Abstract
Purpose
To determine the association of hip circumference with risk of type 2 diabetes in a relatively lean population.
Methods
The relationship between hip circumference for a given waist circumference or BMI and risk of type 2 diabetes was investigated in 56,100 men and 68,273 women, aged 40 to 74, from the Shanghai Men’s Health Study and the Shanghai Women’s Health Study. Cox analyses were used.
Results
During an average of 4.0 years and 7.8 years of follow-up of the men and women, respectively, 2,754 new diabetes cases (955 males; 1,799 females) were documented. After adjustment for BMI, waist circumference, and other potential confounders, the HRs (95% CIs) for type 2 diabetes in quintiles 2 to 5 compared to the first quintile of hip circumference were 0.95 (0.76–1.18), 0.72 (0.57–0.91), 0.83 (0.67–1.04), 0.78 (0.63–0.97), respectively, in men and 0.83 (0.72–0.96), 0.74 (0.64–0.86), 0.72 (0.62–0.84), and 0.65 (0.56–0.75) in women. This relationship was stronger in men and women with a BMI ≤ the median (23.6 kg.m2 in each sex; interaction p-value = 0.04 for men and 0.01 for women).
Conclusion
A larger hip circumference for a given waist circumference and BMI is associated with a reduced risk of type 2 diabetes.
Keywords: type 2 diabetes, hip circumference, waist circumference, body mass index
Type 2 diabetes is becoming an increasing problem worldwide. The rise in Asian populations is particularly alarming, with China accounting for 12% of the global annual increase in diabetes and arguably the highest prevalence worldwide (1). The increased risk of diabetes associated with body mass index (BMI) and abdominal adiposity is well established and is thought to be primarily mediated through increased circulating levels of non-esterified fatty acids, or free fatty acids (FFA), released from the more lipolytically active visceral and abdominal subcutaneous adiposity depots (2–5). FFA interfere with intracellular insulin signaling and increase hepatic gluconeogenesis (6); overexposure of the liver and muscle tissue to FFA leads to hepatic (3, 7) and muscle insulin resistance (3, 8). In addition to the insulin resistance resulting from long term exposure to high FFA levels, high FFA levels are also thought to be toxic to the pancreatic islets (2, 9). In contrast to abdominal fat, gluteofemoral subcutaneous fat is less lipolytically active and appears to serve as a metabolic sink, trapping excess FFA and thus protecting other body tissues and organs from chronic exposure to high levels of FFA. Increased thigh fat is associated with increased insulin sensitivity (10).
A few reports indicate that an increased hip circumference for a given waist circumference is associated with a decreased risk of type 2 diabetes (11–14). However, these reports have been based on European populations. To our knowledge, there has only been one report on the relationship of hip circumference with the risk of type 2 diabetes in a Chinese population; however neither waist circumference or BMI were accounted for (15). Furthermore, it remains unknown whether the association between hip circumference and type 2 diabetes risk may vary by BMI. We report here a comprehensive evaluation of the relationship of hip circumference with the incidence of type 2 diabetes in two large cohort studies of over 130,000 Chinese men and women. Because of the sexual dimorphism in fat distribution (16) and FFA metabolism (16–17), analyses were conducted sex-specifically.
Methods
The Shanghai Women’s Health Study (SWHS) and the Shanghai Men’s Health Study (SMHS) are population-based prospective studies of Chinese women aged 40 to 70 years and men aged 40 to 74 years living in urban Shanghai. The two cohort studies were designed to investigate the association of diet and lifestyle factors with chronic diseases. Details of the study design, methods, and baseline questionnaires have been previously described (18–19). Briefly, trained interviewers recruited 74,942 women between March 1997 and May 2000 and 61,500 men between March 2002 and June 2006 from urban Shanghai and conducted in-person interviews following a standard protocol. The overall response rates were 92% for women and 75% for men. Study protocols were approved by the institutional review boards of all institutes involved; written informed consent was obtained from all participants prior to interview. During the interview, data on dietary habits, physical activity, reproductive history, educational attainment, income, occupation, and physician diagnosis of specific chronic diseases, including diabetes, were collected using structured questionnaires (18, 20). The cohorts were followed by biennial in-person survey and record linkage with the Shanghai vital statistic registry. During follow-up, each participant was asked whether he/she had been diagnosed by a physician to have diabetes since study enrollment. Participants who reported having been diagnosed with diabetes by a physician were further asked to provide information on symptoms, diagnostic tests, the date of first diagnosis, and the use of oral hypoglycemic medications and insulin. The specific information asked for from the diagnostics were a blood glucose concentration ≥11.1 mmol/L, or on two separate occasions a fasting glucose concentration of ≥7 mmol/L. Type 2 diabetes cases included in this study met at least one of the following criteria: reporting a fasting glucose concentration of ≥7 mmol/L on two separate occasions, reporting ≥11.1 mmol/L glucose concentration on an oral glucose tolerance test and/or reporting use of a hypoglycemic agent. All other self-reported cases of diabetes were excluded from analyses.
Anthropometric data was collected during the baseline interview. Height, weight, and waist and hip circumferences were measured by trained interviewers. Measurements of waist circumference were taken 2.5 cm above the umbilicus and hip circumference at the maximum width of the buttocks. Circumferences were measured to the nearest 0.1 cm. Weight was measured to the nearest 0.1 kg. BMI was calculated (kg/m2).
Detailed assessment of physical activity was obtained using a validated questionnaire (PAQ) (21–22), which evaluated regular exercise and sports participation during the last 5 years. Exercise/sports energy expenditure was estimated, using standard metabolic equivalent values (METs) (23), by the weighted average of energy expended in all activities reported over the last 5 years preceding the survey (MET-hours/day/year). Information on daily life activities was also collected and METs calculated using a compendium of physical activity values (23). Total physical activity (MET/hr/day/year) was calculated by combining leisure time physical activity, daily living physical activity and commuting physical activity. Energy intake was estimated based on a validated food frequency questionnaire (24) and nutrient information from the Chinese Food Composition Tables (25).
