20; however, since ultrasound is less sensitive in detecting mild or even moderate steatosis than it is for detecting severe steatosis,21 the impact of milder forms of NAFLD on liver test elevations in our study population may be underestimated. Further, some of our participants who had elevated liver tests (n=11) did not have the ultrasound examination completed, which also may have underestimated NAFLD as the cause of liver disease.
Although all classes of antiretroviral medications have been described to potentially cause liver dysfunction,7,22,23 the use of these medications in our study were not significantly associated with liver abnormalities. These data are reassuring, given the increasing use of HAART among HIV-patients as recommended by recent guidelines.24 Protease inhibitor use was associated with liver abnormalities in the univariate models, but after adjusting for lipid levels, was no longer significant. This suggests that liver test abnormalities among HIV patients receiving PIs may be largely related to increased lipid levels, hence, adequate control of lipid levels may be useful in preventing liver test elevations, and perhaps ongoing liver dysfunction among this patient population. We did not find a relationship between NRTIs and transaminase abnormalities; prior studies have shown that nucleoside agents may cause direct hepatotoxicity and steatosis due to inhibition of mitochondrial DNA polymerase-γ;25 our lack of association between NRTIs and liver abnormalities may be attributed to few study participants receiving the NRTIs (e.g., zalcitabine, stavudine or didanosine) most associated with adverse liver effects.11,25
We did not find that demographic information predicted liver test abnormalities. Studies within the US population have shown that male sex, Mexican American ethnicity, and decreasing age were associated with aminotransferase elevations.6,9,26 Concurrent with our results, these associations were not found in a recent study examining HIV-infected persons.17 It is possible that these factors do not play a role in elevated liver tests among this population; alternatively, our study could have missed these associations, since we evaluated a predominantly male population with few Hispanic/Latino participants.
We undertook an extensive evaluation to identify more obscure causes of liver disease. Of those with abnormal liver tests (n=80), two were found to have relatively rare conditions, hemochromatosis and autoimmune hepatitis. Both cases had only mildly elevated ALT values of 93 IU/L and 147 IU/L, respectively. Although identifying more unusual causes of liver disease was uncommon (2.5%), their diagnosis resulted in potentially life-saving therapies (phlebotomy and corticosteroids, respectively). The utility and cost-effectiveness of this aggressive approach is unknown, especially since liver test abnormalities may be transient in nature in up to 30% of the general population;27 longitudinal evaluations of liver tests abnormalities among HIV-infected persons are needed.
On the other hand, reliance on liver test abnormalities alone may lead to under-recognition of liver disease. For example, the majority of our patients with NAFLD and chronic hepatitis B had normal liver tests. A recent study showed that nearly 80% of those with radiologically confirmed NAFLD had a normal ALT level.28 Data in the general population have suggested the use of revised standards for normal transaminase levels (ALT 30 mg/dl for men and 19 mg/dl for women) may be more sensitive for the detection of liver disease.29 We repeated our analyses and found that by this criteria, 58% of HIV patients with hepatitis B had an abnormal ALT, 71% of those with NAFLD, 52% of those with alcohol overuse, and 64% of those with any underlying liver disease. For this study, we utilized our clinical laboratory’s guidelines for abnormal values as these are used in clinical practice; whether revised values would lead to a more accurate detection of liver disease among HIV-infected persons in clinical practice requires further study. Investigations into the ideal screening method(s) and laboratory cut-off values for detecting liver disease among HIV patients are needed.
Our finding of only 4% of HIV-infected persons had an elevated alkaline phosphatase level was lower than that seen in other cohorts, including among HIV patients17. Since high alkaline phosphatase levels may be due to NAFLD and our study cohort had a relatively high prevalence of NAFLD, this finding was surprising. In one study among HIV patients, alkaline phosphatase was linked specifically to diabetes mellitus and elevated BMI; perhaps the low number of patients with abnormal alkaline phosphatase levels was related to our low prevalence of these conditions in our cohort. We also did not find isolated alkaline phosphatase levels in our study which have been noted in patients with NAFLD, although this finding appears more common among females30 and we studied a male-predominant population.
As with all studies, ours has potential limitations. We evaluated HIV patients over a six-month window for liver test abnormalities; additional longitudinal follow-up would help determine the incidence of liver abnormalities and to establish causal relationships. Of note, establishing causality between liver damage and the medications under consideration is difficult, as patients are often treated with many potentially hepatotoxic medications or have other possible causes for liver dysfunction.22 Given that some liver test elevations are transient or may be due to laboratory error or muscle trauma, the natural history of abnormalities and their impact on morbidity and mortality deserves further study. Since our population consisted of mostly non-drug users, the impact of hepatitis C on liver disease was likely understated; our study does, however, provide important information regarding liver disease among those who sexually-acquired HIV. Finally, although we collected data on alcohol use, reporting bias is possible; moreover, HIV-infected persons may be more sensitive to alcohol toxicity, potentially underestimating the impact of alcohol on liver dysfunction.7
Strengths of the study include the extensive evaluation to identify the potential cause of liver disease in a clinic population of HIV-infected patients. We are not aware of any other studies within the HIV population that have evaluated patients using a comprehensive set of laboratory data and ultrasound evaluations. Finally, we had detailed records regarding medication use as well as performed confidential screening for alcohol and drug use. As such, our study adds to the literature regarding the prevalence and causes of liver disease among HIV-infected persons in the HAART era.