There were 7,357 (3,865 men; 3,492 women) participants who reported having diabetes at baseline and another 2,081 tested positive for glucose in their urine at baseline; these participants were excluded from analyses. Further exclusions included baseline cancer cases (n=1,415), those with missing follow-up data on diabetes (n=77), self-reported incident diabetes cases not meeting the above listed criteria for a diabetes case (n=980), and individuals with missing anthropometric data (n=2).
The student’s t test and the chi-square test were used to compare differences in continuous and categorical data, respectively. Cox proportional hazards analysis with age as the time scale was used to determine the association of each anthropometric index with diabetes risk. Covariates adjusted in the analysis included educational attainment level, occupational status, income, hypertension, a family history of diabetes, energy intake, total physical activity, current smoking status, and alcohol consumption. To control for BMI and waist circumference in the association of hip circumference with type 2 diabetes, models included both waist circumference and the residuals of BMI after being regressed on waist circumference as covariates. To maximize the number of participants in each group, the median BMI (23.6 kg/m2 in both men women) and median waist circumference (85 cm in men and 77 cm in women) were used in stratified analyses. A high BMI were defined as being above the median BMI and a high waist circumference as above the median waist circumference. The restricted cubic spline function in Cox proportional hazards analyses, with follow-up time as the time scale, was also used to evaluate the shape of the association of hip circumference residuals with type 2 diabetes risk. Knots were placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of hip circumference residuals. The 50th percentile of hip circumference residuals was used as the reference (26). The variance inflation factor (VIF) was used to evaluate multicollinearity in models including more than one anthropometric measurement. The VIF for the various anthropometric variables was ≤ 2, suggesting that multicollinearity was not a significant concern. A p-value of <0·05 was considered statistically significant. All analyses were conducted using SAS 9·1·3 (Cary, N.C.).
Results
During follow-up (an average of 4.0 years in men and 7.8 years in women), 2,760 individuals developed diabetes. Baseline characteristics of study participants are presented in Table 1. Incident cases of type 2 diabetes were older, had a higher BMI, waist circumference, hip circumference, and waist-to-hip ratio, compared with participants who did not develop diabetes. They were less educated, more likely to come from lower income families, and were more likely to have a history of smoking and a family history of type 2 diabetes. Physical activity was slightly lower in men, but not women, who developed type 2 diabetes.
Table 1.
Baseline Characteristics of Study Participants, mean (SE) or % (n)
| Men | Women | |||
|---|---|---|---|---|
| Cases (n=955) | Non-cases (n=55,148) | Cases (n=1799) | Non-cases (n=66,474) | |
| Age, yr | 56.9 (0.31) | 54.4 (0.04) § | 56.0 (0.20) | 51.4 (0.03) § |
| BMI, kg/m2 | 26.0 (0.10) | 23.6 (0.01) § | 26.8 (0.09) | 23.8 (0.01) § |
| Waist, cm | 91.3 (0.27) | 84.7 (0.04) § | 85.6 (0.21) | 77.2 (0.03) § |
| Hip, cm | 98.2 (0.21) | 94.3 (0.03) § | 101.2 (0.20) | 95.6 (0.03) § |
| WHR | 0.93 (0.002) | 0.90 (0.0002) § | 0.85 (0.001) | 0.81 (0.0002) § |
| Education | ||||
| College+ | 24.5 (229) | 23.6 (12,818) | 10.3 (186) | 13.9 (9,267) § |
| <Middle school | 7.3 (68) | 6.3 (3,422) | 35.7 (643) | 19.3 (12,823) |
| Middle/high school | 68.2 (638) | 70.1 (38,141) | 53.9 (970) | 66.8 (44,372) |
| Income | ||||
| High income | 9.7 (92) | 9.7 (5,352)* | 28.0 (504) | 34.4 (22,883) § |
| Low income | 51.2 (488) | 55.2 (30,369) | 33.2 (598) | 26.7 (17,744) |
| Middle income | 39.1 (373) | 35.1 (19,307) | 38.7 (697) | 38.9 (25,832) |
| Occupation | ||||
| Professional | 28.9 (276) | 26.1 (14,375)* | 25.3 (455) | 29.0 (19,275) § |
| Clerical | 23.5 (224) | 21.9 (12,055) | 18.8 (339) | 20.8 (13,810) |
| Manual laborer | 47.6 (454) | 52.0 (28,654) | 55.5 (998) | 49.9 (33,161) |
| Housewife | N/A | N/A | 0.39 (7) | 0.34 (228) |
| Current smoker | 51.7 (494) | 59.8 (32,988) § | 2.5 (44) | 2.2 (1,490) |
| Ever smoker | 65.6 (626) | 70.2 (38,732) † | 3.5 (62) | 2.6 (1,721) * |
| Current drinker | 24.8 (237) | 29.9 (16,477) ‡ | 1.8 (32) | 2.0 (1,335) |
| Tertiles of total physical activity (mets) | ||||
| Tertile 1 | 36.7 (350) | 32.9 (18,162) * | 32.0 (575) | 33.0 (21,956) |
| Tertile 2 | 31.6 (302) | 33.0 (18,206) | 34.7 (625) | 33.0 (21,909) |
| Tertile 3 | 31.7 (303) | 34.1 (18,780) | 33.0 (599) | 34.0 (22,609) |
| Energy intake, kcal | 1935.5 (16.03) | 1917.2 (2.06) | 1,696.3 (9.9) | 1,680.0 (1.6) |
| Family history of diabetes | 26.0 (246) | 16.0 (8,782) § | 29.1 (502) | 15.2 (9,344) § |
p<0.05
p<0.01
p<0.001
p<0.0001
Energy intake natural logarithmically transformed before analyses
Table 2 shows the multivariable adjusted risk of type 2 diabetes, by sex, for each of the four anthropometric indices. For each of the anthropometric indices, type 2 diabetes risk increased with increasing adiposity. All four anthropometric measures similarly predicted type 2 diabetes risk.
Table 2.
Risk of Diabetes by Anthropometric Index
| Men | Women | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| BMI, kg/m2* | 1.88 | 1.76–2.01 | 1.70 | 1.63–1.77 |
| Waist circumference, cm* | 1.89 | 1.77–2.03 | 1.88 | 1.80–1.97 |
| Hip circumference, cm* | 1.59 | 1.49–1.70 | 1.56 | 1.50–1.63 |
| WHR * | 1.66 | 1.55–1.77 | 1.50 | 1.45–1.55 |
Expressed as per standard deviation change.
Multivariable analyses also controlled for education, income, occupational status, energy intake, physical activity, current smoker status, alcohol consumption, hypertension, and a family history of type 2 diabetes.
Pearson correlation analyses revealed strong correlations between hip circumference and waist circumference (r=0.81 and 0.82 for men and women, respectively), hip circumference and BMI (r=0.76 and 0.80 for men and women, respectively), and waist circumference and BMI (r=0.84 for both men and women); a moderate correlation between WHR and BMI (r=0.57 and 0.46 for men and women, respectively); and a modest correlation between WHR and hip circumference (r=0.27 and 0.19 for men and women, respectively).
The effect of hip circumference on type 2 diabetes risk after accounting for the effects waist circumference and BMI are presented in Tables 3–5. The first model in each table demonstrates the univariate risk of type 2 diabetes by quintiles of hip circumference. Model 2 demonstrates this risk after the effect of waist circumference has been accounted for. Model 3 shows the effect of hip circumference on risk of type 2 diabetes after accounting for both waist circumference and BMI. The final model further controls for hypertension, physical activity, caloric consumption, smoking, a family history of type 2 diabetes, education, and income.
Table 3.
Risk of Diabetes by Quintiles of Hip Circumference Residuals
| Quintiles of Hip Circumference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||||||
| Referent | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | p-trend | ||
| Men | |||||||||||
| N | 11,101 | 11,060 | 11,003 | 11,069 | 10,901 | ||||||
| Model 1 | Ref | ---- | 1.33 | 0.97–1.82 | 1.51 | 1.12–2.04 | 2.43 | 1.85–3.18 | 4.38 | 3.39–5.65 | <0.0001 |
| Model 2 | Ref | ---- | 1.02 | 0.82–1.26 | 0.78 | 0.62–0.99 | 0.92 | 0.74–1.15 | 0.95 | 0.77–1.17 | 0.46 |
| Model 3 | Ref | ---- | 0.96 | 0.78–1.20 | 0.72 | 0.57–0.90 | 0.82 | 0.65–1.02 | 0.78 | 0.63–0.96 | 0.008 |
| Model 4 | Ref | ---- | 0.95 | 0.76–1.18 | 0.72 | 0.57–0.91 | 0.83 | 0.67–1.04 | 0.78 | 0.63–0.97 | 0.0145 |
| Women | |||||||||||
| N | 13,536 | 13,745 | 13,781 | 13,542 | 13,641 | ||||||
| Model 1 | Ref | ---- | 1.60 | 1.27–2.01 | 1.90 | 1.51–2.40 | 3.01 | 2.45–3.71 | 5.41 | 4.44–6.61 | <0.0001 |
| Model 2 | Ref | ---- | 0.88 | 0.77–1.01 | 0.80 | 0.69–0.92 | 0.80 | 0.70–0.93 | 0.77 | 0.68–0.89 | 0.0001 |
| Model 3 | Ref | ---- | 0.84 | 0.73–0.96 | 0.73 | 0.63–0.85 | 0.72 | 0.62–0.84 | 0.64 | 0.55–0.74 | <0.0001 |
| Model 4 | Ref | ---- | 0.83 | 0.72–0.96 | 0.74 | 0.64–0.86 | 0.72 | 0.62–0.84 | 0.65 | 0.56–0.75 | <0.0001 |
Mean hip circumferences in quintiles 1–5 were 89.6, 92.3, 94.1, 96.0, and 99.8 cm, respectively in men and 90.8, 92.9, 95.1, 97.3, and 102.6 cm, respectively in women.
Model 1: unadjusted model with hip circumference.
Model 2: controlled for waist circumference.
Model 3: controlled for waist circumference and BMI.
Model 4: controlled for waist circumference, BMI, education, income, occupational status, energy intake, physical activity, current smoker status, alcohol consumption, hypertension, and a family history of type 2 diabetes.
Table 5.