In summary, liver abnormalities are common among HIV-infected persons in the HAART era, with one-quarter of patients experiencing at least one liver test elevation during a six-month period. NAFLD and excessive alcohol use were the most common diagnoses among those with elevated liver tests in our study cohort. Owing to the association of high cholesterol levels with liver test abnormalities, fatty deposition may significantly contribute to the high number of ‘unexplained’ liver test abnormalities seen in clinic practice and to the excess burden of liver disease among HIV-infected patients; prospective studies are needed.
Acknowledgments
Support for this work was provided by the Infectious Disease Clinical Research Program (IDCRP), Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD, of which the TriService AIDS Clinical Consortium (TACC) is a component. The IDCRP is a DoD tri-service program executed through USUHS and the Henry M. Jackson Foundation for the Advancement of Military Medicine in collaboration with HHS/NIH/NIAID/DCR through Interagency Agreement HU0001-05-2-0011.
Footnotes
The opinions or ascertains contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Departments of the Army, Navy, or Air Force, or the Department of Defense. The authors have no commercial or other association that might pose a conflict of interest in this work.
This work is original and has not been published elsewhere. Some data contained in this manuscript were presented as abstract #822 at the 14th Conference on Retroviruses and Opportunistic Infections, February 25–28, 2007; Los Angeles, California.
REFERENCES
- 1.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Baker RK, Moorman AC, et al. HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.Soriano V, Martín-Carbonero L, García-Samaniego J, et al. Mortality due to chronic viral liver disease among patients infected with human immunodeficiency virus. Clin Infect Dis. 2001;33:1793–1795. doi: 10.1086/323009. [DOI] [PubMed] [Google Scholar]
- 4.Puoti M, Spinetti A, Ghezzi A, et al. Mortality for liver disease in patients with HIV infection: a cohort study. J Acquir Immune Defic Syndr. 2000;24:211–217. doi: 10.1097/00126334-200007010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficieny virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 6.Ioannou GN, Boyko EJ, Lee SP, et al. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 7.Pol S, Lebray P, Vallet-Pichard A. HIV infection and hepatic enzyme abnormalities: intricacies of the pathogenic mechanisms. Clin Infect Dis. 2004;38:S65–S72. doi: 10.1086/381499. [DOI] [PubMed] [Google Scholar]
- 8.Bonacini M. Hepatobiliary complications in patients with human immunodeficiency virus infection. Am J Med. 1992;92:404–411. doi: 10.1016/0002-9343(92)90271-c. [DOI] [PubMed] [Google Scholar]
- 9.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. N Engl J Med 2000;342:1266–1271. [DOI] [PubMed] [Google Scholar]
- 10.Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250–257. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 11.Núñez M. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol. 2006;44:S132–S139. doi: 10.1016/j.jhep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342:1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 13.Rumack CM, Wilson S, Charboneau WJ, et al., editors. Diagnostic Ultrasound. 3rd ed. Vol 1. St. Louis: Elsevier Mosby; 2005. [Google Scholar]
- 14.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 15.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 51S–209S. [PubMed] [Google Scholar]
- 16.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.Sterling RK, Chiu S, Snider K, et al. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C coinfections. Dig Dis Sci. 2008;53:1375–1382. doi: 10.1007/s10620-007-9999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. [Accessed on August 6];U.S. Obesity Trends. Trends by State 1985–2008. 2009 Available at http://www.cdc.gov/obesity/data/trends.html;
- 19.Joseph AE, Saverymuttu SH, al-Sam S, et al. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol. 1991;43:26–31. doi: 10.1016/s0009-9260(05)80350-2. [DOI] [PubMed] [Google Scholar]
- 20.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 21.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 22.Spengler U, Lichterfeld M, Rockstroh JK. Antiretroviral drug toxicity -- a challenge for the hepatologist? J Hepatol. 2002;36:283–294. doi: 10.1016/s0168-8278(01)00311-7. [DOI] [PubMed] [Google Scholar]
- 23.Savès M, Vandentorren S, Daucourt V, et al. Severe hepatic cytolysis: incidence and risk factors in patients treated by antiretroviral combinations. Aquitaine Cohort, France, 1996–1998. Groupe dEpidémiologie Clinique de Sida en Aquitaine (GECSA) AIDS. 1999;17:F115–F121. doi: 10.1097/00002030-199912030-00002. [DOI] [PubMed] [Google Scholar]
- 24.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Accessed February 1, 2009];Department of Health and Human Services. 2008 November 3;:1–139. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf;
- 25.Bruno R, Sacchi P, Filice G. Mitochondrial toxicity in HIV-HCV coinfection: It depends on the choice of antiretroviral drugs? Hepatology. 2002;35:500–501. doi: 10.1053/jhep.2002.31170. [DOI] [PubMed] [Google Scholar]
- 26.Papatheodoridis GV, Goulis J, Christodoulou D, et al. High prevalence of elevated liver enzymes in blood donors: associations with male gender and central adiposity. Eur J Gastroenterol Hepatol. 2007;19:281–287. doi: 10.1097/MEG.0b013e328011438b. [DOI] [PubMed] [Google Scholar]
- 27.Lazo M, Selvin E, Clark JM. Brief communication: clinical implications of short-term variability in liver function test results. Ann Intern Med. 2008;148:348–352. doi: 10.7326/0003-4819-148-5-200803040-00005. [DOI] [PubMed] [Google Scholar]
- 28.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 29.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–9. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 30.Pantsari MW, Harrison SA. Nonalcoholic fatty liver disease presenting with an isolated elevated alkaline phosphatase. J Clin Gastroenterol. 2006;40:633–635. doi: 10.1097/00004836-200608000-00015. [DOI] [PubMed] [Google Scholar]