Risk of Diabetes by Quintiles of Hip Circumference Residuals Stratified by Median Waist Circumference
| Quintiles of Hip Circumference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||||||
| Referent | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Men | |||||||||||
| Waist circumference ≤ median | |||||||||||
| N | 5,803 | 6,077 | 6,017 | 5,834 | 5,577 | ||||||
| Model 1 | Ref | ---- | 1.05 | 0.72–1.53 | 1.00 | 0.67–1.51 | 1.34 | 0.86–2.10 | 0.65 | 0.20–2.08 | 0.59 |
| Model 2 | Ref | ---- | 0.87 | 0.57–1.31 | 0.51 | 0.32–0.81 | 0.51 | 0.32–0.82 | 0.56 | 0.35–0.87 | 0.0009 |
| Model 3 | Ref | ---- | 0.80 | 0.53–1.21 | 0.45 | 0.28–0.72 | 0.44 | 0.27–0.70 | 0.43 | 0.27–0.68 | <0.0001 |
| Model 4 | Ref | ---- | 0.81 | 0.53–1.23 | 0.44 | 0.27–0.72 | 0.44 | 0.27–0.71 | 0.46 | 0.28–0.71 | <0.0001 |
| Waist circumference > median | |||||||||||
| N | 5,298 | 4983 | 4986 | 5235 | 5,324 | ||||||
| Model 1 | Ref | ---- | 0.74 | 0.38–1.44 | 0.57 | 0.30–1.08 | 0.71 | 0.39–1.31 | 1.14 | 0.63–2.08 | <0.0001 |
| Model 2 | Ref | ---- | 1.05 | 0.82–1.36 | 0.88 | 0.68–1.15 | 1.07 | 0.84–1.38 | 1.10 | 0.87–1.40 | 0.39 |
| Model 3 | Ref | ---- | 1.01 | 0.78–1.30 | 0.81 | 0.63–1.06 | 0.96 | 0.75–1.23 | 0.92 | 0.72–1.17 | 0.45 |
| Model 4 | Ref | ---- | 0.98 | 0.76–1.27 | 0.83 | 0.64–1.09 | 0.98 | 0.76–1.27 | 0.91 | 0.71–1.17 | 0.54 |
| Women | |||||||||||
| Waist circumference ≤ median | |||||||||||
| N | 6,381 | 7,946 | 7,878 | 8,023 | 6,619 | ||||||
| Model 1 | Ref | ---- | 1.29 | 0.98–1.70 | 1.06 | 0.76–1.50 | 1.27 | 0.89–1.82 | 2.52 | 1.41–4.50 | 0.05 |
| Model 2 | Ref | ---- | 0.84 | 0.61–1.15 | 0.67 | 0.48–0.94 | 0.50 | 0.35–0.71 | 0.66 | 0.47–0.93 | 0.0004 |
| Model 3 | Ref | ---- | 0.80 | 0.58–1.10 | 0.62 | 0.44–0.87 | 0.44 | 0.31–0.64 | 0.54 | 0.38–0.78 | <0.0001 |
| Model 4 | Ref | ---- | 0.79 | 0.58–1.10 | 0.63 | 0.45–0.88 | 0.45 | 0.31–0.65 | 0.56 | 0.39–0.80 | <0.0001 |
| Waist circumference > median | |||||||||||
| N | 7,155 | 5,799 | 5,903 | 5,519 | 7,022 | ||||||
| Model 1 | Ref | ---- | 0.87 | 0.55–1.38 | 0.76 | 0.49–1.20 | 0.91 | 0.59–1.40 | 1.36 | 0.89–2.08 | <0.0001 |
| Model 2 | Ref | ---- | 0.89 | 0.77–1.05 | 0.83 | 0.71–0.98 | 0.91 | 0.77–1.06 | 0.82 | 0.70–0.95 | 0.01 |
| Model 3 | Ref | ---- | 0.85 | 0.73–1.00 | 0.77 | 0.65–0.91 | 0.82 | 0.69–0.96 | 0.68 | 0.58–0.80 | <0.0001 |
| Model 4 | Ref | ---- | 0.85 | 0.73–0.99 | 0.77 | 0.66–0.91 | 0.82 | 0.69–0.96 | 0.69 | 0.59–0.81 | <0.0001 |
Model 1: unadjusted model with hip circumference.
Model 2 controlled for waist circumference.
Model 3 controlled for waist circumference and BMI.
Model 4 controlled for waist circumference, BMI, education, income, occupational status, energy intake, physical activity, current smoker status, alcohol consumption, hypertension, and a family history of type 2 diabetes.
Model 1 in Table 3 shows that with each quintile of hip circumference, type 2 diabetes risk increased in both men and women. Model 2 shows that when the risk associated with waist circumference was accounted for, the increased risk associated with hip circumference disappeared in men, while in women a protective effect emerged. Model 3 shows that a protective effect of hip circumference emerged in men after further controlling for BMI, while this additional adjustment appeared to strengthen the protective association in women. Further adjustment for other potential confounders added no additional information (model 4).
When stratified by median BMI (Table 4), the protective effect of a higher hip circumference appeared to be stronger in those with a BMI ≤ the median (p-values for interaction terms=0.04 for men and 0.01 for women), although for men this was only significant in the two highest quintiles (models 2–4). For women, this significant protective effect was observed beginning in quintile 3. Controlling for BMI (model 3) and other potential confounders (model 4) did not appear to have any further effect beyond that of waist circumference (model 2), with exception of that of women above the median BMI, where further controlling for BMI appeared to strengthen the protective association in the highest quintile (model 3).
Table 4.
Risk of Diabetes by Quintiles of Hip Circumference Residuals Stratified by Median Body Mass Index (BMI)
| Quintiles of Hip Circumference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||||||
| Referent | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | p-trend | ||
| Men | |||||||||||
| BMI ≤ median | |||||||||||
| N | 6,309 | 5948 | 5,520 | 5,325 | 4,503 | ||||||
| Model 1 | Ref | ---- | 0.84 | 0.55–1.30 | 1.15 | 0.76–1.76 | 1.56 | 1.00–2.44 | 0.91 | 0.36–2.29 | 0.12 |
| Model 2 | Ref | ---- | 0.95 | 0.62–1.46 | 0.73 | 0.46–1.16 | 0.56 | 0.34–0.94 | 0.59 | 0.35–1.00 | 0.006 |
| Model 4 | Ref | ---- | 0.92 | 0.60–1.41 | 0.69 | 0.44–1.10 | 0.52 | 0.31–0.87 | 0.54 | 0.31–0.92 | 0.002 |
| Model 4 | Ref | ---- | 0.93 | 0.610–1.43 | 0.69 | 0.43–1.11 | 0.51 | 0.30–0.86 | 0.57 | 0.33–0.98 | 0.004 |
| BMI > median | |||||||||||
| N | 4,792 | 5,112 | 5,483 | 5,744 | 6,398 | ||||||
| Model 1 | Ref | ---- | 0.90 | 0.53–1.53 | 0.62 | 0.37–1.03 | 0.83 | 0.51–1.35 | 1.35 | 0.84–2.18 | <0.0001 |
| Model 2 | Ref | ---- | 1.00 | 0.78–1.29 | 0.75 | 0.58–0.98 | 0.96 | 0.75–1.23 | 0.96 | 0.76–1.21 | 0.74 |
| Model 3 | Ref | ---- | 0.97 | 0.76–1.25 | 0.72 | 0.55–0.93 | 0.89 | 0.70–1.15 | 0.84 | 0.67–1.07 | 0.16 |
| Model 4 | Ref | ---- | 0.94 | 0.73–1.22 | 0.73 | 0.56–0.95 | 0.92 | 0.72–1.18 | 0.84 | 0.66–1.08 | 0.23 |
| Women | |||||||||||
| BMI ≤ median | |||||||||||
| N | 7,308 | 7,929 | 7,417 | 6,750 | 4,723 | ||||||
| Model 1 | Ref | ---- | 1.36 | 1.03–1.79 | 1.15 | 0.83–1.61 | 1.39 | 0.98–1.96 | 1.51 | 0.76–3.00 | 0.08 |
| Model 2 | Ref | ---- | 0.88 | 0.66–1.18 | 0.68 | 0.49–0.94 | 0.50 | 0.35–0.74 | 0.55 | 0.36–0.84 | <0.0001 |
| Model 3 | Ref | ---- | 0.86 | 0.65–1.15 | 0.65 | 0.47–0.90 | 0.48 | 0.33–0.70 | 0.51 | 0.33–0.79 | <0.0001 |
| Model 4 | Ref | ---- | 0.86 | 0.65–1.15 | 0.67 | 0.49–0.93 | 0.50 | 0.34–0.73 | 0.53 | 0.34–0.81 | <0.0001 |
| BMI > median | |||||||||||
| N | 6,228 | 5,816 | 6,364 | 6,792 | 8,918 | ||||||
| Model 1 | Ref | ---- | 1.02 | 0.64–1.63 | 1.03 | 0.66–1.62 | 1.33 | 0.87–2.05 | 2.08 | 1.36–3.17 | <0.0001 |
| Model 2 | Ref | ---- | 0.87 | 0.74–1.03 | 0.81 | 0.69–0.96 | 0.85 | 0.72–0.99 | 0.77 | 0.67–0.90 | 0.001 |
| Model 3 | Ref | ---- | 0.84 | 0.72–0.99 | 0.77 | 0.65–0.91 | 0.79 | 0.67–0.93 | 0.68 | 0.58–0.79 | <0.0001 |
| Model 4 | Ref | ---- | 0.84 | 0.72–0.99 | 0.77 | 0.65–0.91 | 0.79 | 0.67–0.93 | 0.69 | 0.59–0.81 | <0.0001 |
Model 1: unadjusted model with hip circumference.
Model 2 controlled for waist circumference.
Model 3 controlled for waist circumference and BMI.
Model 4 controlled for waist circumference, BMI, education, income, occupational status, energy intake, physical activity, current smoker status, alcohol consumption, hypertension, and a family history of type 2 diabetes.
When stratified by median waist circumference (Table 5), in men with a waist circumference below the median, hip circumference demonstrated a linearly protective relationship with type 2 diabetes risk once the effect of waist circumference was accounted for (model 2), which was strengthened after further controlling for BMI (model 3), but no significant association was seen in men with a waist circumference > the median (p-value for interaction term=0.02). In women with a waist circumference ≤ the median, after adjusting for waist circumference (model 2) there was a strong reduction in risk compared to the first quintile, which was strengthened after further controlling for BMI (model 3). In women with a waist circumference > the median, there was also a reduction in risk after adjusting for waist circumference (model 2), which was also strengthened after further controlling for BMI (model 3) (p-value for interaction term=0.13) (Table 5). Further adjustment for other potential confounders added not additional information in either sex (model 4). In all models in which waist circumference or BMI was a covariate, waist circumference and BMI remained positively associated with the incidence of type 2 diabetes.
Ten percent of men and 14% of women had BMI greater than or equal to the World Health Organization’s (WHO) Asian-specific threshold for obesity (27.5 kg/m2), while 29% of the men and 40% of the women had a waist circumference ≥ of the WHO Asian-specific threshold for abdominal obesity (90 cm for men and 80 cm for women). When the data was reanalyzed stratified by these cutpoints, similar results were obtained. Figures 1 and 2, by use of cubic splines, graphically depict the multivariable association of the residuals of hip circumference, after being regressed on waist circumference, with type 2 diabetes risk in men in women, respectively.
Figure 1.
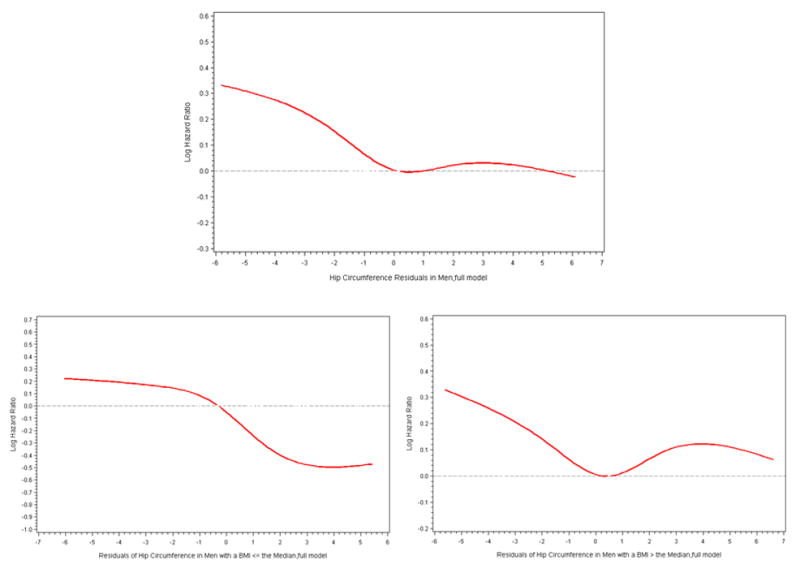
Cubic Splines of Risk Diabetes in Men by the Residuals of Hip Circumference Regressed on Waist Circumference. Knots were placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of hip circumference residuals. The 50th percentile of hip circumference residuals was used as the reference. Multivariable Cox proportional hazards model additionally controlled for waist circumference, residuals of BMI regressed on waist circumference, education, income, occupational status, energy intake, physical activity, current smoker status, alcohol consumption, hypertension, and a family history of type 2 diabetes.
Figure 2.
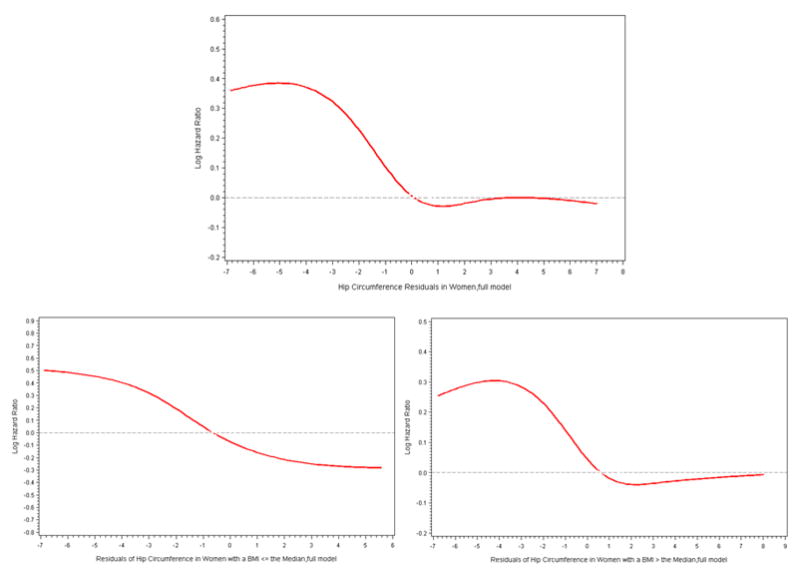
Cubic Splines of Risk of Diabetes in Women by the Residuals of Hip Circumference Regressed on Waist Circumference. Knots were placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of hip circumference residuals. The 50th percentile of hip circumference residuals was used as the reference. Multivariable Cox proportional hazards model additionally controlled for waist circumference, residuals of BMI regressed on waist circumference, education, income, occupational status, energy intake, physical activity, current smoker status, alcohol consumption, hypertension, and a family history of type 2 diabetes.
Discussion
The increased risk of type 2 diabetes associated with increased BMI and abdominal adiposity are well established. Consistent with this, we found that all three of the commonly used anthropometric risk markers, i.e. BMI, waist circumference, and the waist-to-hip ratio, similarly predicted type 2 diabetes (27). In this large Asian population, we also found that a larger hip circumference was associated with a reduced type 2 diabetes risk after accounting for overall and central obesity and also found that this relationship was greater in lean individuals. Furthermore, in mutually adjusted analyses, although a larger hip circumference was associated with a lower risk, waist circumference and BMI remained positive risk factors for type 2 diabetes.
Our results are in agreement with a few European studies that have shown a protective effect of a larger hip circumference on type 2 diabetes risk. In an analysis of 1405 Swedish women, narrower hips were associated with the 24-year incidence of diabetes after controlling for BMI, waist circumference, age, and smoking status (14). A smaller hip circumference for a given waist circumference was also predictive of the incidence of type 2 diabetes in 1357 men and women from the Horne Study (11). To our knowledge, only one other study has looked at hip circumference as a predictor of type 2 diabetes in a Chinese population. Wang et al prospectively followed 2,190 Taiwanese men and women and found that a larger hip circumference was positively associated with the incidence of type 2 diabetes (15). However, waist circumference was not accounted for. In our study, the association of a larger hip circumference with a lower risk of developing type 2 diabetes only emerged after accounting for waist circumference.
The association of a larger hip circumference with a lower risk of developing type 2 diabetes may be attributed to gluteofemoral subcutaneous fat functioning as a ‘metabolic sink’, entrapping FFA (28–29). In women, the increased lipoprotein lipase activity in this region increases the catabolism of triglycerides and their storage into adipocytes. In both men and women, adipocytes in gluteofemoral adipose tissue are less lipolytically active than abdominal visceral or subcutaneous adipose tissue (30–31). Gluteofemoral subcutaneous adipose tissue has been shown to be more insulin sensitive than upper body adipose tissue. Lower fasting insulin levels (32), lower blood glucose concentrations (33), a lower HbA1c (33), and increased insulin sensitivity are observed in those with a larger hip circumference. In particular, greater thigh subcutaneous adipose tissue is associated with lower insulin levels and increased insulin sensitivity (34–35). Thus the reduced incidence of type 2 diabetes associated with a larger hip circumference in our population, when other anthropometric measures were held constant, may relate to protective effects of subcutaneous fat.
The stronger relationship of hip circumference with a reduced risk of type 2 diabetes in those with a BMI below the median may be due to larger hips potentially reflecting increased gluteofemoral muscle mass, particularly in men. Skeletal muscle is the predominant target of insulin action; decreased thigh muscle mass, independent of abdominal fat, is a strong correlate of insulin resistance (36). However, skeletal muscle is also the main site of insulin resistance, postulated to be due to intramyacellular lipid derivatives of FFA interfering with insulin signaling (37–38) and with the oxidative capacity of muscle tissue (39). As muscle mass increases with weight even in the obese, and hip circumference generally increases with waist circumference and BMI, increased intramuscular fat in those with a high BMI or waist circumference may have counteracted the effect of increased muscle mass and/or subcutaneous fat observed in participants with a larger hip circumference in our population.
The sex difference observed in this study, particularly at a high BMI or waist circumference, may be due to hip circumference better reflecting peripheral adipose tissue in women (40) and better reflecting muscle mass in men (41). Lipoprotein lipase activity is increased in the peripheral adipose tissue in women and in abdominal adipose tissue in men. Thus a larger hip circumference may indicate increased storage of FFA in, and slower release from, gluteofemoral subcutaneous adipose tissue in women. In men, a larger hip circumference and a high BMI or waist circumference may reflect increased muscle mass as well as an increase in FFA stored in, and released from, the more lipolytically active abdominal fat, thus offsetting the increased skeletal muscle insulin sensitivity. The lack of a relationship between hip circumference with type 2 diabetes risk in men with a BMI or waist circumference greater than the median seems to support this. In the Atherosclerosis Risk in Communities study, the protective effect of a larger hip circumference for a given waist circumference and BMI was only observed in women (42).
A strength of this study is its prospective design; thus our results are not likely to be due to reverse causality. Additionally, residual analysis was employed in order to avoid the multicollinearity of the anthropometric indices. Finally, all height, weight, and waist and hip circumferences in this large study population were measured by trained personnel. However, a major limitation of this study was that diabetes status was based on self-reported survey responses. Nevertheless, our results are consistent with previous reports showing a protective effect of hip circumference on type 2 diabetes risk determined by an oral glucose tolerance test (11–12). Finally, we had no direct measures of FFA, gluteofemoral fat mass, or muscle mass and therefore could not provide direct evidence supporting the proposed underlying biological mechanisms.
Conclusion
In this report, a larger hip circumference was associated with a reduced type 2 diabetes risk once waist circumference and BMI were accounted for. This association was stronger in women and men below the median BMI or waist circumference. In addition to the established protective effect of greater muscle mass, these findings support the evolving knowledge of the protective role gluteofemoral subcutaneous fat in diabetes.
Acknowledgments
This study was funded by grants and contracts from the National Cancer Institute, US (R37 CA070867, WZ; R01 CA082729 and NO2-CP-11010-66, XOS). The funder played no role in the conduct of the study or development of the manuscript. The authors would also like to thank the participants of the Shanghai Women’s Health Study and Shanghai Men’s Health Study for the invaluable contribution to this work. The authors have no conflicts of interests to declare.
List of abbreviations and acronyms
- BMI
body mass index
- FFA
free fatty acids
- HbA1c
glycosylated hemoglobin A1c
- MET
metabolic equivalents
- SHWS
Shanghai Women’s Health Study
- SMHS
Shanghai Men’s Health Study
- WHR
waist-to-hip ratio
Footnotes
Author contributions
Baqiyyah Conway conducted the literature search, analyzed the data, wrote the manuscript. Yong-Bing Xiang collected the data, reviewed the manuscript for critical intellectual content. Raquel Villegas contributed to the discussion, reviewed/edited the manuscript. Xianglan Zhang contributed to the discussion, reviewed/edited the manuscript. Honglan Li collected the data, reviewed/edited the manuscript. Xiaoyan Wu analyzed the data. Gong Yang provided oversight for the field operations, reviewed/edited the manuscript. Yu-Tang Gao collected the data, reviewed/edited the manuscript. Wei Zheng designed the study, obtained funding, reviewed/edited the manuscript. Xiao Ou Shu designed the study, obtained funding, contributed to the discussion, reviewed/edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of Diabetes among Men and Women in China. N Engl J Med. 2010;362(12):1090–101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 2.Boden G, Shulman G. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32:S14–S23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Boden G. Free fatty acids-the link between obesity and insulin resistance. Endocr Pract. 2001;7(1):44–51. doi: 10.4158/EP.7.1.44. [DOI] [PubMed] [Google Scholar]
- 4.Arner P. Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev. 2002;18(Suppl 2):S5–S9. doi: 10.1002/dmrr.254. [DOI] [PubMed] [Google Scholar]
- 5.Despres J, Lemieux S, Lamarche B, Prud’homme D, Moorjani S, Brun L, et al. The insulin resistance-dyslipidemic syndrome: contribution of visceral obesity and therapeutic implications. Int J Obes Relat Metab Disord. 1995;19(Suppl 1):S76–S86. [PubMed] [Google Scholar]
- 6.Randle P. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev. 1998;14(4):263–83. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Griffin M, Marcucci M, Cline G, Bell K, Barucci N, Lee D, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations inthe insulin signalling cascade. Diaetes. 1999;48(6):1270–4. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 8.Boden GLM. Lipids and glucose in type 2 diabetes: what is the cause and effect? Diabetes Care. 2004;27:2253–9. doi: 10.2337/diacare.27.9.2253. [DOI] [PubMed] [Google Scholar]
- 9.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51(5):1437–42. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 10.Goodpaster B, Thaete F, Kelley D. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–92. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 11.Snijder M, Dekker J, Visser M, Bouter L, Stehouwer C, Kostense P, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr. 2003;77:1192–7. doi: 10.1093/ajcn/77.5.1192. [DOI] [PubMed] [Google Scholar]
- 12.Snijder M, Zimmet P, Visser M, Seidell J, Shaw J. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab Study. Int J Obes. 2004;48(3):402–9. doi: 10.1038/sj.ijo.0802567. [DOI] [PubMed] [Google Scholar]
- 13.Seidell J, Han T, Feskens E, Lean M. Narrow hips and broad waist circumference independently contribute to increased risk on non-insulin-dependent diabetes mellitus. J Intern Med. 1997;242:401–6. doi: 10.1046/j.1365-2796.1997.00235.x. [DOI] [PubMed] [Google Scholar]
- 14.Lissner L, Bjorkelund C, Heitmann B, Seidell J, Bengtsson C. Larger hip Circumference Independently Predicts Health and Longevity in a Swedish Female Cohort. Obes Res. 2001;9:644–6. doi: 10.1038/oby.2001.85. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Pan W, Hwu C, Ho L, Lo C, Lin S, et al. Incidence of NIDDM and the effects of gender, obesity, and hyperinsulinaemia in Taiwan. Diabetologia. 1997;40(12):1431–8. doi: 10.1007/s001250050846. [DOI] [PubMed] [Google Scholar]
- 16.Votruba SB, Jensen MD. Regional fat deposition as a factor in FFA metabolism. Annu Rev Nutr. 2007;27:149–63. doi: 10.1146/annurev.nutr.27.061406.093754. [DOI] [PubMed] [Google Scholar]
- 17.Mittendorfer B. Sexual dimorphism in human lipid metabolism. J Nutr. 2005;135(4):681–6. doi: 10.1093/jn/135.4.681. [DOI] [PubMed] [Google Scholar]
- 18.Zheng W, Chow W, Yang G, Jin F, Rothman N, Blair A, et al. The Shanghai Women’s Health Study: Rationale, Study Design, and Baseline Characteristics. Am J Epidemiol. 2005;162(11):1123–31. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 19.Cai H, Zheng W, Xiang Y, Wang X, Yang G, Li H, et al. Dietary patterns and their correlates among middle-aged and elderly Chinese men: a report from the Shanghai Men’s Health Study. Br J Nutr. 2007;98:1006–13. doi: 10.1017/S0007114507750900. [DOI] [PubMed] [Google Scholar]
- 20.Villegas R, Yang G, Gao Y-T, Cai H, Li H, Zheng W, et al. Dietary patterns are associated with lower incidence of type 2 diabetes in middle-aged women: the Shanghai Women’s Health Study. Int J Epidemiol. 2010 doi: 10.1093/ije/dyq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jurj AL, Wen W, Xiang YB, Matthews CE, Liu D, Zheng W, et al. Reproducibility and validity of the Shanghai Men’s Healht Study physical activity questionnaire. Am J Epidemiol. 2007;165(10):1124–33. doi: 10.1093/aje/kwk119. [DOI] [PubMed] [Google Scholar]
- 22.Matthews CE, Shu XO, Yang G, Jin F, Ainsworth BE, Liu D, et al. Reproducibility and validity of the Shanghai Women’s Health Study physical activity questionnaire. Am J Epidemiol. 2003;158(11):1114–22. doi: 10.1093/aje/kwg255. [DOI] [PubMed] [Google Scholar]
- 23.Ainsworth B, Haskell W, Whitt M, Irwin M, Swartz A, Strath S, et al. Compendium of physical activitiess: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2001;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 24.Shu X-O, Yang G, Jin F, Liu D, Kushi L, Wen W, et al. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women’s Health Study. Eur J Clin Nutr. 2004;58(1):17–23. doi: 10.1038/sj.ejcn.1601738. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Wang G, Pan X. China Food Composition. Beijing: Beijing University Medical Press; 2002. [Google Scholar]
- 26.Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; [Google Scholar]
- 27.Vazquez G, Duval S, Jacobs D, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115–28. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- 28.Lemieux I. Energy Partitioning in Gluteal-Femoral Fat: Does the Metabolic Fate of Trilycerides Affect Coronary Heart Disease Risk? Arterioscler Thromb Vasc Biol. 2004;24:795–7. doi: 10.1161/01.ATV.0000126485.80373.33. [DOI] [PubMed] [Google Scholar]
- 29.Manolopous K, Karpe F, Frayn K. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes. 2010 doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 30.Dowling H, Fried S, Pi-Sunyer F. Insulin resistance in adipocytes of obese women: effects of body fat distribution and race. Metabolism. 1995;44:987–95. doi: 10.1016/0026-0495(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 31.Wahrenberg H, Lonnqvist F, Arner P. Mechanisms underlying regional differences in lipolysis in human adipose tissue. J Clin Invest. 1989;84:458–67. doi: 10.1172/JCI114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seidell J, Perusse L, Despres J, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74(3):315–21. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 33.Snijder M, Dekker J, Visser M, Yudkin J, Stehouwer C, Bouter L, et al. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn Study. Obes Res. 2003;11(1):104–11. doi: 10.1038/oby.2003.18. [DOI] [PubMed] [Google Scholar]
- 34.Schram M, Schalkwajk C, Bootsma A, Fuller J, Chaturvedi N, Stehouwer C, et al. Advanced glycation end products are associated with pulse pressure in type 1 diabetes: the EURODIAB Prospective Complications Study. Hypertension. 2005;46(1):232–7. doi: 10.1161/01.HYP.0000164574.60279.ba. [DOI] [PubMed] [Google Scholar]
- 35.Yim J, Heshka S, Albu J, Heymsfield S, Gallagher D. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J Appl Physiol. 2008;104:700–7. doi: 10.1152/japplphysiol.01035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodpaster B, Theate F, Simoneau J, Kelley D. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–85. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 37.Summers S. Ceramides in insuliin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Summers S, Garza L, Zhou H, Birnbaum M. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457–64. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coen P, Dube J, Amati F, Stefanovic-Racic M, Ferrell R, Toledo F, et al. Insulin Resistance is Associated with Higher Intramyocellular Triglycerides in Type I but not Type II Myocytes Concomitant with Higher Ceramide Content. Diabetes. 2010;59:80–8. doi: 10.2337/db09-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rebuffe-Scrive M, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J Clin Invest. 1985;75:1973–6. doi: 10.1172/JCI111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chowdhury B, Lantz H, Sjostrom L. Computed tomography-determined body composition in relation to cardiovascular risk factors in Indian and matched Swedish males. Metabolism. 1996;45:634–44. doi: 10.1016/s0026-0495(96)90036-0. [DOI] [PubMed] [Google Scholar]
- 42.Parker E, Pereira M, Stevens J, Folsom A. Association of Hip Circumference with Incident Diabetes and Coronary Heart Disease. The Atherosclerosis Risk in Communities Study. 2009;169:837–847. doi: 10.1093/aje/kwn395. [DOI] [PMC free article] [PubMed] [Google Scholar]


